INTRODUCTION
The thornback ray Raja clavata Linnaeus, 1758, is a shallow water demersal skate found in the eastern Atlantic from Norway to South Africa, including the Mediterranean and Black Seas (Stehmann & Bürkel, 1984). This species is widespread in the Mediterranean Sea and is the dominant skate in commercial landings (Garofalo et al., Reference Garofalo, Gristina, Fiorentino, Cigala Fulgosi, Norrito and Sinacori2003; Abella & Serena, Reference Abella and Serena2005). The thornback ray occurs along the Tunisian coasts mainly in the Gulf of Gabes where it is caught throughout the year by commercial trawl fisheries as by-catch, and its commercial value has increased due to the decline of the bony fish stocks (Capapé, Reference Capapé1975).
In previous diet studies carried out in the Atlantic Ocean, the main prey for R. clavata were shrimps and fish (Holden & Tucker, Reference Holden and Tucker1974; Quiniou & Andriamirado, Reference Quiniou and Andriamirado1979; Ajayi, Reference Ajayi1982; Smale & Cowley, Reference Smale and Cowley1992; Ellis et al., Reference Ellis, Pawson and Shackley1996; Morato et al., Reference Morato, Sola, Gros and Menezes2003; Farias et al., Reference Farias, Figueiredo, Moura, Gordo, Neves and Pereira2006). Although abundant along the Mediterranean coasts, there is a paucity of information on thornback ray biology and ecology, particularly its feeding habits. Concerning the diet of this species in the Mediterranean Sea, some studies have provided quantitative descriptions (Capapé, Reference Capapé1975; Vannucci et al., Reference Vannucci, Mancusi, Serena, Cuoco and Volani2006).
In the Gulf of Gabes, central Mediterranean Sea, no studies on feeding habits of R. clavata have been reported. The objectives of this study were: (1) to quantify the diet composition; (2) to examine potential diet differences by sex, size and season; and (3) to qualitatively assess feeding strategy of Raja clavata in the Gulf of Gabes (Tunisia).
MATERIALS AND METHODS
Sampling methods
Samples of R. clavata were regularly collected twice per month, from January to December 2007 in the Gulf of Gabes, by the commercial trawler ‘Said’ using a 22 mm stretched mesh size cod-end. Haul duration was 2 h, through both day and night at depths of 30–150 m (Figure 1). Skates were frozen on-board, then subsequently thawed and analysed in the laboratory. A total of 1280 specimens were collected.

Fig. 1. Map of the Gulf of Gabes (Tunisia, central Mediterranean Sea) showing the sampling localities (black triangles); the specimens of Raja clavata were trawled.
Diet composition and analysis
Once in the laboratory, the skates were sexed and measured (total length, T L) to the nearest millimetre. Stomachs were removed and contents were sorted and identified to the lowest possible taxonomic resolution. Vacuity index (VI) was calculated. The variation in VI was tested by a χ2 test over a contingency table of the number of empty stomachs.
Stomach contents were removed, and ingested prey was identified to the lowest possible taxonomic level using the Riedel (Reference Riedel1963) and Fischer et al. (Reference Fischer, Bauchot and Schneider1987a, Reference Fischer, Bauchot and Schneiderb) manuals.
The importance of different prey items in the diet of R. clavata was determined by calculating the index of relative importance (IRI) of each prey (Pinkas et al., Reference Pinkas, Oliphant and Iverson1971):
where %F, %N and %M are the percentage contributions of a prey category in term of frequency of occurrence, number and mass, respectively, in the stomachs examined.
The IRI values were converted to a percentage to facilitate comparisons between prey items (Cortés, Reference Cortés1997).
 $$\% {\rm IRI} = {\rm 100} \times {\rm IR}{{\rm I}_{\rm i}}{\rm /}\sum\limits_{{\rm i} = {\rm 1}}^{\rm n} {{\rm IR}{{\rm I}_{\rm i}}}$$
$$\% {\rm IRI} = {\rm 100} \times {\rm IR}{{\rm I}_{\rm i}}{\rm /}\sum\limits_{{\rm i} = {\rm 1}}^{\rm n} {{\rm IR}{{\rm I}_{\rm i}}}$$Diet shifts
Dietary shifts with sex, size (T L) and season were evaluated using multivariate analysis of variance (MANOVA) and applying Morisita's index of overlap (CH) (Morisita, Reference Morisita1959) to test for diet similarities between sexes, size-classes and season:where p ij is the proportion of prey category i (based on %IRI) used by size-class j, and p ik is the proportion of prey category i used by size-class k. The degree of overlap was based on the Langton (Reference Langton1982) scale: low (0–0.29), medium (0.30–0.59) and high overlap (>0.60).
Specimens were grouped, based on their T L, in three size-classes: I <50, II 50–75 and III >75 cm. Statistical analyses were carried out using the main prey categories: crustaceans, teleosts, molluscs, polychaetes, gastropods and elasmobranchs. The mass of each prey category was considered the dependant variable and season (winter, spring, summer and autumn), sex (female ‘F’ or male ‘M’) and class (I, II, III) were defined as factors.
The multivariate F value (Wilks' lambda) based on a comparison of the error variance/covariance matrix and the effect variance/covariance matrix was applied to test differences in the diet. An analysis of variance (ANOVA) was further performed to identify the main prey groups responsible for the major differences among factors: sex, season and size. The significance level adopted was 5%.
Trophic position and feeding strategy
To determine the position of R. clavata within the food web, trophic position (TP) was estimated by the method of Cortés (Reference Cortés1999) as:
 $${\rm TP} = {\rm 1} + {\rm \lpar }\sum\limits_{{\rm j} = {\rm 1}}^{\rm n} {\mathop {\rm P}\nolimits_{\rm j} } \times \mathop {{\rm TP}}\nolimits_{\rm j} \rpar$$
$${\rm TP} = {\rm 1} + {\rm \lpar }\sum\limits_{{\rm j} = {\rm 1}}^{\rm n} {\mathop {\rm P}\nolimits_{\rm j} } \times \mathop {{\rm TP}}\nolimits_{\rm j} \rpar$$where TPj is the trophic level of each prey category j, Pj is the proportion of each prey category j (based on %IRI) in the diet of R. clavata, and n is the total number of prey categories. The taxonomic categories used to calculate the standardized trophic level of R. clavata were teleosts, cephalopods, decapods, isopods, polychaetes and amphipods. The trophic level of each prey category was obtained from Ebert & Bizarro (Reference Ebert and Bizzarro2007).
The feeding strategy of R. clavata in terms of specialization and generalization was studied by plotting prey specific-abundance (Pi) against %F (Amundsen et al., Reference Amundsen, Gabler and Staldvik1996). Pi was calculated as the number of a prey i divided by the total number of prey in the stomachs that contained prey i, expressed as a percentage (Amundsen et al., Reference Amundsen, Gabler and Staldvik1996).
Prey diversity (H′) was calculated for each size-class using the Shannon–Weiner diversity index (Shannon & Weiner, Reference Shannon and Weaver1949).
 $${\rm H^{\prime} } = - \sum\limits_{i = 1}^n {\left({{\rm Pi log}{{\rm }_{\rm 2}}{\rm Pi}} \right)}$$
$${\rm H^{\prime} } = - \sum\limits_{i = 1}^n {\left({{\rm Pi log}{{\rm }_{\rm 2}}{\rm Pi}} \right)}$$where Pi is the proportion of individuals in the ith species.
The Schoener's overlap index was used to quantify the dietary overlap between age-classes (Schoener, Reference Schoener1970) (α):
 $$\alpha = 1 - 0.5 \Big( \sum\limits_{i = 1}^n {} \left\lfloor {\,pij - pik} \right\rfloor \Big)$$
$$\alpha = 1 - 0.5 \Big( \sum\limits_{i = 1}^n {} \left\lfloor {\,pij - pik} \right\rfloor \Big)$$(where pij= proportion of the functional group j that consumes the i prey category; pik= proportion of the functional group k that uses the i prey category).
RESULTS
Diet composition
Stomach contents of 750 females and 530 males were analysed in this study. The total length varied between 14.6 and 89 cm for males and between 14 and 110 cm for females. Among the 1280 stomachs examined, 1076 (84.06 %) contained food. The proportion of males (83.01%) with food in stomachs was not significantly different to that of females (84.66%) (χ2 = 0.62, df = 1, P > 0.05).
The diet of the thornback rays included prey belonging to seven families of crustaceans; 16 families of teleosts and three families of cephalopods (Table 1), with a low average number (mean 3.13%) and weight (mean 12.33 g) per stomach. The diet of thornback rays was dominated by teleosts followed by crustaceans (Table 1).
Table 1. Diet composition of Raja clavata off the Gulf of Gabes. %F, frequency of occurrence; %N, percentage in number; %M, percentage in weight; %IRI, index of relative importance of prey item.
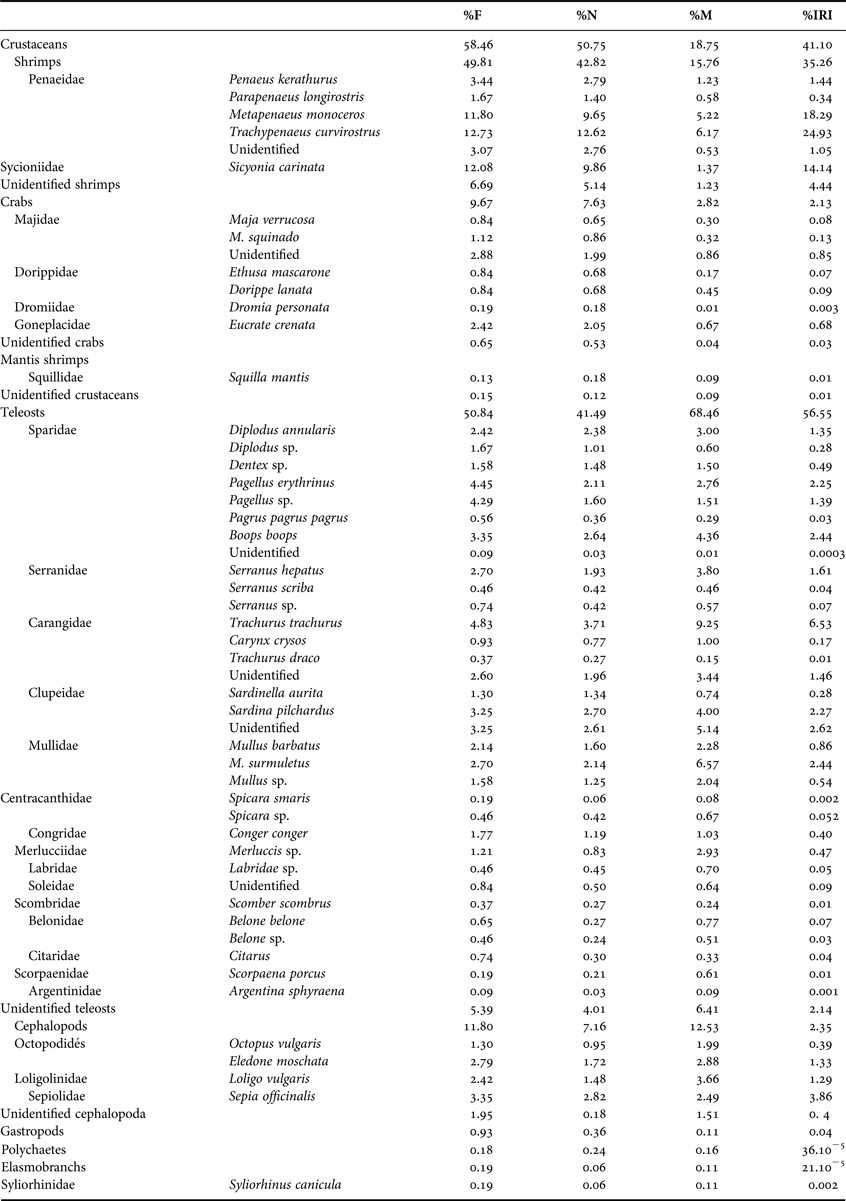
Crustaceans were the dominant food category of number, while teleosts were dominant by weight. The %IRI indicated that teleosts constitute the major part of the diet, followed by crustaceans and cephalopods (Table 1).
Among crustaceans, shrimps were the dominant prey in terms of number, mass and occurrence, followed by crabs, with Majidae predominant in term of occurrence, number and mass (Table 1). The consumed teleosts were mainly represented by demersal species, and a few pelagic species (Clupeidae, Carangidae). Among the teleosts, sparids were the most common followed by carangids (Table 1). The cephalopods were the third prey category, with 11.80% of the full stomachs (Table I). Sepia officinalis occurred most abundantly, followed by Octopus vulgaris.
Polychaetes and gastropods were of minor importance in the diet. Elasmobranchs did not occur in the small and medium size-classes, but constituted a minor portion of the diet of the mature females skates (Table 1).
In cases where a prey item was largely digested, the claws and legs for crustaceans, otoliths for fish and beaks for cephalopods remained for identification. If identification failed, the prey item was included in the category ‘unidentified’.
Diet shifts
The multivariate test revealed significant differences in the diet by sex, size and season with significant sex–season, sex–size size–season and sex–size–season interactions (Table 2). Females consumed a significantly larger quantity of crustaceans and teleosts than did males, whereas cephalopods and gastropods appeared more abundant in the stomachs of males than in those of females (Table 2). The class I of both sexes consumed nearly equal proportions of each category of prey, whereas mature females consumed significantly more of each than mature males.
Table 2. Multivariate analysis of variance (MANOVA) table of Wilks' lambda and group response to analysis of variance (ANOVA). (df, degrees of freedom; W.L, value of Wilks lambda; F, approximate F value; H.df, hypothesis df; E.df, error df; Sig, significant; Cru, crustaceans; Tel, teleosts; Ceph, cephalopods; Gast, gasteropods).

The ANOVA performed for each dependent variable (group taxa) indicated that teleosts and crustaceans were responsible for the difference between sexes, but crustaceans, teleosts and molluscs were responsible for the difference between sizes (Table 2).
Ontogenetic changes in the diet of thornback ray were observed (Figure 2). Rays in the small size-class consumed a larger proportion of crustaceans than rays in the larger two size-classes. Cephalopods occurred more frequently in stomachs of the medium size-classes.

Fig. 2. Percentage index of relative importance (%IRI) contributions of broad taxonomic groups to the diets of different length-classes of males and females Raja clavata off the Gulf of Gabes.
Between seasons, crustaceans were consumed more in the summer. Whereas the teleosts occurred during all seasons, their occurrence decreased during summer in favour of crustaceans. Molluscs occurred frequently in autumn (Figure 3). The sex–season interaction was driven by crustaceans and the size–season interaction was caused by crustaceans and teleosts. Additionally there were significant sex–maturity determined by crustaceans teleots and cephalopods (Table 2).

Fig. 3. Seasonal diet change in percentage index of relative importance (%IRI) by size-classes of males and females Raja clavata from off the Gulf of Gabes.
Dietary overlap values were very high between large and medium rays (Table 3). Diet of specimens in those size-classes consisted mostly of teleosts. Schoener's index indicated high degree of diet overlap between any seasons (Table 3).
Table 3. Schoener's diet overlap index for thornback ray (Raja clavata) estimated between size-classes of both sex and seasons. M, males; F, females.

Trophic position and feeding strategy
The estimated TP for the whole population of R. clavata was 3.83. The TP of R. clavata increased with increasing body length. The three size-classes had similar values of both sex, but skates of class I and class II were identified as secondary consumers (T L <4) whereas individuals of class III were tertiary consumers (T L >4) (Table 4) for both sexes
Table 4. Trophic position for each size-class for thornback ray (Raja clavata) estimated between size-classes of both sexes.

The overall dietary diversity (H′) for the entire samples was 2.53 Shannon–Wiener prey diversity values showed increasing prey diversity from classes I to II and II to III (Figure 4).

Fig. 4. Prey diversity (Shannon–Wiener index, H′) for each size-class.
Prey-specific abundance against frequency of occurrence (%Pi–%F) showed a progressive change in diet of R. clavata with increased size, from one dominated by crustaceans in the class I to a mixed diet composed mostly of crustaceans and teleosts in class II and class III (Figure 5). The graphical method indicated a specialization on crustaceans of class I (Figure 5B(a), 5C(a)). The diet of classes II and III consisted of a mixed diet composed of crustaceans and teleosts with a dominance of crustaceans in class II and teleosts in class III of both sexes. Over 54% of the class II preyed on crustaceans, with 66.23% Pi and 89.65% with 70.16% Pi of males and females, respectively. On the other hand, 86.81% of the class III preyed on teleosts, with 25.90% and 91.97%, with 26.93% Pi, of males and females, respectively. Raja clavata is a genralized feeder with an ontogenetic change on preferred prey from crustaceans to teleosts (Figure 5).

Fig. 5. Prey-specific abundance (%Pi) plotted against frequency of occurrence (%Fi) of prey categories of Raja clavata from off the Gulf of Gabes. A, all specimens; B, females; C, males; (a) class I, <50 cm; (b) class II, 50–75 cm; (c) class III, >75 cm T L.
DISCUSSION
The percentage of empty thornback ray stomachs in the Gulf of Gabes was high when compared to those reported from the North Sea (9% (Daan et al., Reference Daan, Johnson, Larsen and Sparholt1993); 3.7% (Ellis et al., Reference Ellis, Pawson and Shackley1996)), South Wales (4.5% (Ajayi, Reference Ajayi1982)), west coast of southern Africa (4.5%, (Ebert et al., Reference Ebert, Cowley and Compagno1991); 2.6% (Smale & Cowley, Reference Smale and Cowley1992)), Portuguese mainland coast (2.5% (Cunha et al., Reference Cunha, Calvário, Marques and Ré1986)) and the Ligurian Sea (2.33% (Vannucci, Reference Vannucci2005)). The highest percentage of empty stomachs for thornback rays was (37.1%) reported from the Azores Islands (Morato et al., Reference Morato, Sola, Gros and Menezes2003).
The differences in percentage of empty stomachs among region may be related to fishing year and the different distribution patterns observed in these species. The percentage of empty stomachs is usually greater with samples obtained using baited gear (Medved et al., Reference Medved, Stillwell and Casey1985).
Our study shows that teleosts were the most abundant prey of R. clavata from the Gulf of Gabes. This prey group represented more than 50% of the total %IRI and can be classified as main food (Rosecchi & Nouazé, Reference Rosecchi and Nouazé1987). Crustaceans were secondary preys while cephalopods, gastropods and polychaetes were of minor importance in stomachs contents and incidentally consumed. The main diet categories of thornback rays from the Black Sea were crustaceans and secondary consumers (Saglam & Bascinar, Reference Saglam and Bascinar2008).
Off the Tunisian coast Capapé (Reference Capapé1975) found that crustaceans were preferential prey of the thornback rays, in the Ligurian Sea Vannucci (Reference Vannucci2005) found that these species fed upon crustaceans (%IRI = 76.83) while teleosts represented secondary food (IRI%17.40).
Differences in diet composition of several predators may reflect the geographical peculiarities in fauna composition (Kadri et al., Reference Kadri, Marouani, Saïdi, Bradaï, Bouaïn and Morize2014 )
Molluscs were also generally absent in the diets of other skate species and when present contributed little to diet composition (Ebert & Bizzarro, Reference Ebert and Bizzarro2007). Therefore it is likely that molluscs were incidentally ingested. Our study indicates that R. clavata in the Gulf of Gabes feed mainly on benthopelagic (Metapenaeus monoceros) and benthic crustaceans (Sicyonia carinata) and teleosts that live on the sand (Sardinella aurita) and soft bottom sediment (Carynx crysos). The occurrence of pelagic prey in the diet of demersal elasmobranch species may be derived from scavenging of the discard of pilchards by commercial fisheries (Simpfendorfer et al., Reference Simpfendorfer, Goodreid and McAuley2001; Saidi et al., Reference Saidi, Enajjar, Bradai and Bouain2009). In this study some pelagic fish prey were also recorded in the stomachs of thornback rays, confirming previous suggestions (Orlov, Reference Orlov1998; Morato et al., Reference Morato, Sola, Gros and Menezes2003; Saglam & Bascinar, Reference Saglam and Bascinar2008) that thornback rays are active predators and able to feed semi-pelagically. Muto et al. (Reference Muto, Soares and Goitein2001) demonstrated that the rio skate, Rioraja agassizii, feeds mainly on crustaceans (81.26%IRI) and fish (18.23%IRI) in south-eastern Brazil. O'Shea et al. (Reference O'Shea, Thums, van Keulen, Kempster and Meekan2013) established that the Himantura uarnak consumes a larger proportion of crustaceans, notably penaeids. Other authors have reported a dominance of fish and low importance of crustaceans in the diet of the thornback ray and other rays, namely in Azorean waters, the north-eastern Atlantic (Morato et al., Reference Morato, Sola, Gros and Menezes2003) in northern and central Patagonian waters (Koen Alonso et al., Reference Koen Alonso, Crespo, Garcia, Pedraza, Mariotti and Beron2001) and in the Barents Sea (Dolgov, Reference Dolgov2005).
The influence of sex and maturity is probably related to behaviour of the species and to its life cycle, which includes migrations that influence the type of prey caught. In other Rajidae species migration and aggregation have been linked to spatial and temporal variation in prey concentration or mating behaviour (Skjæraasen & Bergstad, Reference Skjæraasen and Bergstad2000). A comparison of the diet between size-classes indicated that R. clavata exhibited ontogenetic changes in diet, with crustaceans decreasing and molluscs and teleosts increasing in importance with ray size.
Changes in dietary composition that accompany growth reflect an increased ability of rays to consume larger preys such as teleosts and molluscs. These changes could also be related to modifications in the environmental conditions or to the energetic requirements of the animals.
Differences in diet by sex have been related to spatial segregation of sexes caused by differing habitats, including feeding and reproductive behaviour (McCord & Campana, Reference McCord and Campana2003). Colin et al. (Reference Colin, Simpfendorfer and McAuley2001) noted differences in diet by sex of Mustelus antarcticus. But Saglam & Bascinar (Reference Saglam and Bascinar2008) indicate no differences between the overall diet of males and females of Raja clavata, and similar findings have been reported in other studies (Braccini & Perez, Reference Braccini and Perez2005; Scenna et al., Reference Scenna, García and Díaz2006; San Martín et al., Reference San Martín, Braccini, Tamini, Chiaramonte and Perez2007). Conversely, sexual differences in the diet have been found in other skate species of Bathyraja in the western Bering Sea (B. parmifera, B. aleutica, B. maculata, B. matsubarai and B. minispinosa) (Orlov, Reference Orlov2001). There are sex-based differences in the diet of Mustelus lenticulatus that are largely the result of males and females having differing distribution patterns, though sexual differences in diets have been observed in some species (King & Clark, Reference King and Clark1984; Gray et al., Reference Gray, Mulligan and Hannah1997). Sexual differences in the food composition of species of Bathyraja from the northern Pacific have been attributed to the existence of sexual size dimorphism (Orlov, Reference Orlov1998). Stillwell & Kohler (Reference Stillwell and Kohler1993) noted some differences in diet between males and females, which may have been due to segregation by sex or to sampling location. Diet shifts with size is a pattern widely observed in elasmobranchs (Ebert, Reference Ebert2002; Treolar et al., Reference Treolar, Laurenson and Stevens2007; Lucifora et al., Reference Lucifora, Garcia, Menni, Escalante and Hozbor2009), but there is no unique explanation for it.
Raja clavata in the Gulf of Gabes exhibited ontogenetic changes in diet, with teleosts increasing in importance with skates' increasing size. The larger skate fed more evenly among prey categories, while the smaller skates foraged on a wider variety within those categories, as seen by the slight decrease in prey diversity with increasing skate size. These ontogenetic changes in diet mostly reflect habitat use, although physiological and morphological constraints certainly play some role.
A seasonal variation in the diet of R. clavata was noted within the study area. In addition, values of Schoener's index indicated high dietary overlap between seasons.
Some studies on the feeding habits of skates have described them as generalist predators (McEachran et al., Reference McEachran, Boesch and Musick1976; Orlov, Reference Orlov1998), although some species have been considered as specialist predators (Ebert et al., Reference Ebert, Cowley and Compagno1991; Braccini & Perez, Reference Braccini and Perez2005; Scenna et al., Reference Scenna, García and Díaz2006; San Martín et al., Reference San Martín, Braccini, Tamini, Chiaramonte and Perez2007). The graphical method used by Amundsen et al. (Reference Amundsen, Gabler and Staldvik1996) indicated that Raja clavata in the Gulf of Gabes is a generalized feeder.
ACKNOWLEDGEMENTS
We are grateful to the commercial fishermen for sample collection. Thanks also to the anonymous referees for comments that greatly improved the submitted manuscript.











