Pregnant women are often and understandably anxious about the health effects of environmental exposures on the fetus and particularly concerned about exposure to radiation. Unfortunately, many in the medical community are unfamiliar with the topic (as was abundantly clear following the Chernobyl nuclear power plant event in 1986, when many European women had abortions on the advice of their physicians, although the fetal radiation doses to these patients were far below any level of concern).Reference Castronovo1 This lack of understanding may lead clinicians to provide poor advice and may leave patients confused and fearful.
Because radiation sources are ubiquitous in nature (eg, radon, cosmic radiation), all humans are exposed to a small amount of natural background radiation (approximately 0.36 rem or 0.0036 Sv/y on average).2 (For a brief review of ionizing radiation, visit http://www.remm.nlm.gov/remm_RadPhysics.htm.) In addition to natural background radiation, radiation doses to the fetus are typically low, usually the result of diagnostic medical procedures3 or occupational radiation exposures within the regulatory limits set by the US Nuclear Regulatory Commission (NRC) of 5 rem (0.05 Sv) per year for workers who have not declared themselves pregnant and 0.5 rem (0.005 Sv) for declared pregnant workers during the entire pregnancy.4 (For more information on women's occupational health, visit http://www.cdc.gov/niosh/topics/women.) Nevertheless, some radiation incidents could expose an expectant mother to doses large enough to be of health concern. Interventional medical procedures (eg, “procedures comprising guided therapeutic and diagnostic interventions, by percutaneous or other access, usually performed under local anesthesia and/or sedation, with fluoroscopic imaging used to localize the lesion/treatment site, monitor the procedure, and control and document the therapy”),5 radiation therapy,3 or a nuclear or radiological incident (including terrorism)Reference Donnelly, Farfan and Parker6 could result in the fetus receiving high doses of radiation.
The health consequences of high radiation doses to an embryo or fetus (possibly large enough to result in acute radiation syndrome in the expectant mother) can result in intrauterine fetal demise. Even at doses too low to affect the mother immediately, radiation consequences can include growth retardation, gross congenital organ malformations (including microcephaly, hydrocephalus, porencephaly, skull malformations, hypoplastic genitalia, cleft palate, hypospadius, eye defects, skeletal defects, and neurological and motor deficiencies),Reference Mettler and Upton7 impaired brain function, and cancer.38Reference Gusev, Guskova and Mettler910Reference Otake, Schull and Lee1112Reference Schull1314Reference Sasaski, Kasuga and Sato1516
In an attempt to assist clinicians in treating patients who have been exposed to radiation, the Centers for Disease Control and Prevention has produced a variety of products, including fact sheets, satellite broadcasts, videos, and CD-ROM training materials (available at http://www.bt.cdc.gov/radiation/toolkit.asp). One product under development is an all-encompassing reference handbook and self-study guide to be entitled “Clinical Response to Nuclear and Radiological Incidents Including Terrorism,” which will contain a chapter on prenatal radiation exposure. The present article summarizes that chapter, providing clinicians with background information regarding prenatal radiation exposure. The article is intended as an aid in counseling pregnant patients who have been exposed to radiation.
REVIEW OF FETAL DEVELOPMENT
A conceptus develops in 3 distinct stages. These are described in Table 1.
TABLE 1 Review of Fetal Development

CHEMICAL TOXICITY TO THE FETUS FROM RADIONUCLIDES
In almost all cases, the toxicity of the radioactive isotopes of an element derives from the radiation emitted, not from the chemical properties of the element. For example, an ingested amount of only 100 μg of a common isotope such as cesium 137 could deliver a lethal radiation dose, but this would be far too little mass of cesium to be chemically toxic.17 For an isotope such as polonium 210, it would require a factor of 1000 less mass to give a lethal dose from the radiation than from the chemical toxicity.Reference Harrison, Leggett, Lloyd, Phipps and Scott18Reference Scott19Reference Brosh-Nissimov, Havkin, Davidovitch, Poles and Shapira20 The rare exceptions to this principle are for such isotopes as uranium 238, with a half-life greater than 1 billion years.21 Only in those cases of extremely long half-lives are the toxic chemical properties of an element of greater significance than the toxicity due to its emitted radiation. Such long-lived radionuclides have the additional challenge (depending on their chemical form) of crossing the placental barrier in quantities sufficient to be of concern to the fetus. (Only 1.8% of uranium reaching maternal blood will cross the placental barrier.)Reference Sikov and Hui22
ESTIMATING THE RADIATION DOSE TO THE EMBRYO OR FETUS
Adverse health effects in the fetus depend on the fetal radiation dose and the gestational stage at the time of exposure. The fetal dose and gestational age must therefore both be estimated before potential health effects can be assessed.
Fetal dose estimations from medical exposures to pregnant women, as calculated in detail by Russell et al,Reference Russell, Stabin, Sparks and Watson23 are found in the International Commission on Radiological Protection publication 84, Pregnancy and Medical Radiation.3 As shown in the first 2 tables of that publication,3 typical fetal doses from diagnostic radiology or diagnostic nuclear medicine rarely exceed 0.025 Gy (2.5 rad) for a single procedure (including a pelvic computed tomographic scan). Fetal doses below 0.05 Gy (5 rad) are considered to be low-risk exposures for which the potential risks to the fetus are likely outweighed by the benefit to the mother.
In radiotherapy (or interventional medical procedures), the dose to the fetus is dependent largely on the distance of the fetus from the radiation field. In addition, the dose to the mother from an interventional medical procedure can vary broadly, depending on the duration of the procedure.5 Thus, there are no typical fetal doses from radiation therapy or interventional procedures to the mother. For example, fetal doses for a typical treatment regimen for brain cancer are in the magnitude of 0.03 Gy (3 rads), whereas for anterior and posterior mantle treatments of the chest for Hodgkin disease, they can be as much as 0.4 to 0.5 Gy (40-50 rad).3 Doses for other interventional or therapeutic procedures may exceed the doses cited here and pregnant patients should discuss any risk to the fetus with their health care providers.
Resources for Fetal Dose Estimation
Therefore, for radiotherapy, as well as for nonclinical nuclear or radiological exposure incidents (including terrorism) in which fetal doses can range from no exposure to 100 Gy (10 000 rad), clinicians should consult with experts in radiation dosimetry about fetal dose estimation. Hospital medical physicists and health physicists are good resources for radiation dose estimation. In addition, in the United States, the Conference of Radiation Control Program Directors maintains a list of state radiation control/radiation protection contact information at http://www.crcpd.org/Map/map.html. The American Board of Health Physics maintains a listing of active certified health physicists at http://www.hps1.org/aahp/members/members.htm, and the American Board of Medical Physicists maintains a list of diplomates in medical physics specialties at http://www.abmpexam.com. Clinicians should contact these or other radiation protection organizations for assistance in estimating fetal radiation dose.
POTENTIAL NONMALIGNANT HEALTH EFFECTS OF PRENATAL RADIATION EXPOSURE
Table 238Reference Gusev, Guskova and Mettler910Reference Otake, Schull and Lee1112Reference Schull1314Reference Sasaski, Kasuga and Sato1516 summarizes potential noncancer health risks of prenatal radiation exposure. This table can assist clinicians in understanding the potential harm that can result from high exposures to radiation and in providing care to pregnant women who may have been exposed to radiation (recommendations in individual cases should be made in consultation with a hospital medical physicist or health physicist). The indicated doses and times postconception, or gestational age, are approximations.
TABLE 2 Potential Health Effects (Other Than Cancer) of Prenatal Radiation Exposure
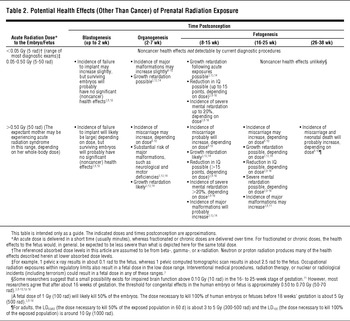
Failure to Implant
Failure of a blastocyst to implant in the uterine wall is a common occurrence. Even in the absence of radiation exposure, implementation failure is on the order of 30% to 50%. After the embryo implants, however, the miscarriage rate decreases to about 15% for the remainder of the pregnancy. The cells then begin to differentiate into various stem cells that eventually form all the organs in the body.8Reference Gusev, Guskova and Mettler91012
During blastogenesis, the embryo is comprised of only a few cells. Damage to a single cell, the progenitor of many other cells, can cause the embryo's death, and the blastocyst will fail to implant in the uterus. This can occur at doses above 0.5 Gy (50 rad). Embryos that survive radiation exposure during blastogenesis likely will not have been damaged by that radiation exposure at all and, consequently, will have low risk of congenital abnormalities.3812
Growth Retardation
Ionizing radiation can interfere with the development of the central nervous system or other major organ systems. In addition, atomic bomb survivor data show that a fetus exposed to high doses of radiation may experience permanent retardation of physical growth, and the likelihood increases with increasing dose (due to cell death), particularly above 1 Gy (100 rad). This growth retardation is most pronounced when the exposure occurs in the first 13 weeks postconception, due to the criticality of cellular activities and the high proportion of radiosensitive cells. When the dose is greater than 1 Gy (100 rad), about a 3% to 4% reduction of height is evident at age 17 years.3Reference Schull1314
Brain Damage
Radiation may significantly affect global brain development in a fetus exposed at 8 to 25 weeks postconception. This is due to neuron loss during the important stages of neuronal migration.
Brain Damage in the 8 to 15 Weeks Gestational Period
Atomic bomb survivor data indicate that exposures at this stage result in an average intelligence quotient (IQ) loss of approximately 25 to 31 points per Gy (100 rad) above 0.1 Gy (10 rad). Because IQ measurement sensitivity is limited in the dose range below about 0.1 Sv (10 rad), this is viewed essentially as the threshold dose below which there is little effect on IQ. When the dose is greater than 0.1 Gy (10 rad), the risk for severe mental retardation is approximately 40%/Gy (100 rad).38Reference Gusev, Guskova and Mettler910Reference Otake, Schull and Lee1112Reference Schull1314Reference Sasaski, Kasuga and Sato1516
For studies of Japanese atomic bomb victims, severe mental retardation was related not to IQ but to clinical observation: “unable to perform simple calculations, to make simple conversation, to care for himself or herself, or if he or she was completely unmanageable or had been institutionalized.”Reference Schull13 This corresponds to an IQ<50 (0.4% prevalence in the unexposed population), although there was 1 subject whose IQ was eventually measured as high as 64.Reference Otake, Schull and Lee11Reference Schull1314
Brain Damage in the 16 to 25 Weeks Gestational Period
The central nervous system is less sensitive in the 16- to 25-week stage postconception. Still, at higher doses, the same effects seen in the 8- to 15-week stage may also occur. Although some researchers suggest that a small possibility exists for impaired brain function above 0.10 Gy (10 rad) in the 16- to 25-week stage postconception,Reference Otake, Schull and Lee11 most agree that the threshold for observably impaired brain function in this period is approximately 0.50 to 0.70 Gy (50-70 rad).38Reference Gusev, Guskova and Mettler91012Reference Schull1314Reference Sasaski, Kasuga and Sato15 The average IQ loss is approximately 13 to 21 points per Gy (per 100 rad) at doses above 0.7 Gy (70 rad), and the risk for severe mental retardation is approximately 9%/Gy (100 rad) above 0.7 Gy (70 rad)3Reference Gusev, Guskova and Mettler910Reference Otake, Schull and Lee1112Reference Schull1314Reference Sasaski, Kasuga and Sato1516 in the 16- to 25-week stage. From about 16 weeks postconception to birth, radiation-induced noncancer health effects are unlikely to occur below about 0.50 Gy (50 rad).
Figure 1 depicts the degree of IQ detriment as a function of fetal dose for these 2 gestational periods.

FIGURE 1 Estimated IQ loss of 2 gestational periods based on fetal dose
Thyroid Damage From Radioactive Iodine
The fetal thyroid is capable of taking up radioiodines beginning approximately 70 to 80 days after conception, with uptake increasing to the time of birth.24 If internal uptake of radioactive iodine occurs during the 16- to 25-week stage (or if an internal uptake of radioactive iodine occurs in an earlier stage and has not cleared from the mother's body by this stage), then the long-term health consequences to the offspring's thyroid (eg, spontaneous abortion, hypothyroidism, hyperthyroidism, cretinism)Reference Mettler and Upton724 should be considered. The fetal thyroid is extremely active during this period of development; if the mother ingests or inhales radioactive iodine, it will concentrate in the fetal thyroid as well as in the mother's thyroid unless the thyroid has been blocked through the use of stable iodine prophylaxis.824 For information about the use of potassium iodide to protect the fetus from radioactive iodine uptake, visit http://www.bt.cdc.gov/radiation/ki.asp.
Late-Stage Miscarriage
The sensitivity of the fetus to the noncancer health effects of radiation exposure is lower during the third trimester (after about 26 weeks) than in other stages of pregnancy. Nevertheless, at doses >1 Gy (100 rad), the risks for miscarriage and neonatal death (ie, infant death within 28 days after birth, including stillbirth) increase.3Reference Gusev, Guskova and Mettler910Reference Otake, Schull and Lee1112Reference Schull1314Reference Sasaski, Kasuga and Sato1516
Most researchers agree that a dose of <0.05 Gy (5 rad) represents no measurable noncancer risk to the embryo or fetus at any stage postconception.3816Reference Brent25 Research on rodents suggests a small risk for external malformations or skeletal defects as well as effects on the central nervous system in the 0.05 to 0.10 Gy (5-10 rad) range for some stages postconception.8Reference Otake, Schull and Lee11 A practical threshold for any type of congenital effects in the human embryo or fetus is, however, most likely between 0.10 to 0.20 Gy (10-20 rad).3
POTENTIAL CARCINOGENIC EFFECTS OF PRENATAL RADIATION EXPOSURE
Cancer risk from radiation exposure is generally considered to be proportional to dose. Latency between exposure and disease depends on many factors, but there is ample evidence that exposure in childhood reduces the time to onset.26 Cancer at a specific time of life (eg, childhood) is generally assessed separately from a person's lifetime cancer risk. The background incidence of childhood cancer (onset up through age 15) is 0.3%.3 The additional risk of developing a childhood cancer as a result of in utero radiation exposure is shown in Table 3; the table is based on the estimated risk of 12%/Gy (0.12%/rad) above the background incidence.3Reference Gusev, Guskova and Mettler9Reference Sasaski, Kasuga and Sato1516 The background incidence of lifetime cancer is approximately 41% (using rates from 2001-2003, 41.28% of males and females born in the United States today will be diagnosed with cancer at some time during their lifetime).Reference Ries, Harkins and Krapcho27 Although the lifetime cancer risks from prenatal radiation exposure are not yet known because the population of atomic bomb survivors currently alive who were in utero when the exposure occurred are only 63 years old, the lifetime cancer incidence risk of 34%/Gy (0.34%/rad) above the background incidence for exposure at age 10 years also is shown in Table 3 for estimation purposes.28
TABLE 3 Estimated Risk for Cancer From Prenatal Radiation Exposure

In addition, it has not been determined whether the carcinogenic effects for a given dose vary with the timing of the exposure. At this time, carcinogenic risks are assumed to be constant throughout pregnancy.12 Analysis of animal data suggests that, although there is a strong sensitivity to carcinogenic effects in late fetal development, exposure during blastogenesis and organogenesis is unlikely to lead to malignancy.Reference Sasaski, Kasuga and Sato15
Studies are under way to determine the lifetime cancer risk from prenatal radiation exposure. Early indications are that lifetime cancer risk from prenatal radiation exposure is similar to, or slightly lower than,Reference Preston, Cullings and Suyama29 lifetime cancer risk from exposure in childhood. Therefore, lifetime cancer risk from childhood radiation exposure may provide a good approximation of the prenatal risk.312Reference Ries, Harkins and Krapcho2728
CONCLUSIONS
The health consequences of high radiation doses (possibly large enough to result in acute radiation syndrome in the expectant mother) to an embryo or a fetus can be severe. Interventional medical procedures,5 radiation therapy,3 or a nuclear or radiological incident (including terrorism)Reference Donnelly, Farfan and Parker6 are examples of incidents that could result in high doses to the fetus. Fetal doses deriving from diagnostic studies or occupational radiation exposure, however, are usually expected to cause little or no harm.
Because fetal sensitivity to radiation-induced health effects is related to gestational age as well as to fetal dose, a visual aid may be beneficial when one is counseling pregnant patients who have been exposed to radiation.Figure 2 depicts the developmentally sensitive periods of gestation and shows the potential health effects for 3 different dose ranges.

FIGURE 2 Effects of radiation exposure on fetal development
Author Disclosures: The author reports no conflicts of interest.
Acknowledgment: The authors thank Fred A. Mettler Jr, MD, MPH, for assistance with this manuscript.







