INTRODUCTION
The small sponge order Homoscleromorpha, with a single family Plakinidae, seven genera and approximately 60 species, has recently become the subject of an increasing interest by biologists. This is due to the discovery that its species share characters with other metazoan phyla that are absent in other sponges: the presence of an acrosome in spermatozoa (Baccetti et al., Reference Baccetti, Gaino and Sarà1986) and a basal lamina underlining choanocytes and pinacocytes both in adults and in the larvae (Boute et al., Reference Boute, Exposito, Boury-Esnault, Vacelet, Nono, Miyazaki, Yoshizato and Garrone1996; Boury-Esnault et al., Reference Boury-Esnault, Ereskovsky, Bézac and Tokina2003). In addition, phylogenetic analyses of 18S rRNA, LSU rDNA and COI sequences suggest that the Homoscleromorpha may not form a monophyletic group with other Demospongiae, appearing instead more closely related to the Calcarea (Borchiellini et al., Reference Borchiellini, Chombard, Manuel, Alivon, Vacelet and Boury-Esnault2004; Nichols, Reference Nichols2005; Boury-Esnault, Reference Boury-Esnault2006).
Our understanding of the phylogenetic relationships of the Homoscleromorpha is partly hampered by the limited knowledge on its diversity. The low number of species currently known largely reflects insufficient sampling due to small size, rarity and cryptic habits of many species, together with the existence of several unresolved complexes of allegedly cosmopolitan species (e.g. Oscarella lobularis, Plakina monolopha, Plakina trilopha and Plakortis simplex), rather than a really low diversity. Also, sponges of the cosmopolitan plakinid genus Plakortis Schulze, Reference Schulze1880 are known to produce several interesting natural products and bioactive compounds such as cyclic peroxides (e.g. plakorstatins), pyrroloacridine alkaloids (e.g. plakinidines and amphiasterins) and polyketides (e.g. plakortides), which display antibacterial, antifungal, cytotoxic, antineoplastic and antineuroinflammatory activities (e.g. Higgs & Faulkner, Reference Higgs and Faulkner1978; Davidson, Reference Davidson1991; Yao & Steliou, Reference Yao and Steliou2002; Pettit et al., Reference Pettit, Nogawa, Knight, Doubek and Hooper2004; Rahm et al., Reference Rahm, Hayes and Kitching2004; Laroche et al., Reference Laroche, Imperatore, Grozdanov, Costantino, Mangoni, Hentschel and Fattorusso2007; Ralifo et al., Reference Ralifo, Sanchez, Gassner, Tenney, Lokey, Holman, Valeriote and Crews2007; Mayer et al., Reference Mayer, Kossuga, Veloso, Ferreira, Hajdu and Berlinck2008). This chemical diversity makes the genus Plakortis an interesting target group for pharmacological studies.
Despite its importance and wide distribution, the genus Plakortis has many unsolved taxonomic problems, such as cryptic speciation and alleged but unlikely cosmopolitanism of some species (e.g. P. angulospiculatus (Carter, Reference Carter1879) and Plakortis simplex Schulze, Reference Schulze1880). The spiculation of Plakortis is simple, composed almost exclusively of irregular diods and triods, making the distinction of closely related species very difficult (Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002). Only in a few Plakortis species there are other spicules such as microrhabds and quasiamphiasters to help in identification (P. lita de Laubenfels, Reference Laubenfels1954, P. microrhabdifera Moraes & Muricy, Reference Moraes and Muricy2003 and P. quasiamphiaster Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994). The architecture of the aquiferous system and of both choanosomal and tangential ectosomal skeletons provides useful taxonomic characters (cf. Lévi, Reference Lévi1952; Moraes & Muricy, Reference Moraes and Muricy2003), but it has been rarely described in detail. Furthermore, as few descriptions have been documented with underwater photographs, there is little information available on external morphological characters observed in situ, which could also provide useful information for species discrimination (e.g. Zea, Reference Zea1987; Bakus & Nishiyama, Reference Bakus and Nishiyama2000; Moraes & Muricy, Reference Moraes and Muricy2003; Cruz-Barraza & Carballo, Reference Cruz-Barraza and Carballo2005). In this paper, particular attention is given to the architecture of the tangential ectosomal skeleton and, when possible, to the morphology of living specimens.
So far, 15 valid species of Plakortis have been described worldwide: P. albicans Cruz-Barraza & Carballo, Reference Cruz-Barraza and Carballo2005; P. angulospiculatus (Carter, Reference Carter1879), P. copiosa Pulitzer-Finali, Reference Pulitzer-Finali1993, P. erythraena Lévi, Reference Lévi1958, P. galapagensis van Soest & Desqueyroux-Faúndez, Reference van Soest and Desqueyroux-Faúndez1997, P. insularis Moraes & Muricy, Reference Moraes and Muricy2003, P. japonica (Hoshino, Reference Hoshino1977), P. kenyensis Pulitzer-Finali, Reference Pulitzer-Finali1993, P. lita de Laubenfels, Reference Laubenfels1954, P. halichondrioides (Wilson, Reference Wilson1902), P. microrhabdifera Moraes & Muricy, Reference Moraes and Muricy2003, P. nigra Lévi, Reference Lévi1953, P. quasiamphiaster Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994, P. simplex Schulze, Reference Schulze1880 and P. zyggompha (de Laubenfels, Reference Laubenfels1934). Most of these species have restricted distributions, with the exception of P. simplex, P. angulospiculatus and, to a lesser extent, P. nigra.
After its description from the Mediterranean (Schulze, Reference Schulze1880), the type species P. simplex has been recorded from distant regions such as the Mediterranean Sea, north-east Atlantic, east Atlantic, south-west Atlantic, Caribbean Sea, Indo-Pacific, north-west Pacific, central Pacific and west Indian Ocean (e.g. Schulze, Reference Schulze1880; Topsent, Reference Topsent1897, Reference Topsent1901, Reference Topsent1928, Reference Topsent1934; Hentschel, Reference Hentschel1912; Burton, Reference Burton1930; de Laubenfels, Reference Laubenfels1950; Lévi, Reference Lévi1952; Boury-Esnault, Reference Boury-Esnault1973; Thomas, Reference Thomas1973; Vacelet et al., Reference Vacelet, Vasseur and Lévi1976; Cruz & Bacallado, Reference Cruz and Bacallado1981; Hoshino, Reference Hoshino1981; Pulitzer-Finali, Reference Pulitzer-Finali1986). However, in view of the low dispersal abilities of sponge larvae, the apparent co-specificity of distant, disjunct populations probably only reflects the difficulty of taxonomists to discriminate between closely related species due to the simple and non-diagnostic nature of their spiculation and external morphology (Lévi, Reference Lévi1953; Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002), similarly to other allegedly cosmopolitan species such as Chondrilla nucula, Clathrina clathrus, Oscarella lobularis and Plakina trilopha (Solé-Cava et al., Reference Solé-Cava, Klautau, Boury-Esnault, Borojevic and Thorpe1991; Muricy et al., Reference Muricy, Boury-Esnault, Bézac and Vacelet1996a, Reference Muricy, Solé-Cava, Thorpe and Boury-Esnaultb, Reference Muricy, Boury-Esnault, Bézac and Vacelet1998; Klautau et al., Reference Klautau, Russo, Lazoski, Boury-Esnault, Thorpe and Solé-Cava1999). Therefore, Plakortis simplex sensu lato most probably defines a complex of sibling species rather than a single biological species and will be treated here accordingly. Here I will use the expression ‘species complex’ for such groups composed of sibling species that are currently not discriminated by morphological methods, and ‘species group’ for groups of similar but clearly distinct species (see Discussion).
As described by Schulze (Reference Schulze1880) and Muricy & Diaz (Reference Muricy, Diaz, Hooper and van Soest2002), Plakortis simplex sensu stricto is a thin encrustation (maximum 5 mm thick), light-coloured (light brown, tan, yellow or white), with smooth surface, few small oscules (1 mm in diameter) and a compressible consistency. The aquiferous system has both ectosomal inhalant cavities and basal exhalant cavities; the skeleton is confused in both the choanosome and ectosome. The spicules are diods 60–150 µm long and triods with actines 20–50 µm long. In contrast, the Plakortis simplex species complex (or P. simplex sensu lato) in the Pacific and Indian Oceans is a heterogeneous group that includes dull olive brown, dull grey, pale yellow brown, greenish, bluish black externally and white internally, and dark blue sponges, 0.2–4.0 cm thick, with surface smooth or tuberculated, and with oscules up to 5 mm in diameter. The skeleton is usually described as confused and the aquiferous system has not been described at all. The spicules are diods in one size-class (varying from 40–250 µm) or in two size-classes (25–40 µm and 80–140 µm), alone or accompanied by triods with actines measuring 10–100 µm long (Topsent, Reference Topsent1897, Reference Topsent1928; Hentschel, Reference Hentschel1912; de Laubenfels, Reference Laubenfels1950; Thomas, Reference Thomas1973; Vacelet et al., Reference Vacelet, Vasseur and Lévi1976; Desqueyroux-Faúndez, Reference Desqueyroux-Faúndez1981; Lévi & Lévi, Reference Lévi and Lévi1983). Due to this high heterogeneity and to insufficient descriptions, comparisons to P. simplex will be based here on the original and subsequent descriptions from the type locality (Naples, Italy) and nearby areas (viz., Adriatic and Mediterranean Seas), here referred to as Plakortis simplex sensu stricto.
The sponge fauna of Australia and the Indo-Pacific is relatively well known (e.g. van Soest, Reference van Soest1989; Hooper, Reference Hooper2005), but to date only four species of Plakortis were described from this region: the species complex P. simplex from Indonesia (Aru Island and Amboine), Papua New Guinea and New Caledonia, Plakortis aff. angulospiculatus from Indonesia and Papua New Guinea, P. lita from the Caroline Islands, Indonesia, Papua New Guinea and Philippines, and P. quasiamphiaster from Vanuatu and Fiji (Topsent, Reference Topsent1897; Hentschel, Reference Hentschel1912; de Laubenfels, Reference Laubenfels1954; Desqueyroux-Faúndez, Reference Desqueyroux-Faúndez1981; Lévi & Lévi, Reference Lévi and Lévi1983; Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994; Pulitzer-Finali, Reference Pulitzer-Finali1996; Bakus & Nishiyama, Reference Bakus and Nishiyama2000; Longakit et al., Reference Longakit, Sotto and Kelly2005). Several other records of P. angulospiculatus, P. simplex, P. lita, P. nigra, P. cf. nigra and Plakortis sp. were reported without description, mostly in checklists, ecological, chemical and pharmacological studies (e.g. van Soest, Reference van Soest1989, Reference van Soest and Rützler1990; West et al., Reference West, Mayne, Ireland, Brinen and Clardy1990; Hooper et al., Reference Hooper, Kennedy and van Soest2000; Pettit et al., Reference Pettit, Nogawa, Knight, Doubek and Hooper2004, Hooper & Ekins, Reference Hooper and Ekins2004; Pangan et al., Reference Pangan, Uy and Oclarit2007). Here I describe a collection of six species of Plakortis from Australia and the Indo-Pacific, four of which are new to science, and discuss the usefulness of different morphological characters for the taxonomy of Plakortis. A key to Indo-Australian species of Plakortis is provided.
MATERIALS AND METHODS
The specimens studied were housed in the sponge collections of the Western Australian Museum, Perth, Australia (WAM) and in the Queensland Museum, Brisbane, Australia (QM) and were kindly sent on loan by their curators, Dr Jane Fromont from WAM and Drs Steve Cook and Merrick Ekins from QM. Type specimens of Plakortis quasiamphiaster and P. lita were loaned from the Porifera collections of the Zoological Museum of Amsterdam, The Netherlands (ZMAPOR) and The Smithsonian Institution (USNM), Washington DC, USA, by Dr Rob van Soest and Dr Klaus Rützler, respectively. Specimens were collected from October 1990 to July 1999 by different researchers (see ‘Specimens examined’ sections). Collections were made through SCUBA and free diving from 7–42 m depth in Australia (western and eastern coasts), Vanuatu, Fiji, Tonga, Indonesia, Philippines, Palau, and Papua New Guinea. Specimens collected were preserved in ethanol 70%. Spicule slides were prepared by dissociation of a small fragment of the sponge in boiling nitric acid. Hand-made, thick sections of the skeleton were observed under light microscope; transversal sections were made in paraffin-embedded specimens, and tangential sections of the ectosome were made in wet or dry specimens. Two to 20 spicules of each kind, depending on their abundance, were measured per individual. Spicule measurements are expressed as minimum–medium–maximum length/minimum–maximum width in μm (number of measurements). Other abbreviations used: MNRJ, Museu Nacional, Universidade Federal do Rio de Janeiro, Brazil; MNHN, Muséum National d'Histoire Naturelle, Paris, France; UFRJPOR, Porifera collection of the Universidade Federal do Rio de Janeiro, Brazil; USNM, United States National Museum, Smithsonian Institution, Washington DC. Scanning electron microscopy (SEM) of spicules was made in Jeol JSM-5310 and Jeol JSM-6390 electron microscopes after metallization with gold.
DEFINITION
Plakinidae with a skeleton formed by diods and triods in varying abundance. Diactine-derived microscleres (microrhabds) spheres and spined diods (amphiaster-like spicules) may be present in some species (emended from Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002).
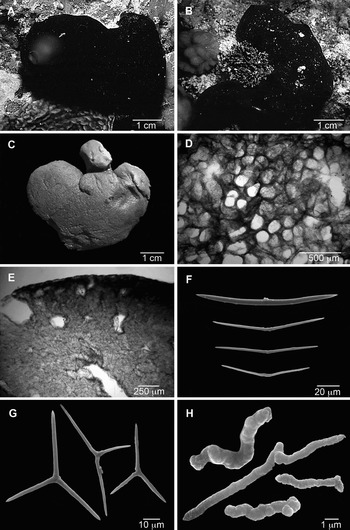
Fig. 1. Plakortis lita. (A, B) Specimens in situ: (A) GQM-315197 from Fiji; (B) GQM-315928 from Osprey Reef, Coral Sea, north-eastern Australia (photographs by the collectors); (C) preserved specimen (GQM-305093); (D) ectosome (tangential section); (E) choanosome and ectosome (transverse section); (F) diods; (G) triods; (H) microrhabds.

Fig. 2. Distribution of Plakortis lita. White circles, previous records; black circles, new records.
Table 1. Intraspecific variation of spicule measurements of Plakortis lita (length minimum–medium–maximum in μm). h, holotype.

DIAGNOSIS
Brown or black, cushion-shaped Plakortis with microrhabds, an irregular tangential ectosomal reticulation, a confused choanosomal skeleton and thin, irregular, acerate diods and triods.
SPECIMENS EXAMINED
Holotype: USNM 23069 (by original designation; cf. de Laubenfels, Reference Laubenfels1954: 247), Micronesia: Caroline Islands: Truk Atoll: Moen Islet, 7°28′0.84″N–151°50′33.04″E, 10 August 1949, 3 m depth.
ADDITIONAL SPECIMENS
Australia: Queensland: GQM-305093, mid east-side lagoon, Osprey Reef, Coral Sea, 13°53.3′S–146°39.1′E, J.N.A. Hooper coll., 24 February 1995, 26 m depth; GQM-315928, Lagoon entrance, Osprey Reef, Coral Sea, 13°53′47.10″S–146°38′50.30″E. J.A. Kennedy and D.W. Edson coll., 13 December 1999, 20 m depth; GQM-305715, Bacci Cay, Riversong Cays, Swain Reefs, 21°38.4′S–152°23.1′E, J.N.A. Hooper, S.D. Cook, J.A. Kennedy, P.A. Tompkins coll., 29 July 1995, 28 m depth; GQM-314186, Fitzroy Reefs, Bunker Group, 23°37.88′S–152°07.84′E, J.N.A. Hooper, S.D. Cook, J.A. Kennedy, A. Carrol coll.; 26 February 1998, 7.7 m depth; GQM-314454, Davies Reefs, 18°49.555′S–147°37.561′E, S.D. Cook, J.A. Kennedy, C.I. Adams, G. Wörheide coll., 24 January 1998, 24 m depth; GQM-314532, GQM-314534, Stanley Reef, 19°18.83′S–148°02.56′E, S.D. Cook, J.A. Kennedy, C.I. Adams, G. Wörheide coll., 25 January 1999, 30 m depth; GQM-315094, Inner Gneerings, Sunshine Coast, 26°39.19′S–153°10.996′E, S.D. Cook, J.A. Kennedy, J.N.A. Hooper, G. Wörheide coll., 14 October 1998, 19 m depth; GQM-315240, Hook Reef Lagoon, 19°45.229′S–149°10.753′E; S.D. Cook, J.A. Kennedy, C.I. Adams, G. Wörheide, D. Edson coll., 5 June 1999, 9.4 m depth; GQM-315311, Edgell Reef, 20°08.879′S–149°55.152′E, S.D. Cook, J.A. Kennedy, C.I. Adams, G. Wörheide, D. Edson coll., 6 July 1999, 18 m depth. Vanuatu: Mota Lava Island: GQM-315558, Banks Territory, 13°43.130′S–167°37.423′E, J.A. Kennedy, G. Wörheide coll., 15 July 1999, 27 m depth. Fiji: Viti Levu: GQM-315197, north side of Òné Island, off Rakiraki, 17°16′48.40″S–178°12′55.77″E, J.N.A. Hooper, A. Carrol, A. Wright coll., 5 March 1999, 19.8 m depth.
DESCRIPTION (FIGURE 1A–C)
Sponge thickly encrusting, irregular. Size up to 18 cm wide and 1–2 cm thick. Size of collected fragments up to 6.0 × 5.0 cm wide by 2 cm thick. External colour in vivo black, dark brown or grey-brown; internal colour brown or grey-brown. Preserved specimens are reddish to dark greyish brown. Surface smooth, slimy or sticky, sometimes with large tubes with small terminal oscules, which are contracted in preserved specimens. Consistency soft, liver-like, easily torn.
SKELETON (FIGURE 1D, E)
Ectosomal skeleton dense, forming an irregular reticulation with round or elliptical meshes, 110–165 µm in diameter (Figure 1D). Subectosomal lacunae often higher than wide, 80–200/100–700 µm, separated by spicule columns 50–150 µm thick. Choanosomal skeleton is a confused, low-density mass of diods dispersed between choanocyte chambers and canals (Figure 1E). Choanosome with large canals, 200–600 µm in diameter, and spherical to ovoid choanocyte chambers, 30–60 µm in diameter.
SPICULES (FIGURE 1F–H; TABLE 1)
Diods abundant, slightly curved, smooth or irregular, with a well-marked centre; the central irregularity varies from a barely detectable thickening to a series of 1–4 short bends which look like bumps or thicker rings (Figure 1F). Endings acerate; styloid modifications are rare: 23–87–145/0.5–8.0 µm (N = 188).
Triods relatively rare, absent in one specimen, irregular, with high variation in angles between actines and in size of actines in the same spicule, usually with acerate endings (Figure 1G): actines 1–20–66/0.5–2.5 µm (N = 60).
Microrhabds irregular, strongyloid (Figure 1H), sometimes vaguely triactinal: 1–25 × 0.5–2.0 µm (N = 60).
REPRODUCTION
Ovoid or spherical larvae 140–1000 µm in diameter were found in specimens collected in January, February and August.
HABITAT
Coral reefs: fore-reef, back-reef, boomies and sand, gulleys, spur and groove, patch reef in lagoon, pinnacles, Acropora thickets, vertical walls, sheer slope, and terraces, 3–30 m depth.
DISTRIBUTION
Indo-West Pacific: Caroline Islands, Micronesia (Truk Atoll and Ponape; de Laubenfels, Reference Laubenfels1954), Indonesia, Papua New Guinea (Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994), Vanuatu, Fiji and Eastern Australia (Great Barrier Reef, Queensland) (present study; Figure 2).
REMARKS
In the original description, de Laubenfels (Reference Laubenfels1954) reported the absence of triods in all his specimens, but close observation showed that they are present both in the holotype and in most specimens examined here. Although rare, triods were also observed by Diaz & van Soest (Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994). Plakortis lita is easily recognizable by the presence of microrhabds, which are so far only shared in the genus with P. microrhabdifera from the central Western Atlantic (Atol das Rocas, north-east Brazil; Moraes & Muricy, Reference Moraes and Muricy2003) (see the description of P. hooperi sp. nov. below). Plakortis lita and P. microrhabdifera strongly differ in colour (black, dark reddish or greyish brown in P. lita, light brown in P. microrhabdifera), ectosomal skeleton (with irregular, mostly elliptical meshes in a single size category in P. lita and with regular, rounded meshes in two size-classes in P. microrhabdifera), and in diod shape (with acerate endings in P. lita, often strongyloid in P. microrhabdifera). So far, triods have not been found in P. microrhabdifera (Moraes & Muricy, Reference Moraes and Muricy2003; Muricy et al., Reference Muricy, Esteves, Moraes, Santos, Silva, Almeida, Klautau and Lanna2008).
Plakortis lita sensu Bakus & Nishiyama (Reference Bakus and Nishiyama2000) and Longakit et al. (Reference Longakit, Sotto and Kelly2005) from Cebu Island, Philippines, differs from Plakortis lita sensu de Laubenfels, Reference Laubenfels1954 in the firm consistency, more regular diods, and absence of microrhabds; it therefore must be assigned to a different species. It is similar to P. nigra in its dark colour, thick encrusting habit and firm consistency; however, its colour is dark brown versus black in P. nigra, its oscules are smaller and more numerous than in P. nigra (which has a single apical oscule, 3 mm in diameter) and its spicules are larger than those of P. nigra (90–148 versus 20–90 µm). The dark brown Plakortis from Cebu is therefore provisionally included in the Plakortis simplex species complex, but it is probably a new species.
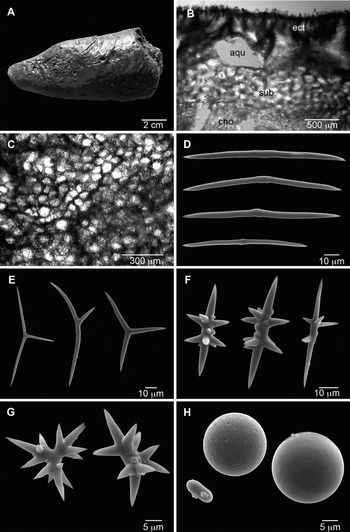
Fig. 3. Plakortis quasiamphiaster. (A) Preserved specimen (GQM-306925); (B) choanosome and ectosome (transverse section; ect, ectosome; sub, subectosomal region; aqu, aquiferous cavities; cho, inner choanosome); (C) ectosome (tangential section); (D) diods; (E) triods; (F) diactinal quasiamphiasters; (G) triactinal quasiamphiasters; (H) spheres and microstrongyle.

Fig. 4. Distribution of Plakortis quasiamphiaster. White circles, previous records; black circles, new records.
Table 2. Intraspecific variation of spicule measurements of Plakortis quasiamphiaster (length minimum–medium–maximum, in μm). h, holotype.

DIAGNOSIS
Plakortis with quasiamphiasters and a skeleton with a distinct subectosomal region.
SPECIMENS EXAMINED
Holotype: ZMAPOR 7777, Vanuatu, P. Crews coll., 20 m depth.
ADDITIONAL SPECIMENS
Vanuatu: Espiritu Santo: GQM-306848, Malvioror Reef, Hog Harbour, 15°08′S–167°06′E, J.N.A. Hooper coll., 28 June 1996, 42 m depth. Malicolo: GQM-306864, GQM-306907, outer Reef, Benehour Point, South-West Bay, 16°30′S–167°24′E, J.N.A. Hooper coll., 4 July 1996, 32 m depth. Emae: GQM-306925, 17°03′S–168°21′E, ORSTOM Nouméa coll., 9 July 1996, 20 m depth. Malekula Island: GQM-312976, 21 May 1997, 21.7 m depth; GQM-313029, 22 May 1997, 35.5 m depth; both from Dixon Reef, 16°21′S–167°21′E, J.N.A. Hooper coll. Fiji: Motua Levu: GQM-312813, Goldea Wall, 16°41′S–179°38′E, J.N.A. Hooper coll., 30 October 1996, 34.2 m depth. Tonga: GQM-313236, Hunga Lagoon, 18°42′S–174°07′W, J.N.A. Hooper coll., 9 November 1997, depth unknown; GQM-313281, Fotula, rock just off west coast of Vava'u Island, 18°38′S–174°04′W, J.N.A. Hooper coll., 13 November 1997, 32 m depth.
DESCRIPTION (FIGURE 3A)
Sponge thickly encrusting to massive, columnar or lobate. Size up to 5 cm wide by 12 cm high. Colour in vivo usually reddish-brown, sometimes paler internally, but one specimen was deep blue. Preserved specimens are light or dark reddish-brown, and often produce a dark red-brown exudate. The sponge is firmly attached to the substrate through a large base. Surface uniform, smooth or microhispid, often striated or with superficial canals converging to the oscules. Oscules rounded, up to 5 mm in diameter, surrounded by a membrane, contracted in 70% ethanol. Consistency compressible but resistant in spirit; dry specimens are brittle.
SKELETON (FIGURE 3B, C)
Ectosome distinct, 100–1012 µm thick, with parallel, ascending multispicular tracts 19–98 µm in diameter and 5–200 µm apart, slightly protruding through the surface (Figure 3B). The ectosome has a tangential reticulation of spicule tracts forming rounded meshes (Figure 3C). In most specimens there is a well-marked subectosomal region, 250–1265 µm thick, composed of an irregular reticulation of multispicular tracts 20–80 µm thick, forming rounded or irregular meshes 49–200 µm in diameter (Figure 3B). Within the subectosomal region and between it and the ectosome, there are elongated or elliptical aquiferous cavities, 50–1012 µm long. Inner choanosome with a dense, confused reticulation of diods (Figure 3B).
SPICULES (FIGURE 3D–H; TABLE 2)
Abundant diods, robust, straight or curved, smooth, relatively uniform, with a lightly marked centre, just slightly thickened or with 1–3 very short, almost imperceptible bends; endings acerate: 28.7–94.8–179.6/0.8–7 µm (N = 166; Figure 3D).
Triods relatively abundant, smooth, with variable angles between actines and size of actines in the same spicule, usually with acerate endings: actines 10.5–34.3–78.4/1–5 µm (N = 121; Figure 3E).
Diactinal quasiamphiasters relatively abundant to rare, straight or curved, with thick, irregular centre and acerate endings (Figure 3F). Each spicule has 2–15 spines, 1–45 µm long, usually concentrated in two rows in opposite sides near the centre: 9.0–33.3–91.0/1.5–6.0 µm (N = 117).
Triactinal quasiamphiasters rare or absent in most specimens (Figure 3G): actines 7.8–11.3–16.0/1–4 µm, with spines 2–7 µm long (N = 9).
In all specimens studied there are smooth or irregular spheres, 2–15 µm in diameter, which are sometimes elongated to form short thick microstrongyles, 4–10/2–4 µm (Figure 3H).
REPRODUCTION
Ovoid larvae, 200–393 µm long, were found in a few specimens collected in July and October.
HABITAT
Coral reefs, 20–42 m depth.
DISTRIBUTION
Vanuatu (Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994); Fiji and Tonga are new records (Figure 4).
REMARKS
Plakortis quasiamphiaster is easily distinguishable from all other species of Plakortis by the peculiar amphiaster-like spicules called quasiamphiasters (spined diods and triods), and by the distinct subectosomal region with a reticulation of multispicular tracts. These traits are very unusual in the genus Plakortis, and could justify its placement in a separate, monotypic genus. This species could also be considered to belong to the closely related genus Plakina due to the resemblance between its triactinal quasiamphiasters and trilophose triods of, e.g. Plakina weinbergi Muricy et al., Reference Muricy, Boury-Esnault, Bézac and Vacelet1998. However, the lophose calthrops diagnostic of Plakina are absent in P. quasiamphiaster. This species is left in the genus Plakortis until more information is available to solve its phylogenetic relationships.
The distinctive skeletal architecture and the spheres were not reported previously (Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994). The spheres may be an ecomorphological, variable trait depending on silicate levels of seawater (see Discussion section), but since they are present in all specimens examined including the holotype, it cannot be ruled out that they can be a genetically determined, constant part of the spiculation of the species.

Fig. 5. Plakortis communis sp. nov. (A) Specimen in situ (GQM-300346) (photograph by the collectors); (B) preserved specimen (WAM Z-14); (C) ectosome (tangential section); (D) choanosome and ectosome (transverse section); (E) diods; (F) triod.

Fig. 6. Distribution of Plakortis communis sp. nov.
Table 3. Intraspecific variation of spicule measurements of Plakortis communi s sp. nov. (length minimum–medium–maximum in μm). h, holotype.

DIAGNOSIS
Plakortis greyish-brown to black externally and greyish or light brown internally, with a tangential ectosomal reticulation forming circular meshes approximately 50–100 µm in diameter. In transverse sections, the ectosome is differentiated, with columns and subectosomal lacunae. Choanosome confused or vaguely reticulated. Microrhabds absent.
SPECIMENS EXAMINED
Holotype: Australia: Western Australia: GQM-301057, Cartier Island, 12°32.2′S–123°31.9′E, J.N.A. Hooper coll., 4 May 1992, 12 m depth.
Paratypes: Australia: Western Australia: WAM Z-1272, 19 March 1997, 25 m depth, WAM Z-14, 20 March 1997, 18 m depth, both from Houtman Abrolhos, Beacon Island, Goss Passage, 28°29′S–113°46′E, J. Fromont coll. GQM-304653, Houtman Abrolhos, near to Rat Island, 28°40.0′S–113°50′E, C. Bryce coll., depth and date unknown. Queensland: GQM-314832, Alcyonarian Point, Hook Island, Whitsunday Group, 20°03.932′S–148°55.408′E, S.D. Cook, J.A. Kennedy, C.L. Adams, G. Wörheide, D. Edson coll., 3 June 1999, 15 m depth. Philippines: Cebu Island: GQM-300346, south-west tip, 9°24′1.81″N–123°17′57.59″E, NCI, Australian Institute of Marine Science coll., 26 April 1991, 25 m depth. Fiji: Taveuni: GQM-312793, Robert's Reef, Nggamea Island, 16°42.9′S–179°48.2′E, J.N.A. Hooper coll., 29 October1990, 42 m depth.
ETYMOLOGY
This species is named after its apparent abundance and wide distribution in the Indo-Australian region.
DESCRIPTION (FIGURE 5A, B)
Sponge thickly encrusting to cushion-shaped, irregular, up to 6.5 × 4.5 cm wide by 3 cm thick. Colour in vivo dark or light brown, khaki-brown, greyish-brown, rusty-brown, fawn with purple eggs or black with whitish eggs; internal colour usually lighter (greyish or light brown); preserved specimens are beige to light brown. Surface smooth, regular. Oscules flush or elevated, contracted in preserved specimens. Consistency variable from firm to soft, liver-like; sponge rather easy to tear off.
SKELETON (FIGURE 5C, D)
Ectosomal skeleton with a tangential reticulation, with pauci- to multispicular tracts forming irregularly elliptical meshes around 40–100 µm in diameter (Figure 5C). In one of the specimens studied, uni- to paucispicular tracts may form, inside the main reticulation, smaller, irregular meshes, with approximately 30 µm in diameter. The ectosome is usually well differentiated from the choanosome, most often showing, in transverse sections, ascending multispicular tracts (columns) and irregular subectosomal lacunae (Figure 5D). Choanosomal skeleton confused or vaguely reticulate, with irregular meshes. Choanosome usually with large canals. One specimen (WAM Z-14) has abundant spherulous cells dispersed in the choanosome.
SPICULES (FIGURE 5E, F; TABLE 3)
Diods abundant, slightly curved or straight, thin, regular; centre slightly thickened or with 1–3 short irregular, barely visible bends; endings acerate (Figure 5E): 27–105–143/1–6 µm (N = 90).
Triods irregular, often sagittal, rare or absent (Figure 5F): actines 30–47–60/1–5 µm (N = 22).
Some specimens have smooth or irregular spheres, 1–12 µm in diameter (not shown).
REPRODUCTION
Spherical or ovoid larvae, 450–800 µm in diameter, were found in specimens collected in March and April.
HABITAT
Outer patch reef, fringing coral reef, spurs and grooves, caves, overhangs, tunnels near shore, euryhaline lake surrounded by limestone cliffs, sometimes encrusting on coral branches, from 5–42 m depth.
DISTRIBUTION
Cartier and Houtman Abrolhos Islands (Western Australia), Great Barrier Reef, Queensland (Eastern Australia), Cebu and Fiji (Figure 6).
REMARKS
Plakortis communis sp. nov. is part of the P. simplex species group, which is characterized by the absence of any clearly distinctive traits such as microrhabds, quasiamphiasters, or large spicules; the group includes 10 species (P. albicans, P. communis sp. nov., P. copiosa, P. erythraena, P. galapagensis, P. insularis, P. japonica, P. nigra, P. simplex and P. zyggompha; see Discussion). The new species could have been included in the P. simplex complex, but Mediterranean specimens of Plakortis simplex sensu stricto (cf. Schulze, Reference Schulze1880) differ from P. communis sp. nov. in external characters such as a thinly encrusting shape and light colours (white, yellow and light brown) versus a thickly encrusting to cushion shape usually with dark colours in the new species. Furthermore, the ectosomal skeleton of the new species has a tangential reticulation of pauci- to multispicular tracts forming irregularly elliptical meshes, whereas P. simplex sensu stricto seems to have no ectosomal specialization (Schulze, Reference Schulze1880; Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002). The other species of this group also differ from Plakortis communis sp. nov. by few, rather subtle characters: Plakortis zyggompha, P. galapagensis and P. erythraena are thinly encrusting; furthermore, P. galapagensis has diods in two size-categories and P. erythraena has smaller diods (maximum 90 µm; Lévi, Reference Lévi1958; van Soest & Desqueyroux-Faúndez, Reference van Soest and Desqueyroux-Faúndez1997). Plakortis albicans and P. japonica are thinly encrusting and respectively white or pinkish-white externally (Hoshino, Reference Hoshino1977). Plakortis copiosa from Kenya was poorly described, without information on skeletal architecture, but its diods are smaller than those of P. communis sp. nov. (maximum size 110 µm) and its triods are regularly symmetrical and very abundant (Pulitzer-Finali, Reference Pulitzer-Finali1993). Plakortis insularis from north-east Brazil differs from the new species by its softer consistency and absence of ectosomal reticulation (Moraes & Muricy, Reference Moraes and Muricy2003). Finally, P. nigra from the Red Sea differs from P. communis sp. nov. in the bluish black colour both externally and internally, single large apical oscules with tangential exhalant canals, and in the massive, lobate shape (Lévi, Reference Lévi1953). Also, its diods are thinner and smaller (<20–50–90 µm versus 27–105–143 µm), and triods are completely absent.
Unfortunately, several characters vary within the new species, including shape, colour, oscules, consistency and presence of spheres. Some characters co-vary, allowing the distinction of two groups: (1) dark brown, thick encrusting to massive sponges, with rounded borders (GQM-301057 (holotype), GQM-300346, GQM-304653, WAM Z-14); and (2) light brown sponges with shiny smooth surface (GQM-312793, WAM Z-1272, GQM-314832). These groups might deserve status of distinct species, but I consider that the differences between them are not conspicuous enough to allow unequivocal distinction of individuals at the species level and are based in a small number of characters. Most other characters appear to vary randomly respective to each other and are within the range of variation of Plakortis simplex sensu lato. I have preferred to group all these specimens under the single name Plakortis communis sp. nov. to avoid the enlargement of the Plakortis simplex species complex or excessive splitting without good characters to distinguish the species. The problem of determining the limits between intra- and interspecific variation in Plakortis simplex sensu lato and in the new species can be solved only by careful underwater examination of living populations together with histological, cytological or molecular analyses.

Fig. 7. Plakortis bergquistae sp. nov. (A) Preserved specimen (GQM-301163); (B) ectosome (tangential section); (C) choanosome and ectosome (transverse section); (D) small diods; (E) large diods; (F) triods.

Fig. 8. Distribution of Plakortis bergquistae sp. nov.
DIAGNOSIS
Plakortis with diods in two size-classes, the larger one up to 330–356 µm long, and large triods (actines up to 75–121 µm long).
SPECIMENS EXAMINED
Holotype: GQM-312579, Indonesia: N. Sulawesi: Bitung, 1°28′N–125°13′E, L. & D. Tackett coll., 16 August 1995, 38 m depth.
Paratypes: GQM-301163 (2 specimens): Australia: Western Australia: Hibernia Reef, 11°58.2′S–123°21.8′E, J.N.A. Hooper coll., 12 March 1992, 24 m depth.
ETYMOLOGY
This species is named in honour of Dame Professor Patricia Bergquist, a remarkable taxonomist and biologist of sponges, unfortunately deceased in September 2009.
DESCRIPTION (FIGURE 7A)
Sponge thickly encrusting, up to 6 × 5 cm wide by 2 cm thick. Colour in vivo unknown; preserved specimens are light brown to orange brown. Surface smooth but irregular, sometimes with pore-fields or star-shaped canals converging to contracted oscules. Consistency firm, cartilaginous.
SKELETON (FIGURE 7B, C)
Ectosomal skeleton reticulate, with multispicular tracts forming circular or irregular meshes, 100–300 µm in diameter (Figure 7B). The ectosome is poorly differentiated, slightly denser than the choanosome, without subectosomal lacunae (Figure 7C, transverse section). Choanosomal skeleton confused to vaguely reticulate. Choanosome with large canals.
SPICULES (FIGURE 7D–F)
Diods abundant, straight or slightly curved, smooth, regular, with slightly thickened centre, and in two size-classes: the smaller measures 91–133–163/2–5–6 µm (N = 30) (Figure 7D) and the larger 202–280–356/5–9–11 µm (N = 28) (Figure 7E).
Triods large, mainly sagittal, smooth, regular; actines 30–72–122/5–11–20 µm (Figure 7F) (N = 8).
REPRODUCTION
The specimens examined, collected in May and August, have ovoid to spherical larvae.
HABITAT
Patch reef, inner reef slope, from 24–38 m depth.
DISTRIBUTION
Hibernia Reef (Western Australia) and North Sulawesi (Indonesia) (Figure 8).
REMARKS
In the specimens from Hibernia Reef, the ectosomal reticulation forms larger meshes (approximately 300 µm diameter) than in the specimen from Sulawesi, with meshes 100–150 µm in diameter. In transverse sections, the distinction between ectosomal and choanosomal skeletons is clearer in the Sulawesi specimen than in Hibernia Reef specimens. Such differences were here considered intraspecific in view of the similarity of the three specimens in the large size of diods, with two size-classes, the larger one up to 350 µm long. This character is exclusive of this species and allows easy distinction of Plakortis bergquistae sp. nov. from all other species of Plakortis. The lack of calthrops distinguishes it from species of Plakinastrella, which have diods in a similar size-range.

Fig. 9. Plakortis fromontae sp. nov. (A) Preserved holotype (WAM Z-1280); (B) ectosome (tangential section); (C) choanosome and ectosome (transverse section); (D) diods; (E) triod; (F) spheres.

Fig. 10. Distribution of Plakortis fromontae sp. nov.
DIAGNOSIS
Plakortis with curved diods in a wide size-range (20–220 µm long), rare triods, and a double ectosomal reticulation with circular meshes 30 and 120 µm in diameter.
SPECIMENS EXAMINED
Holotype: WAM Z-1280, Australia: Western Australia: Rat Island, Houtman Abrolhos, 28°43′S–113°46′E, J. Fromont coll., 4 December 1996, 14 m depth.
ETYMOLOGY
This species is named in honour of Dr Jane Fromont, from the Western Australian Museum, who collected the holotype and who first drew my attention to the great diversity of Australian Plakortis.
DESCRIPTION (FIGURE 9A)
Sponge thickly encrusting to cushion-shaped, up to 7.5 × 4 × 2 cm. Colour in vivo black with cream interior. Preserved specimens are light orange-brown. Surface smooth, regular. Oscules contracted, sometimes elevated, 1–2 mm in diameter. Consistency firm, cartilaginous, or compressible.
SKELETON (FIGURE 9B, C)
Ectosomal skeleton with a double reticulation. Pauci- to multispicular tracts form larger circular meshes, 120–400 µm in diameter, inside which uni- to paucispicular tracts form smaller meshes, circular to irregular, 30–100 µm in diameter (Figure 9B). In transverse sections, the ectosomal skeleton appears differentiated, with columnar spicule tracts. Subectosomal lacunae relatively abundant, spherical or irregular, 150–500 µm in diameter. Choanosome with a confused to irregularly reticulate skeleton and large canals, 140–800 µm in diameter (Figure 9C).
SPICULES (FIGURE 9D–F)
Diods abundant, irregular, smooth, with centre only slightly thickened (not s-bent) and straight to strongly curved actines, acerate, with wide variation in size (Figure 9D): 25–124–223/2–6–10 µm (N = 40).
Triods rare, regular, mostly equiangular and equiradiate (Figure 9E): 6–24–44/0.5–1.6–5.0 µm (N = 10).
Spheres, relatively rare, hollow, never modified to microstrongyles (Figure 9F): 10–15 µm in diameter (N = 10).
REPRODUCTION
Ovoid larvae 350–600 µm in diameter were found in the single specimen, collected in December.
HABITAT
Cave and reef, 14–37 m depth.
DISTRIBUTION
Houtman Abrolhos (Western Australia) (Figure 10).
REMARKS
Plakortis fromontae sp. nov. is part of the P. angulospiculatus species group, characterized by a simple spiculation of diods and triods as in the P. simplex species-group, but with maximum diod size ranging between 190 and 300 µm long (see Discussion). Within this group, it shares a reticulate ectosomal skeleton only with P. angulospiculatus and P. halichondrioides. It differs in the external colour from P. halichondrioides (which is black) and from P. angulospiculatus in having a double tangential ectosomal reticulation versus a simple reticulation. The new species is similar to Plakortis kenyensis in shape and size, but they differ in consistency, colour and in spicule size (slightly larger in P. kenyensis). Plakortis fromontae sp. nov. differs from all other species of Plakortis by its irregularly curved diods, ranging in length from 25 to 223 µm. It is unclear whether the spheres are a constant part of the spiculation of this species or a variable trait depending on silicate levels of seawater (see Discussion).

Fig. 11. Plakortis hooperi sp. nov. (A) Preserved holotype (GQM-312880); (B) ectosome (tangential section); (C) choanosome and ectosome (transverse section); (D) diods; (E) triod; (F) spheres and microrhabds.

Fig. 12. Distribution of Plakortis hooperi sp. nov.
DIAGNOSIS
Plakortis with diods, triods and microrhabds, with confused ectosomal skeleton. Shape thinly encrusting.
SPECIMENS EXAMINED
Holotype: GQM-312880, Papua New Guinea: Motupore Island: Round Hill Barrier Reef, south-east of Motupore Island, 9°57.1′S–147°28′9E, J.N.A. Hooper coll., 12 December 1996, 39 m depth.
ETYMOLOGY
This species is named in honour of Dr John N.A. Hooper, from the Queensland Museum, Brisbane, Australia, who collected the holotype and who has been greatly contributing to the progress of sponge taxonomy, especially of Australian and Indo-Western Pacific areas.
DESCRIPTION (FIGURE 11A)
Three fragments, with size up to 3 × 3 cm. Sponge thinly encrusting to cushion-shaped; studied fragments up to 5 mm thick. Colour in vivo brown-beige. Preserved specimens are cream to light brown externally and internally. Surface smooth, but irregular. Oscules large in vivo, contracted in alcohol. Consistency slightly compressible. The sponge releases abundant mucous and has a typical acetone smell.
Skeleton (Figure 11B, C): Ectosomal skeleton confused, without any trace of reticulation (Figure 11B). Ectosome undifferentiated, except for irregular, sparse subectosomal lacunae. Choanosomal skeleton confused (Figure 11C).
SPICULES (Figure 11D–F)
Diods abundant, relatively thick, centrotylote, regular, acerate (Figure 11D): 79–111.6–148/2–5 µm (N = 20).
Triods irregular, inequirradiate, relatively rare (Figure 11E): actines 17–29.9–61/2–3 µm (N = 10).
Microrhabds abundant, irregular, strongylote or deformed (Figure 11F): 2–5.5–8/0.5–1.0 µm (N = 17).
Spheres, relatively common, spherical or ovoid to irregular, sometimes elongated, forming microstrongyles (Figure 11F): 5–20 µm in diameter (N =20).
REPRODUCTION
Unknown.
HABITAT
Barrier Reef, 39 m depth.
DISTRIBUTION
Known only from Papua New Guinea.
REMARKS
Plakortis hooperi sp. nov. is related to P. lita and P. microrhabdifera by the presence of irregular microrhabds. It differs from both however in its confused ectosomal skeleton, whereas the other two species have a reticulated ectosome. The light beige colour in alcohol and the slightly compressible consistency are also distinctive traits of the new species. It differs from all other species of Plakortis in the presence of irregular microrhabds. The spheres and microstrongyles may be a constant part of the spiculation of this species or a variable trait depending on silicate levels of seawater (see Discussion).
DISCUSSION
The genus Plakortis is characterized by a simple spiculation, composed almost exclusively of very irregular diods and triods, and by high uniformity of external morphological characters, with several species with brown colour and encrusting shape. This morphological homogeneity makes the identification of species of Plakortis extremely hard (see also Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002). Furthermore, most descriptions of Plakortis species lack information on external morphology in vivo and on skeletal and aquiferous system architecture, and few studies show details of spicule shape in SEM micrographs. Such characters can be very useful for the distinction of species of Plakortis (Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002; Moraes & Muricy, Reference Moraes and Muricy2003; Cruz-Barraza & Carballo, Reference Cruz-Barraza and Carballo2005) and must be included in future descriptions to allow better species discrimination. Revision of these characters is especially needed for P. zyggompha, P. copiosa and P. kenyensis, whose descriptions totally lack such information, and for several records of P. simplex, P. nigra and P. angulospiculatus worldwide, including the Indo-Australian region (e.g. Topsent, Reference Topsent1897; Hentschel, Reference Hentschel1912; van Soest, Reference van Soest1989, 1990; Desqueyroux-Faúndez, Reference Desqueyroux-Faúndez1981; Lévi & Lévi, Reference Lévi and Lévi1983; Pulitzer-Finali, Reference Pulitzer-Finali1996; Hooper et al., Reference Hooper, Kennedy and van Soest2000; Hooper & Ekins, Reference Hooper and Ekins2004; Pangan et al., Reference Pangan, Uy and Oclarit2007). Designation of a neotype for the type species Plakortis simplex is also essential to achieve taxonomic stability in the genus.
The architecture of the tangential ectosomal skeleton is particularly useful for the taxonomy of Indo-Australian Plakortis, with three major types of arrangement: (1) reticulated, with multispicular tracts forming simple, rounded or elliptical meshes; (2) reticulated, with multispicular tracts forming rounded or elliptical meshes inside which paucispicular tracts form a secondary reticulation with smaller rounded meshes; and (3) confused, with no trace of reticulation. The same patterns were found in Brazilian and Mexican species (Moraes & Muricy, Reference Moraes and Muricy2003; Cruz-Barraza & Carballo, Reference Cruz-Barraza and Carballo2005). However, most species of Plakortis are compressible and their oscules contract after fixation; some, such as Plakortis lita, can change shape and thickness remarkably after collection. Such changes might affect, to a limited extent, the ectosomal organization of these sponges, but as all descriptions and comparisons are based on preserved specimens, they do not reduce the usefulness of this character. Examination of living specimens in situ or in aquarium would be necessary to ascertain the degree of modification of the ectosomal skeleton after collection and fixation.
The finding of large diods (up to 350 µm long) and triods (actines up to 120 µm long) in P. bergquistae sp. nov. was unexpected. All other species of the genus have diods not longer than 260 µm, and usually shorter than 150 µm. Large diods and triods are found in the closely related genus Plakinastrella Schulze, Reference Schulze1880, but the two genera are distinguished by the presence of well-formed calthrops only in Plakinastrella (Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994; Muricy & Diaz, Reference Muricy, Diaz, Hooper and van Soest2002; Moraes & Muricy, Reference Moraes and Muricy2003). Although the distinction between Plakortis and Plakinastrella is probably artificial, it is followed here for the sake of stability of the classification until a phylogenetic analysis including the two genera is available.
The presence of spheres in four species of Plakortis (P. quasiamphiaster, P. communis sp. nov., P. fromontae sp. nov. and P. hooperi sp. nov.) was also surprising, although spheres were previously reported in a deep-sea species from New Caledonia identified as Plakortis simplex (Lévi & Lévi, Reference Lévi and Lévi1983). Lévi (Reference Lévi1953) also reported abundant ovoid or 8-shaped elements in P. nigra, without identifying them as spicules, cells or other structures. Beyond the genus Plakortis, smooth spheres have been found in species of the orders Astrophorida (Isops apiarum (Schmidt, Reference Schmidt1870); Caminus albus Pulitzer-Finali, Reference Pulitzer-Finali1996; C. chinensis Lindgren, Reference Lindgren1897; Penares dendyi (Hentschel, Reference Hentschel1912)), Hadromerida (Tethya ingalli Bowerbank, Reference Bowerbank1858 (Lendenfeld, Reference von Lendenfeld1888 as Tethya laevis); T. monstrosa (Burton, Reference Burton1924); T. omanensis Sarà & Bavestrello, Reference Sarà and Bavestrello1995; Sarà & Sarà, Reference Sarà and Sarà2004; van Soest & Beglinger, Reference van Soest and Beglinger2008), and Poecilosclerida (Biemna megalosigma Hentschel, Reference Hentschel1912; Guitarra flamenca Carballo & Uriz, Reference Carballo and Uriz1998). In the latter species, the perfectly spherical or lobate, smooth silica structures were considered as aberrant silica formations associated to silica-rich environments such as those off the coast of Namibia (Carballo & Uriz, Reference Carballo and Uriz1998). This is a plausible explanation, since the occurrence of these spheres is rare and dispersed in few species from widely divergent taxonomic groups. Furthermore, several of these species also occur in Indo-Australian waters (e.g. Caminus albus, Penares dendyi, Tethya laevis and T. monstrosa). However, the relationship between silicate concentration and sphere production remains to be demonstrated experimentally. For the time being, these spheres are considered as a variable part of the spiculation of the species in which they occur, since it is likely that their presence varies depending on the locality.
Indo-Australian species of Plakortis seem to have no marked reproductive period, since embryos and larvae were found in most specimens, irrespectively of the period of collection. In contrast, most Mediterranean plakinids reproduce preferably during summer and autumn (Schulze, Reference Schulze1880; Muricy et al., Reference Muricy, Boury-Esnault, Bézac and Vacelet1996a, Reference Muricy, Boury-Esnault, Bézac and Vacelet1998; Maldonado & Riesgo, Reference Maldonado and Riesgo2008). This difference might be related to the low seasonal temperature variation in the tropics compared to temperate areas such as the Mediterranean Sea.
It is very difficult to evaluate the identity of previous Indo-Australian records of P. nigra and of the Plakortis simplex complex due to the lack of detailed descriptions of their external morphology in vivo, skeleton architecture and aquiferous system organization. In fact, the shortness of most descriptions is one of the main reasons for the very existence of the P. simplex species complex. The records of P. nigra from the Indo-Australian region cannot be objectively evaluated because there are no descriptions of the species from the area; the only descriptions of this species so far are from the Red Sea (Lévi, Reference Lévi1953, Reference Lévi1958; Diaz & van Soest, Reference Diaz, van Soest, van Soest, van Kenpen and Braeckman1994). In his first description of P. simplex from Amboine (Indonesia), Topsent (Reference Topsent1897) made no mention of its skeleton or spicules. In subsequent re-descriptions, the same specimen was described as having triods and microcalthrops (Topsent, Reference Topsent1901) and later as having diods in two size-classes (Topsent, Reference Topsent1928; Desqueyroux-Faúndez, Reference Desqueyroux-Faúndez1981). In addition, the sponge is thickly encrusting and its colour is bluish-black externally and white internally (Topsent, Reference Topsent1897). It is thus clearly not co-specific with P. simplex sensu stricto, neither with any of the species described here. If it possesses true calthrops it should be transferred to Plakinastrella Schulze, Reference Schulze1880. In any case, it probably should receive a new species name, but more detailed examination of the specimen is needed before this can be done. Plakortis simplex sensu Vacelet et al. (Reference Vacelet, Vasseur and Lévi1976), from Madagascar, differs from both P. communis sp. nov. and P. simplex sensu stricto in the massive shape, greenish colour, firm consistency and the large size of diods (40–250 µm long); it may either belong to P. kenyensis (cf. Pulitzer-Finali, Reference Pulitzer-Finali1993) or to an undescribed species. Plakortis simplex sensu Thomas (Reference Thomas1973) from the Seychelles is close to P. communis sp. nov., from which it differs by a thinner shape (up to 10 mm thick), lighter colour and slightly smaller diods and triods. The record of P. simplex from Hawaii (de Laubenfels, Reference Laubenfels1950) is close to P. simplex sensu stricto in the thinly encrusting shape and light colours; however, its triods are much larger than those of both P. communis sp. nov. and P. simplex sensu stricto (actines average 100 µm long versus 30–60 and 25–50 µm, respectively). Plakortis simplex sensu Hentschel (Reference Hentschel1912) from Aru Island, Indonesia, and sensu Lévi & Lévi (Reference Lévi and Lévi1983) from New Caledonia have spicules in the same size-range of P. communis sp. nov. and P. simplex sensu stricto. Lévi & Lévi (Reference Lévi and Lévi1983) did not mention the colour of their specimens; de Laubenfels (Reference Laubenfels1950) did not describe the size of diods; and no previous study has described the arrangement of the tangential ectosomal skeleton. In the lack of such information, these records are provisionally left in the Plakortis simplex species complex, although most of them probably deserve new species names. Due to these problems, it is currently impossible to estimate the actual diversity of Plakortis in the Indo-Australian region. Judging from the morphological heterogeneity of the P. simplex species complex and the large number of unnamed records (as Plakortis sp.) both in Australia (Hooper & Ekins, Reference Hooper and Ekins2004) and Indonesia (Poppe & Poppe, Reference Poppe and Poppe2010), it is clear that there are many species yet to be described in this area.
As the number of species and the information on their morphology increase, three groups of species can now be distinguished within the genus Plakortis, based on spicule types and size:
(1) P. simplex species group—includes the nominal species (which is itself a complex of sibling species) and all other species with spiculation composed of only diods and triods, and whose diods are smaller than 190 µm long (P. albicans, P. communis sp. nov., P. copiosa, P. erythraena, P. galapagensis, P. insularis, P. japonica, P. nigra and P. zyggompha);
(2) P. angulospiculatus species group—also with a nominal species complex and including species with only diods and triods, but the largest diods vary between 190 µm and 300 µm long (P. angulospiculatus, P. fromontae sp. nov., P. halichondrioides and P. kenyensis);
(3) P. lita species group—includes the species with microrhabds (P. hooperi sp. nov., P. lita and P. microrhabdifera).
Two species do not fit in any group: P. bergquistae, characterized by the presence of very large diods (up to more than 300 µm long) and triods (with actines up to more than 100 µm long); and P. quasiamphiaster, characterized by the presence of diactinal and triactinal quasiamphiasters. In the absence of a cladistic analysis, no phylogenetic value is assumed to these groups, neither are they proposed as subgenera, but they may be useful to help species identification in the genus. Within each group, species discrimination can be made by details of skeletal architecture, external morphology and spicule shape. As in other plakinid genera such as Plakina and Oscarella (Muricy et al., Reference Muricy, Boury-Esnault, Bézac and Vacelet1996a, Reference Muricy, Solé-Cava, Thorpe and Boury-Esnaultb, Reference Muricy, Boury-Esnault, Bézac and Vacelet1998), the taxonomy of Plakortis would greatly benefit from the inclusion of histological, cytological and molecular characters. With the data currently available, however, it is now clear that the morphological diversity within the genus, although lower than in most demosponges, is higher than previously thought.
IDENTIFICATION KEY FOR INDO-AUSTRALIAN SPECIES OF PLAKORTIS
1. Quasiamphiasters present………P. quasiamphiaster Quasiamphiasters absent………2
2. Microrhabds present………3
Microrhabds absent………4
3. Colour dark brown or black; ectosomal reticulation simple, with irregularly elliptical meshes………P. lita
Colour brown or light brown; ectosomal skeleton confused; sponge producing mucus………P. hooperi sp. nov.
4. Diods up to 350 µm long, triod actines up to 120 µm long………P. bergquistae sp. nov.
Diods less than 300 µm long, triod actines less than 80 µm long………5
5. Largest diods 190 µm long or more; tangential ectosomal reticulation double-meshed………P. fromontae sp. nov.
6. Largest diods less than 190 µm long; tangential ectosomal reticulation single-meshed………P. communis sp. nov.
ACKNOWLEDGEMENTS
I thank Jane Fromont (Western Australian Museum, Perth, Australia), Stephen Cook and Merrick Ekins (Queensland Museum, Brisbane, Australia), Rob van Soest (Zoological Museum of Amsterdam, The Netherlands) and Klaus Rützler (Smithsonian Institution, Washington DC, USA) for the kind loan of the specimens. The comments of two anonymous referees greatly improved the manuscript. Financial support was provided by Centro de Pesquisas e Desenvolvimento Leopoldo Américo Miguez de Mello (CENPES/PETROBRAS) through the project ‘Desenvolvimento da Taxonomia de esponjas marinhas (Porifera) no Brasil’, by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). I am grateful to Dr Marcia Attias and Noêmia Rodrigues (Laboratório de Ultraestrutura Celular Herta Meyer, UFRJ) and to Elivaldo de Lima for help with SEM, and to Renata Silvano for the preparations for LM and SEM. The project ‘Modernização, informatização e infra-estrutura das coleções marinhas do Museu Nacional/UFRJ (Setores de Carcinologia, Celenterologia, Malacologia e Peixes) e Desenvolvimento do Centro de Microscopia Eletrônica de Varredura’ (SAPE 460022548-3) of CENPES-PETROBRAS/UFRJ allowed the use of SEM facilities at Museu Nacional, Rio de Janeiro.

















