INTRODUCTION
The microsporidian Thelohania contejeani commonly causes porcelain disease in populations of indigenous endangered white-clawed (Austropotamobius pallipes) and vulnerable noble (Astacus astacus) crayfish throughout Europe (e.g. Mazylis, Reference Mazylis1978; Diéguez-Uribeondo et al. Reference Diéguez-Uribeondo, Pinedo-Ruíz and Muzquiz1997). Porcelain disease is a chronic infection that results in the deaths of infected crayfish, and has been implicated in past crayfish mass mortalities (Henneguy and Thélohan, Reference Henneguy and Thélohan1892; Duffield, Reference Duffield1933; Pixell Goodrich, Reference Pixell Goodrich1956). Commonly, prevalence in white-clawed crayfish populations range from 0·2 to 10% (Cossins and Bowler, Reference Cossins and Bowler1974; O‘Keefe and Reynolds, Reference O'Keefe and Reynolds1983; Mori and Salvidio, Reference Mori and Salvidio2000; Hutchings, Reference Hutchings, Brickland, Holdich and Imhoff2009), but higher rates can occur (e.g. 30%, Schäperclaus, Reference Schäperclaus1954; 18%, Pixell Goodrich, Reference Pixell Goodrich1956; 30%, O‘Keefe and Reynolds, Reference O'Keefe and Reynolds1983; 18–50%, Imhoff et al. Reference Imhoff, Mortimer, Christmas, Dunn, Brickland, Holdich and Imhoff2009; >80%, J. Brickland, personal communication).
The primary site of infection is the muscle cells, where sporogony occurs, although the mature spores may be found in other body tissues extracellularly (Cossins and Bowler, Reference Cossins and Bowler1974; Oidtmann et al. Reference Oidtmann, El-Matbouli, Fischer, Hoffmann, Klärding, Schmid and Schmidt1996). Infected muscle becomes filled with spores and takes on an opaque white colouration which is visible externally and gives rise to the common name of microsporidiosis in crayfish – porcelain disease. Lom et al. (Reference Lom, Nilson and Dykova2001) found that T. contejeani exhibits 2 different routes of sporogony simultaneously within the same host tissue. The first route occurs inside a sporophorous vesicle and results in the formation of 8 uninucleated (haploid) spores with 9–10 turns of the polar filament. The second route occurs in ‘vacuole-like compartments’ and results in the formation of single diplokaryotic spores with 5–7 turns of the polar filament (Lom et al. Reference Lom, Nilson and Dykova2001). Molecular and ultrastructural data indicate that T. contejeani is closely related to T. montirivilorum and T. parastaci which occur in Australian crayfish species and which have similar developmental patterns in the muscle tissue (Moodie et al. 2003Reference Moodie, Le Jambre and Katza, Reference Moodie, Le Jambre and Katzb).
The route of transmission of T. contejeani between hosts is not well understood. A proposed life cycle of the congeneric parasite T. parastaci was provided by Moodie et al. (Reference Moodie, Le Jambre and Katz2003b), who proposed that diplokaryotic spores may be involved in vertical transmission and that uninucleate spores may be involved in horizontal transmission. Observations of T. contejeani in haemolymph (Cossins and Bowler, Reference Cossins and Bowler1974; Oidtmann et al. Reference Oidtmann, El-Matbouli, Fischer, Hoffmann, Klärding, Schmid and Schmidt1996) suggest that, following intrusion of the parasite into the crayfish, the parasite is transported via the haemolymph to the muscle cells (Moodie et al. Reference Moodie, Le Jambre and Katz2003b). However, the method of transmission to new hosts remains poorly understood. A number of transmission routes have been suggested, including direct cannibalism of infected crayfish tissue (Voronin, Reference Voronin1971), uptake of spores released from dead and decaying infected crayfish (Cossins and Bowler, Reference Cossins and Bowler1974), the use by the parasite of an intermediate host (Graham and France, Reference Graham and France1986; Lom et al. Reference Lom, Nilson and Dykova2001) and vertical (transovarial) transmission from mother to eggs (Vey and Vago, Reference Vey and Vago1973; Cossins and Bowler, Reference Cossins and Bowler1974; Moodie et al. Reference Moodie, Le Jambre and Katz2003b).
The white-clawed crayfish is indigenous to Europe and was recently classified as Endangered on the International Union for Conservation of Nature Red List (IUCN, 2010). A greater understanding of the transmission of T. contejeani may help instruct conservation efforts, because fatal porcelain disease caused by T. contejeani is common in populations of these crayfish and can occur at high rates (Imhoff et al. Reference Imhoff, Mortimer, Christmas, Dunn, Brickland, Holdich and Imhoff2009). Furthermore, the parasite has recently been found in populations of the invasive, non-indigenous signal crayfish (Pacifastacus leniusculus) at high prevalence (25–75%), and sequence comparison suggests that the invader has acquired the parasite from its new range (Dunn et al. Reference Dunn, McClymont, Christmas and Dunn2009).
This study investigates horizontal transmission between white-clawed crayfish by experimentally exposing crayfish to spores through feeding of infected tissue and through contaminated water. To determine whether signal crayfish are able to acquire the parasite from the indigenous white-clawed crayfish, signal crayfish were also exposed to infection.
MATERIALS AND METHODS
Experimental animals
A total of 98 juvenile white-clawed crayfish were collected from the River Fury in Northern Ireland and 98 juvenile signal crayfish were collected from the River Wharfe in England. White-clawed crayfish that were used to initiate the infection were visibly infected with T. contejeani and were collected from Wyke Beck, England. Signal crayfish were held at the University of Leeds under license from CEFAS. White-clawed crayfish ranged from 7 to 18·2 mm in carapace length (mean = 13·8 mm) at the beginning of the experiment, and signal crayfish from 8·4 to 27·2 mm (mean = 20 mm). The crayfish were housed in plastic tubs measuring 58 cm by 74 cm, divided into 35 individual 10 cm by 10 cm units. De-chlorinated tap water was provided, with constant aeration, at 6 cm deep and was changed every 2 weeks. The crayfish unit divider had a mesh bottom to allow water circulation. Crayfish were fed crustacean food pellets (Hikari Crab Cuisine) and detrital leaves throughout the experiment.
Horizontal transmission treatments
To test for direct horizontal transmission between A. pallipes hosts, crayfish were exposed to T. contejeani spores through feeding of infected tissue and through contaminated water. To determine whether signal crayfish are able to acquire the parasite from the indigenous white-clawed crayfish, signal crayfish were also exposed to infection. As limited juvenile white-clawed crayfish were available, transmission from signal to white-clawed crayfish was not tested. Each crayfish was randomly assigned to 1 of 3 experimental groups. To test for transmission via cannibalism/predation or scavenging, crayfish were fed fresh abdominal muscle tissue from heavily infected adult white-clawed crayfish. Feeding of T. contejeani-infected tissue occurred every 2 weeks, with 6 tissue feedings in total. At each feeding, each individual crayfish was provided with approximately 100 mg of tissue. All crayfish readily consumed the infected tissue. To test for transmission via spores released from infected crayfish into the water, crayfish were exposed to water from an aquarium housing multiple infected adult white-clawed crayfish in various stages of infection. Three hundred ml of this water was poured directly into the appropriate experimental tanks following the water change performed every 2 weeks. The control group did not receive tissue or exposure to contaminated water, but were otherwise treated the same as the other groups. Thus, the control group provided an estimate of the base rate of infection for the populations (juvenile crayfish were not screened for parasitism at the onset of the experiment owing to the likelihood that tissue sampling would lead to mortality in these small individuals (Imhoff et al. Reference Imhoff, Mortimer, Christmas and Dunn2010)). All groups were monitored for 26 weeks and overall growth and survival data were recorded. To determine overall growth rate, the carapace length of each crayfish was measured at the beginning and end of the experimental period (or following death), growth rate was estimated as the total change in length divided by the number of weeks the crayfish was housed. At the end of the experiment all remaining crayfish were frozen individually and 24 crayfish were randomly selected (using www.random.org) from each treatment to be screened for T. contejeani by PCR.
Parasite screening
To screen the animals for infection with T. contejeani, tissue samples (7–15 mg) were removed from each crayfish's abdominal muscle taking care to avoid the gut and cuticle. Individuals were also examined for visible signs of infection. DNA was extracted from each sample using a phenol:chloroform method based on that of Kocher et al. (Reference Kocher, Thomas, Meyer, Edwards, Paabo, Villablanca and Wilson1989). A nested PCR to amplify a portion of microsporidian small subunit ribosomal DNA was carried out using general microsporidian primers (all primers presented in 5′ – 3′ orientation) V1f (CACCAGGTTGATTCTGCCTGAC) and 1492r (GGTTACCTTGTTACGACTT) for the outer nest (Weiss et al. Reference Weiss, Zhu, Cali, Tanowitz and Wittner1994), and T. contejeani-specific primers F3 (AGCTAGTATGTAGGGTAAGGGC) and B3 (ACTCTTGGAGCTGGAATTACCG) for the inner nest (El-Matbouli et al. 2006). See Table 1 for PCR protocols. Multiple negative and positive controls were included in each reaction. To confirm successful extraction of DNA, PCRs using host primers HCO2198 (TAAACTTCAGGGTGACCAAAAAATCA) and LCO1490 (GGTCAACAAATCATAAAGATATTGG) were also carried out (Folmer et al. Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994). All PCR products were visualized by electrophoresis in 2% agarose gels stained with ethidium bromide. All samples were screened twice, and were considered positive when both screenings yielded a readily discernible band of the expected number of base pairs on the gel. Two white-clawed and 2 signal crayfish samples were sequenced to further confirm the presence of T. contejeani.
Table 1. PCR protocols showing primers and expected fragment sizes, reagent quantities/concentrations, and PCR cycles for each reaction

Data analysis
Statistical analyses were performed using the programs Excel (Microsoft, 2007) and R 2.10.1 (www.r-project.org). To determine predictors of survival and infection, generalised linear models (GLM, logistic regression type) were constructed and tested. Explanatory variables tested for survival included species and treatment group, and variables for infection included sex, growth rate, and treatment group. To examine any differences in growth among the experimental groups and between uninfected and infected (those which screened positive by PCR) crayfish, ANOVA tests were performed after examining the data to ensure the appropriate assumptions were met. Significance was accepted at probabilities of 0·05 or less in all original test analyses. Where post-hoc multiple comparisons were required, alpha was adjusted using the Bonferroni method.
RESULTS
Parasite screening
For both white-clawed and signal crayfish, the frequency of infection was higher in crayfish exposed to infected tissue or to contaminated water than in the control treatments (Fig. 1), providing evidence for horizontal transmission of T. contejeani. None of the signal or white-clawed crayfish which tested positive or negative for T. contejeani infection by PCR showed visible signs of infection. Of the control animals, 1 white-clawed crayfish and 2 signal crayfish tested positive for T. contejeani, indicating low parasite prevalence in the field.
Among white-clawed crayfish, each treatment group had significantly different rates of infection. The tissue-fed group had a higher rate of infection than the group exposed to contaminated water, which in turn had a higher rate of infection than the control group (Fig. 1, Table 2). There was no difference in the sex ratios assigned to the different treatments, (χ 2 = 0·99, d.f. = 2, P = 0·605). However, females were more likely to become infected than males (deviance = 7·61, d.f. = 1, P = 0·0058).

Fig. 1. Horizontal transmission of Thelohania contejeani to Austropotamobius pallipes and Pacifastacus leniusculus. The graph shows the numbers of crayfish in each treatment group that tested positive for T. contejeani by PCR; (24 individuals per treatment group were screened for infection. Treatments; Tissue-fed; animals were fed infected muscle tissue from parasitized A. pallipes, Contaminated water; animals were exposed to water from tanks housing infected A. pallipes.
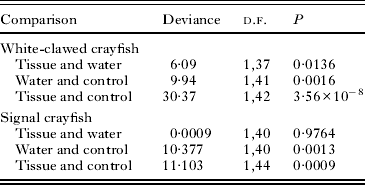
Table 2. Results of comparisons of infection status between treatment groups for white-clawed and signal crayfish samples
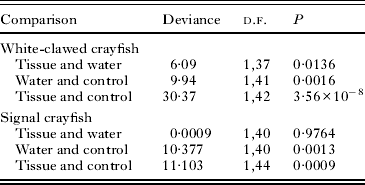
Among signal crayfish, treatment group was the only significant predictor of infection status (deviance = 13·89, d.f. = 2, P = 0·00096). Infection was significantly higher in animals exposed to infected tissue or to contaminated water than in animals from the control group. However, there was no difference in the infection rate in animals exposed to infected tissue or to contaminated water (Fig. 1, Table 2). Sex was not a significant predictor of infection status (deviance = 1·387, d.f. = 1, P = 0·239).
Growth and survival
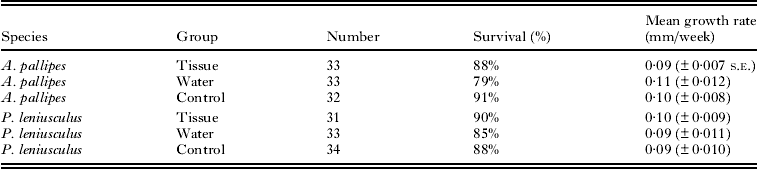
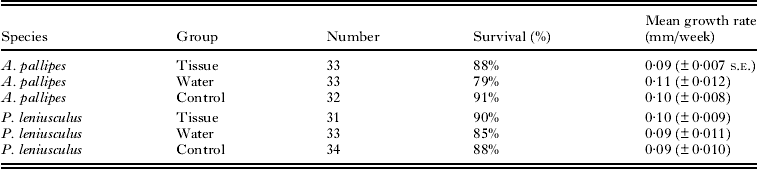
Growth rates did not differ among the treatment groups for either crayfish species (Table 3). White-clawed crayfish had a mean growth rate (±s.e.) of 0·102 ± 0·005 mm/week and growth rate did not differ among the three treatments (F 2,83 = 0·774, P = 0·46). Signal crayfish had a mean growth rate of 0·095 ± 0·006 mm/week and growth rate did not differ significantly among treatment groups (F 2,85 = 0·415, P = 0·66). As not all crayfish became infected following exposure, growth rate was also compared between crayfish that were found to be infected and uninfected at the end of the experiment. There was no difference in growth rate between white-clawed crayfish which screened positive or negative for parasite presence at the end of the experiment (0·102 ± 0·008 mm/week and 0·105 ± 0·008 mm/week; F 1,64 = 0·066, P = 0·80). Likewise there was no significant difference between signal crayfish which screened positive or negative (0·108 ± 0·010 mm/week and 0·104 ± 0·007 mm/week, F 1,63 = 0·016, P = 0·90).
Table 3. Survival and growth rates of crayfish from the 3 experimental groups; tissue; fed with tissue from Thelohania contejeani infected Austropotamobius pallipes, water; exposed to contaminated water from tanks that housed infected A. pallipes, control animals were not fed infected tissue or exposed to contaminated water
Survival was greater than 78% in all treatments (Table 3) and did not differ between the treatments (deviance = 2·01, d.f. = 2, P = 0·4) or between white-clawed and signal crayfish (deviance = 0·18, d.f. = 1, P = 0·7).
DISCUSSION
Thelohania contejeani was transmitted experimentally between white-clawed crayfish via ingestion of infected tissue as well as through exposure to contaminated water, with direct ingestion of infected tissue leading to a higher rate of transmission (83% vs 42%). This study presents an improvement in the understanding of the transmission route of T. contejeani in freshwater crayfish. There will be frequent opportunities for direct horizontal transmission of T. contejeani in the wild. Cannibalism is common in crayfish, with larger adults and juveniles preying on smaller individuals (Abrahamsson, Reference Abrahamsson1966; Mason, Reference Mason1977), providing opportunities for ingestion of infected tissue. Crayfish use refuges in close proximity to each other as well as coming into contact when foraging and mating (Peay, Reference Peay2002), providing opportunities for spore transmission via the water. This study also provides the first evidence for direct transmission of T. contejeani from infected white-clawed crayfish to the invasive signal crayfish, both by consumption of infected tissue and through exposure to contaminated water. Transmission success to the signal crayfish was high (50%) in both treatments.
Previous attempts to transmit T. contejeani to healthy crayfish have been of mixed success. Voronin (Reference Voronin1971) and Mazylis (Reference Mazylis1978) may have induced infection in the noble crayfish Astacus astacus by injection of spores into the stomach and by feeding infected tissue to apparently healthy crayfish. However, others have been critical of these results, suggesting that the infections which arose were likely present in the crayfish prior to experimental treatment (Graham and France, Reference Graham and France1986). Later experiments were unable to replicate the findings of Voronin and Mazylis. Fischer (Reference Fischer1992) and Hoffman et al. (Reference Hoffman, El-Matbouli, Oidtmann and Fischer1999) attempted transmission in noble crayfish via cannibalism and injection of spores into the stomach, as well as via a snail intermediate host, but no transmission of the parasite was observed. Similarly, Graham and France (Reference Graham and France1986) found no evidence of direct or indirect transmission of T. contejeani to the North American crayfish species, Orconectes virilis.
These earlier studies relied on visual examination and light microscopy for parasite detection, and may have missed infections at low burden and low pathogenicity. The use of PCR in the current study increases the sensitivity of detection (Imhoff et al. Reference Imhoff, Mortimer, Christmas and Dunn2010), as well as providing an estimate for the base rate of infection in the field population. None of the crayfish tested showed visible signs of infection, suggesting that parasite burden is low during the initial months of infection. This is supported by our observation that growth and survival did not differ between infected and uninfected crayfish of either species over the 6-month study period.
There is considerable current interest in the creation of ‘ark sites’ as refuges for the endangered white-clawed crayfish in the United Kingdom (Kemp et al. Reference Kemp, Birkinshaw, Peay and Hiley2003; Horton, Reference Horton, Brickland, Holdich and Imhoff2009). Crayfish populations are moved from a location where they are threatened by non-indigenous species or habitat degradation to a water body deemed safe and appropriate for the population's survival (the ‘ark site’). Our findings lead us to recommend that infected individuals are removed from the population before translocation, in agreement with Diéguez-Uribeondo et al. (Reference Diéguez-Uribeondo, Pinedo-Ruíz and Muzquiz1997). Mazylis (Reference Mazylis1978) recommended quarantining a population for 6 months prior to introduction to an ark site, to allow visual identification and removal of infected individuals, but for many ark site translocations this is not feasible due to the number of crayfish involved. Non-lethal molecular screening of individuals provides a more sensitive method of parasite detection (Imhoff et al. Reference Imhoff, Mortimer, Christmas and Dunn2010), although it may be beyond the scope of conservationists. In such a case, critical visual inspection and subsequent removal of infected crayfish must suffice. There has also been recent interest in using captive-reared white-clawed crayfish to supplement wild populations (Nightingale, Reference Nightingale, Brickland, Holdich and Imhoff2009). Again we recommend the removal of any infected crayfish from the breeding programme, as our findings indicate that they can transmit the parasite to healthy juveniles via shared water supply.
Parasites can play a key role in mediating interspecific interactions including competition and thus can influence the success and outcome of biological invasions (Prenter et al. Reference Prenter, MacNeil, Dick and Dunn2004; Tompkins et al. Reference Tompkins, Dunn, Smith and Telfer2011; Dunn et al. Reference Dunn, Torchin, Hatcher, Kotanen, Blumenthal, Byers, Coon, Frankel, Holt, Hufbauer, Kanarek, Shierenbeck, Wolfe and Perkins2012). Invaders may introduce parasites to a biological community, or may acquire parasites in their new range. The current study provides evidence for efficient, direct horizontal transmission of the endemic parasite T. contejeani to the invasive crayfish P. leniusculus through ingestion of infected A. pallipes tissue and through contaminated water. A. pallipes and P. leniusculus frequently occur in sympatry at the edges of the invasion zone and intraguild predation is common among crayfish, hence opportunities for transmission will be common in the field, and previous studies have reported the infection in wild P. leniusculus populations (Dunn et al. Reference Dunn, McClymont, Christmas and Dunn2009). The outcome of this shared infection will depend on the relative competence of the native and invading host species (Dunn, Reference Dunn2009; Hatcher et al. Reference Hatcher and Dunn2012). One the one hand, if the invasive species is a less competent host, it may act as a sink for the parasite, leading to a reduction in prevalence in native hosts. For example, Solter and Maddox (Reference Solter and Maddox1998) investigated transmission of endemic N. American microsporidia to the invasive gypsy moth Lymantria dipar. Although gypsy moth larvae that were experimentally exposed to microsporidia spores developed infections, subsequent transmission to new hosts was very low. If the signal crayfish acts as a sink for T. contejeani then the tendency of signal crayfish to form dense populations may lead to a reduction in the impact of T. contejeani on the native white-clawed crayfish when the species occur in sympatry. Conversely, an invasive host species may act as a reservoir for transmission of the infection into native hosts, leading to an increase in parasite prevalence. The process of spillover (where the reservoir host is the original host species) or spillback (where the reservoir is a more recently acquired host, Poulin et al. Reference Poulin, Paterson, Townsend, Tompkins and Kelly2011; Hatcher et al. Reference Hatcher, Dick and Dunn2012) can also mediate invasion success (Dunn, Reference Dunn2009; Tompkins et al. Reference Tompkins, Dunn, Smith and Telfer2011). For example, the invasion of the signal crayfish and resulting extirpation of the native European crayfish has been facilitated in many populations by outbreaks of crayfish plague, caused by the fungus Aphanomyces astaci. The disease was co-introduced with North American crayfish species, in which it causes little pathogenicity, but spillover to native crayfish causes high mortality (Holdich and Reeve, Reference Holdich and Reeve1991; Alderman, Reference Alderman1996). Although transmission from the invasive to the native species remains to be tested, it is possible that P. leniusculus may act as a reservoir for spillback of the native parasite T. contejeani to A. pallipes, potentially facilitating extirpation of the native species. The potential for spillback from the invasive signal crayfish to the endangered native species is of concern as signal crayfish reach higher population densities than do white-clawed crayfish (Arrignon and Roché, Reference Arrignon and Roché1983; Holdich and Domaniewski, Reference Holdich and Domaniewski1995; Guan and Wiles, Reference Guan and Wiles1996). Current policies to restrict the movement of signal crayfish and promote conservation of the white-clawed crayfish in isolated habitats (Holdich et al. Reference Holdich, Sibley and Peay2004; Peay, Reference Peay, Brickland, Holdich and Imhoff2009) may therefore be important to control transmission of T. contejeani as well as plague.
ACKNOWLEDGEMENTS
The authors thank N. Wilson and J. Dick of Queens University, Belfast for assistance in collecting and caring for the Irish white-clawed crayfish. A. Dubuffet and N. Haddaway of University of Leeds assisted with molecular work and care of signal crayfish. H. Soliman provided advice regarding the use of T. contejeani-specific primers and J. E. Smith on PCR. J. Brickland provided data on a population of crayfish with high parasite prevalence.
FINANCIAL SUPPORT
E. M. Imhoff acknowledges support from the Marie Curie Early Stage Training and the Environment Agency; A. M. Dunn acknowledges support from NERC NE/D011000/1.