INTRODUCTION
Macrobenthic communities are often used as bioindicators (e.g. Pearson & Rosenberg, Reference Pearson and Rosenberg1978; Shin et al., Reference Shin, Huang and Wu2004; Gaudêncio & Cabral, Reference Gaudêncio and Cabral2007), and the attributes of a macrobenthic community (species composition, quantitative parameters, various biotic indices, e.g. B-IBI, AMBI, BENTIX, m-AMBI, BOPA, ITI) may reflect natural or anthropogenic perturbations in the marine environment (Weisberg et al., Reference Weisberg, Schaffner, Ranasinghe and Diaz1997; Borja et al., Reference Borja, Franco and Pérez2000; Simboura & Zenetos, Reference Simboura and Zenetos2002; Belan, Reference Belan2003; Dauvin & Ruellet, Reference Dauvin and Ruellet2007; Weise et al., Reference Weise, Cromey, Callier, Archambault, Chamberlain and McKindsey2009). The long generation time of many macrobenthic taxa make it possible to reflect the environmental conditions integrated over a long period of time, rather than reflecting the conditions at the time of sampling (Belan, Reference Belan2003). Marine ecologists have reached a consensus that the macrobenthos is a powerful tool to gauge organic enrichment or monitor environmental disturbance (Pearson & Rosenberg, Reference Pearson and Rosenberg1978; Henderson & Ross, Reference Henderson and Ross1995; Lee et al., Reference Lee, Bailey-Brock and McGurr2006; Tomassetti et al., Reference Tomassetti, Persia, Mercatali, Vani, Marussso and Porrello2009). However, the classical microscopy identification of macrobenthos is extremely time-consuming and requires specialized taxonomists and a high degree of taxonomic expertise given the large number of macrobenthic taxa (Warwick, Reference Warwick1988; Olsgard et al., Reference Olsgard, Somerfield and Carr1998; Anderson et al., Reference Anderson, Connell, Gillanders, Diebel, Blom, Saunders and Landers2005; Elias et al., Reference Elias, Palacios, Rivero and Vallarino2005; de-la-Ossa-Carretero et al., Reference de-la-Ossa-Carretero, Del-Pilar-Ruso, Giménez-Casalduero and Sánchez-Lizaso2012a). Consequently, the identification of an enormous macrofauna is always the major bottleneck in this field (Tataranni et al., Reference Tataranni, Maltagliati, Floris, Castelli and Lardicci2009; de-la-Ossa-Carretero et al., Reference de-la-Ossa-Carretero, Simboura, Del-Pilar-Rusoa, Pancucci-Papadopoulou, Giménez-Casalduero and Sánchez-Lizasoa2012b).
The use of feeding guilds in environmental assessments can decrease the taxonomic effort and provide value-added information. Guilds are defined as a set of organisms that act on or exploit environmental resources in a similar manner, regardless of their phylogenetic relationships (Root, Reference Root1967; Fauchald & Jumars, Reference Fauchald and Jumars1979; MacDonald et al., Reference MacDonald, Burd, MacDonald and van Roodselaar2010; Manokaran et al., Reference Manokaran, Khan, Lyla, Raja and Ansari2013; Jumars et al., Reference Jumars, Dorgan and Lindsay2015). Feeding guilds, which can link community structure to ecological function (Jumars et al., Reference Jumars, Dorgan and Lindsay2015), have proven to be valuable for generalizations and for continuing investigations in various animals and plants (Fauchald & Jumars, Reference Fauchald and Jumars1979). We adopted ‘feeding guilds’ because of the term's popularity, instead of the term ‘functional groups’ that some scientists have proposed (Schaffner, Reference Schaffner1990; Rosenberg, Reference Rosenberg2001). The composition of feeding guilds can be affected by many biotic or abiotic factors, such as total organic carbon (TOC) (Denisenko et al., Reference Denisenko, Denisenko, Lehtonen, Andersin and Laine2003; Taurusman, Reference Taurusman2010), food availability (Dauwe et al., Reference Dauwe, Herman and Heip1998; Rosenberg, Reference Rosenberg2001), depth and salinity (Rosenberg, Reference Rosenberg2001), hydrodynamic processes (Sanders, Reference Sanders1958) and the physical characteristics of the substrates (Arruda et al., Reference Arruda, Domaneschi and Amaral2003). The definition of a feeding guild is very helpful for interpreting the ecological functioning or rationale behind guilds, and the use of a feeding guild is crucial for furthering our understanding of the benthic process (e.g. commensalism), the role of benthos and community energy flux (Warwick, Reference Warwick1988; Pearson, Reference Pearson2001; Gray & Elliott, Reference Gray and Elliott2009; Chaudhuri et al., Reference Chaudhuri, Mukherjee and Homechaudhuri2014). They are also used to explain changes in the macrobenthic community along an environmental gradient, e.g. organic enrichment in shellfish aquaculture (Pearson, Reference Pearson2001; Gray & Elliott, Reference Gray and Elliott2009).
The polychaete is one of the most important components of the marine macrobenthic community in coastal and marine environments, in terms of high diversity and abundance (Fauchald & Jumars, Reference Fauchald and Jumars1979; Grassle & Maciolek, Reference Grassle and Maciolek1992; Hutchings, Reference Hutchings1998). Polychaetes are among the most speciose benthic taxa (Fauchald & Jumars, Reference Fauchald and Jumars1979), with over 13,000 species described to date (Minelli, Reference Minelli1993; Jumars et al., Reference Jumars, Dorgan and Lindsay2015), comprising over one third of the total macrobenthic species number and up to 80% of the total abundance (Fauchald & Jumars, Reference Fauchald and Jumars1979; Belan, Reference Belan2003; Manokaran et al., Reference Manokaran, Khan, Lyla, Raja and Ansari2013). They also exhibit a high diversity of feeding modes, reproductive strategies and different levels of tolerance to the negative impacts induced by pollution and natural perturbations, and many species can be utilized as indicators for the accumulation of organic matter or other disturbances (Giangrande et al., Reference Giangrande, Licciano and Musco2005; MacDonald et al., Reference MacDonald, Burd, MacDonald and van Roodselaar2010; Sivadas et al., Reference Sivadas, Ingole and Nanajkar2010). Polychaetes have characteristic feeding structures and can be easily identified as carnivores, herbivores, surface deposit feeders, filter feeders or subsurface deposit feeders (burrowers) (Fauchald & Jumars, Reference Fauchald and Jumars1979; Gray & Elliott, Reference Gray and Elliott2009; Jumars et al., Reference Jumars, Dorgan and Lindsay2015).
Various feeding guilds of polychaetes have been well studied (e.g. Fauchald & Jumars, Reference Fauchald and Jumars1979, etc.) and recently updated (e.g. Jumars et al. Reference Jumars, Dorgan and Lindsay2015). This information allows for the analysis of feeding guilds of polychaete communities, and could provide us with accurate and useful information for understanding the local benthic environmental and ecological quality. Since the use of feeding guilds has been proven a faster, easier and more effective way to detect or assess biological impacts, these studies are crucial for both science and management, especially for northern China, which is subjected to extensive anthropogenic perturbation and environmental pollution. In the present study, field samples were collected bimonthly in northern China in 2011 to demonstrate the distribution and composition of polychaete feeding guilds and reveal how the polychaete feeding guilds responded to environmental change.
MATERIALS AND METHODS
Study area
In 2011, field sampling was conducted bimonthly in the Sishili Bay. The Sishili Bay is situated in the northern Yellow sea, China. A total of four sites from each of four transects were linearly defined, outward from the Kongtong Island (Figure 1). The stations were named after the combination of transect numbers and site numbers. The average water depth was approximately 10 m in transects 2, 3 and 4, and 20 m in transect 1. The particle size of sediment in the Sishili bay showed over 70% similarity, and the substrate was primarily composed of fine sand and coarse silt (Liu et al., Reference Liu, Shen, Wang, Chen and Li2012).

Fig. 1. Locations of the sampling transects and stations around the Kongtong Islands in the northern Yellow Sea.
Collection of polychaetes
Samples were collected bimonthly in 2011. At each site, four samples were collected using a 0.05 m2 Van Veen Grab. Three of these samples were used to study the composition of the macrobenthos. These samples were washed in situ using a 0.5 mm mesh screen and then preserved in 95% ethyl alcohol. The polychaetes were sorted through a stereo binocular microscope after staining with Rose Bengal. The polychaete was identified to the species level, and then quantified and weighed.
Environmental parameters
Environmental parameters, including temperature, depth, salinity, dissolved oxygen (DO) and pH, were examined using YSI 660 (Yellow Springs, OH). At each site, the 4th sample was used to analyse the characteristics of sediment, including TOC, grain size, total nitrogen (TN) and total sulphur (TS).
The sediment samples were air-dried at room temperature. To remove the organic matter, 10 mL 10% H2O2 were added to the air-dried samples, and then they were heated at 60°C for 2 h. The samples were treated with NaPO3 (0.05 mol dm−3) after centrifugation. Finally, the grain size was measured using a Mastersizer 2000 Laser Particle Sizer (Malvern Instruments Ltd, UK), and the detailed grain sizes were referred to by the terms sand (64–2000 µm), coarse silt (16–64 µm), fine silt (4–16 µm) and clay (<4 µm) (Folk & Ward, Reference Folk and Ward1957).
The samples were treated with a diluted HCl solution to remove carbonates from the soil and were washed with MilliQ water 3 times after acid washing. TOC, TN and TS were examined using a vario MICRO CUBE (Elementar, Germany).
Polychaete feeding guild assignments
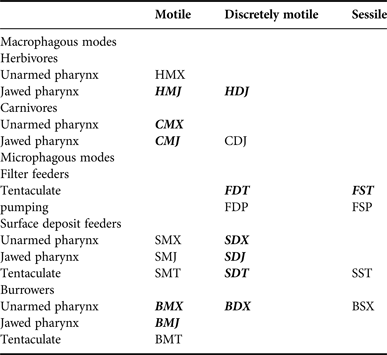
A feeding guild can be defined as a set of organisms that exploit food resources through a similar intake mechanism, independent of their phylogenetic relationship (Fauchald & Jumars, Reference Fauchald and Jumars1979; Manokaran et al., Reference Manokaran, Khan, Lyla, Raja and Ansari2013). Fauchald & Jumars (Reference Fauchald and Jumars1979) proposed a classification of polychaetes into 22 feeding guilds based on their feeding mode, motility pattern and morphological feeding structure, and recently Jumars et al. (Reference Jumars, Dorgan and Lindsay2015) made an emendation to the diet of these marine worms. The assignments of feeding guilds of polychaetes were guided by the conclusion of Fauchald & Jumars (Reference Fauchald and Jumars1979), MacDonald et al. (Reference MacDonald, Burd, MacDonald and van Roodselaar2010) and Jumars et al. (Reference Jumars, Dorgan and Lindsay2015). The feeding guilds of polychaetes were divided into two modes (macrophagous and microphagous) and five submodes (herbivores, carnivores, filter feeders, surface deposit feeders and burrowers), according to their major feeding modes (Table 1). The motility pattern was classified into motile, discretely motile and sessile, and the morphological structure used in feeding was divided into jawed, pumping, tentaculate or other structure, which are usually eversible sac-like pharynges (Fauchald & Jumars, Reference Fauchald and Jumars1979; MacDonald et al., Reference MacDonald, Burd, MacDonald and van Roodselaar2010; Jumars et al., Reference Jumars, Dorgan and Lindsay2015). If the feeding guild of a particular species was unknown, it was assumed to feed in a similar manner as congeneric or confamilial species or species within the same major groups after the assignments of Fauchald & Jumars (Reference Fauchald and Jumars1979), MacDonald et al. (Reference MacDonald, Burd, MacDonald and van Roodselaar2010) and Jumars et al. (Reference Jumars, Dorgan and Lindsay2015).
Table 1. Feeding guilds of polychaetes after Fauchald & Jumars (Reference Fauchald and Jumars1979), Pagliosa (Reference Pagliosa2005) and Jumars et al. (Reference Jumars, Dorgan and Lindsay2015). The feeding guilds found in this study are in italics and bold. In the three letter codes, the letter in the first position indicates major mode, the second the motility pattern, and the last letter indicates the morphological structure used in feeding; in position 1-B, subsurface deposit feeder (burrower); C, carnivore; F, filter feeder; H, herbivore, S, surface deposit feeder; in position 2-D, discretely motile; M, motile; S, sessile; in position 3-J, jawed; P, pumping; T, tentaculate; X, other structures, usually eversible sac-like pharynges.
Statistical analyses
The abundance data were standardized (ind. m−2) and then processed using both univariate and multivariate statistical analyses. PRIMER (Plymouth Routines in Multivariate Ecological Research, version 6.0) and PASW Statistics (version 18.0) were used for statistical analyses. Abundance data were square root transformed to reduce the contribution of dominant taxa and, therefore, increase the importance of less abundant species before all subsequent analyses. The Bray–Curtis similarity for abundance and biomass data and Euclidean distance for environmental data were used to construct resemblance matrices among samples before further analyses. Non-metric multidimensional scaling (MDS) and hierarchical cluster analysis (CLUSTER) were performed to analyse the community structure. An Analysis of Similarities (ANOSIM) was conducted to statistically test whether two or more sample groups were significantly dissimilar. The Similarity Percentage Routine (SIMPER) was used to examine which variable (feeding guild) contributed the most to the within-group similarities at every site. Correlation coefficients (Spearman) between environmental factors and the feeding guilds of the polychaetes were analysed using PASW Statistics.
RESULTS
Taxonomic composition of Polychaeta
We collected the macrobenthic organisms during six expeditions in Sishili Bay in 2011. As the most abundant macrobenthic taxa in this area, polychaetes comprised 62.76% of the total individuals. A total of 12,845 polychaete specimens were classified and identified as 80 species that belonged to 73 genera and 37 families. The abundance of the polychaetes varied from 313.49 to 2407.87 ind. m−2. The most speciose families were Spionidae (seven species), Nereidae (six species) and Polynoidae (five species). The most abundant families were Lumbrineridae and Capitellidae, which accounted for 42.75 and 14.98%, respectively, of the total abundance. The polychaetes Lumbrineris latreilli Audouin & Edwards, 1834 and Heteromastus filiformis (Claparède, 1864) were the two most abundant and prevalent species and comprised 42.68 and 11.33%, respectively, of the total abundance across all of the sampling sites.
The dominant polychaete L. latreilli is a very common carnivore in the northern Yellow Sea and the Bohai Sea. They construct semi-permanent burrows, from which they selectively feed upon small particles of subsurface deposits, microfauna, meiofauna and macrofauna (Watson et al., Reference Watson, Robertson and Littlejohn1984; Petch, Reference Petch1986; Renaud et al., Reference Renaud, Wlodarska-Kowalczuk, Trannum, Holte, Weslawsk, Cochrane, Dahle and Gulliksen2007; MacDonald et al., Reference MacDonald, Burd, MacDonald and van Roodselaar2010; Han et al., Reference Han, Wang, Zhang, Keesing and Liu2013). The worm L. latreilli was unrelated to TOC and not susceptible to the organic matter content (Han et al., Reference Han, Wang, Zhang, Keesing and Liu2013), but it was significantly positively correlated to the content of coarse silt (P < 0.01).
The common bristle worm, H. filiformis, is an omnivorous subsurface deposit feeder that has a clear preference for fine sediments with high mud content (Degraer et al., Reference Degraer, Wittoeck, Appeltans, Cooreman, Deprez, Hillewaert, Hostens, Mees, Vanden Berghe and Vincx2006; MacDonald et al., Reference MacDonald, Burd, MacDonald and van Roodselaar2010). This species has a high relative occurrence (>20%) in sediments with a median grain size of 100–200 µm (Degraer et al., Reference Degraer, Wittoeck, Appeltans, Cooreman, Deprez, Hillewaert, Hostens, Mees, Vanden Berghe and Vincx2006). This species was positively correlated with TN (P < 0.05), TOC (P < 0.05) and coarse silt (4–16 µm, P < 0.01). The total abundance across all of the sampling transects and months was negatively correlated with the clay content (P < 0.01) but positively correlated with the coarse silt content (P < 0.01).
Comparison of polychaete community structure
Before the analyses of the polychaete community structure, we verified whether the assessment of polychaete community structure, instead of the whole macrobenthic community, could be used to detect environmental disturbance or ecological change. We calculated the ecological indices of both the polychaete community and the macrobenthic community (e.g. total species, total individuals, species richness, Pielou's evenness and Shannon diversity). All of the correlations between these pairwise groups were significant at the 0.01 level (Table 2).
Table 2. The test of correlation between macrobenthos and polychaete community, including the significance level and Pearson's correlation.

The non-metric multi-dimensional scaling (MDS) and hierarchical cluster analysis (CLUSTER) showed the difference in the species composition of polychaetes in different months (Figure 2). The CLUSTER output showed that the sampling sites appeared to cluster into five groups at a 50% similarity level, and the one-way ANOSIM analysis confirmed this result (R = 0.857, P < 0.01). The samples clustered consistently according to the sampling location rather than the sampling time (month). This indicates that spatial heterogeneity is a stronger predictor of polychaete community structure than is month.

Fig. 2. Dendrogram of hierarchical clustering (CLUSTER) using group-average linking across all sampling transects and months.
Two-way analysis of similarities (ANOSIM) and pairwise test were adopted to test whether the polychaete community showed significant spatial and temporal changes. The results showed that the community structure was strongly related to both site (R = 0.385, P < 0.01) and sampling season (R = 0.241, P < 0.01). However, the pairwise test indicated that the polychaete communities were relatively homogenous between May and July, and September and November (Table 3).
Table 3. One-way ANOSIM using PRIMER 6.0. Statistically significant values are in bold.

*Significant at 95% confidence level.
**Significant at 99% confidence level.
To clearly demonstrate the similarity level of the macrobenthic communities, MDS and CLUSTER were performed on the polychaete abundance data for every sampling month, and a one-way ANOSIM was performed on the transects (Table 4). These analyses revealed that significant differences occurred between the transect groups in every month. The results of the CLUSTER analysis showed that the community structures of transects 3 and 4 were more similar, with the value of their Bray–Curtis similarity reaching 70%.
Table 4. One-way ANOSIM of every sampling months using PRIMER 6.0. Statistically significant values are in bold.

*Significant at 95% confidence level.
**Significant at 99% confidence level.
Similarity percentages (SIMPER) analysis was also performed to determine which species were responsible for the differences among different CLUSTER groups. The outputs of the SIMPER analysis showed that the average dissimilarity between transects 3 and 4 was 36.51%, which was much lower than other pairwise comparison of different transects, which varied from 44.45–47.86%. Four species were always the most important contributor to average similarity, they were L. latreilli, H. filiformis, Nephthys polybranchia Southern, 1921 and Pista cristata (Müller, 1776). This result coincided with the output of the CLUSTER analyses.
Feeding guilds composition
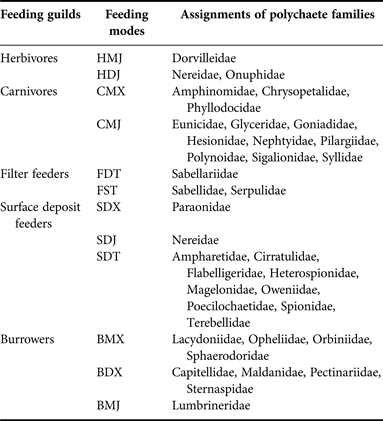
A total of 80 polychaete species were classified into five feeding modes (subsurface deposit feeder or burrower, carnivore, filter feeder, herbivore and surface deposit feeder) and 12 submodes (HMJ, HDJ, CMX, CMJ, FDT, FST, SDX, SDJ, SDT, BDX, BMX, BMJ) (Table 5). Carnivores and surface deposit feeders were the dominant feeding guilds in the study area in terms of species number, comprising 29 species and 24 species, respectively. Filter feeders and herbivores were the least diverse, and represented by only five and four species, respectively. Among all 12 of the feeding submodes, SDT and CMJ were the most speciose feeding guilds, together constituting ~50% of the total species across all of the sampling seasons and transects.
Table 5. Feeding guilds assignment for polychaete families, after Fauchald & Jumars (Reference Fauchald and Jumars1979), MacDonald et al. (Reference MacDonald, Burd, MacDonald and van Roodselaar2010) and Jumars et al. (Reference Jumars, Dorgan and Lindsay2015).
With respect to abundance, the polychaete community was composed of primarily burrowers (61.48%), surface deposit feeders (21.20%), carnivores (16.80%), herbivores (0.37%) and filter feeders (0.16%) (Figure 3). Among all of the feeding submodes, BDX (16.42%), BMJ (42.76%), CMJ (14.43%) and SDT (17.58%) were found to be the most common. Feeding guilds without strong representation were FDT (0.05%, with only one species Lygdamis giardi (McIntosh, 1885)); FST (0.10%); HMJ (0.09%, with only one species Dorvillea pseudorubrovittata Berkeley, 1927); and HDJ (0.29%). The worms H. filiformis, L. latreilli, Nephthys polybranchia Southern, 1921 and P. cristata were the most important abundance contributors to the dominant feeding submodes (BDX, BMJ, CMJ and SDT), accounting for 99.82, 68.99, 36.81 and 43.91%, respectively, of the total submode abundance they belonged in. A one-way ANOVA was conducted to examine the spatial and temporal differences in PRIMER 6.0. The results showed that the feeding submodes had both significant spatial (between different transects, global R = 0.423, P = 0.1% < 0.05) and temporal (between different sampling months, global R = 0.185, P = 0.1% < 0.05) differences.

Fig. 3. The temporal and spatial variation of abundance percentages of feeding guilds.
The aggregation data of the different motion patterns were counted and analysed. The motile taxa included 35 species of 18 families, accounting for 61.94% of the total abundance. The most abundant species, L. latreilli, comprised 69.42% of the motile polychaetes. Forty-one species of 17 families were involved in discretely motile locomotion and accounted for 37.96% of the total abundance. Only 4 species of 2 families were involved in the sessile locomotion pattern, accounting for 0.10% of the total abundance. The results of the one-way ANOSIM showed that the feeding submodes had both significant spatial (between different transects, global R = 0.351, P = 0.1% < 0.05) and temporal (between different sampling months, global R = 0.073, P = 0.6% < 0.05) differences.
The polychaetes in the research area fed with three types of feeding structures. Twenty-seven species, belonging to 13 families, fed using their jawed structure, which constituted 59.19% of the total abundance. Tentacular structures were found in 28 species of 12 families, and they accounted for approximately 17.74% of the total abundance. Other structures, typically eversible sac-like pharynges, were used by 25 species in 12 families, constituting 23.07% of the total abundance. The results showed that the feeding submodes had both significant spatial (between different transects, global R = 0.383, P = 0.1% < 0.05) and temporal (between different sampling months, global R = 0.093, P = 0.2% < 0.05) differences.
Relationship between feeding guilds and environmental factors
A Spearman rank correlation was performed to analyse the relationships between the abundance of feeding groups (feeding guilds, aggregation of motility patterns or feeding structures) and the environmental parameters (TN%, TOC%, TS%, depth, amount of clay, fine silt, coarse silt and sand) in PASW Statistics 18.0 (Table 6). The results indicated that the amount of coarse silt was the most influential environmental factor, and was positively correlated with the abundance of burrowers, carnivores, surface deposit feeders, motile polychaetes, discretely motile polychaetes and all of the aggregations of feeding structures but was negatively correlated with herbivores. The amount of fine silt was a negatively influential environmental factor to the abundance of carnivores, surface deposit feeders and organisms fed with tentacles. The amount of clay was negatively correlated with the abundance of carnivores, surface deposit feeders, motile polychaetes, discretely motile polychaetes and all of the aggregations of different feeding structures. The depth was negatively correlated with the abundance of herbivores, surface deposit feeders and tentacular animals. The TS amount was negatively correlated with the abundance of carnivores, herbivores, surface deposit feeders and tentacular animals. The TOC content can only affect the abundance of carnivores. The TN content was proved to be a positively influential environmental factor for the abundance of carnivores, surface deposit feeders, motile polychaete, jawed or tentacular animals. The amount of sand had a negligible influence on the distribution or composition of the feeding guilds. The TOC distribution followed the distribution of clay (Pearson correlation = 0.251, P = 0.016 < 0.05) and the fine silt fraction (Pearson correlation = 0.284, P = 0.006 < 0.01), and it avoided the high percentage of sand (Pearson correlation = −0.332, P = 0.001 < 0.01).
Table 6. Correlation matrix with P values between feeding guilds and environmental variables.

TOC, total organic carbon; TN, total nitrogen; S, sulphur.
*Significant at 95% confidence level.
**Significant at 99% confidence level.
DISCUSSION
In the present study, the abundance and composition of soft bottom polychaete assemblages in a northern China coastal bay were described, together with the analyses of feeding guilds of polychaete assemblages. The results showed that the polychaete community and the feeding guilds exhibited significant spatial and temporal differences. This conclusion coincided with the previous study derived from the analysis of the macrobenthic community that macrobenthic communities can be significantly affected by the sampling months and transects (Han et al., Reference Han, Wang, Zhang, Keesing and Liu2013).
The results obtained using polychaetes or macrofauna were exactly the same, which indicated that polychaete assemblages alone should be sufficient for environmental monitoring without losing important information. Studies of taxonomic sufficiency showed that information loss was relatively low because the data were aggregated at higher taxonomic levels, particularly family- or even order-level resolution (e.g. Warwick, Reference Warwick1988; Vanderklift et al., Reference Vanderklift, Ward and Jacoby1996; Olsgard et al., Reference Olsgard, Somerfield and Carr1998; Karakassis & Hatziyanni, Reference Karakassis and Hatziyanni2000; Lampadariou et al., Reference Lampadariou, Karakassis and Pearson2005; Wlodarska-Kowalczuk & Kedra, Reference Wlodarska-Kowalczuk and Kedra2007; Tataranni et al., Reference Tataranni, Maltagliati, Floris, Castelli and Lardicci2009). The feeding guilds are mainly classified at the family-level resolution based on a joint consideration of food, feeding habits and locomotory pattern (Fauchald & Jumars, Reference Fauchald and Jumars1979; MacDonald et al., Reference MacDonald, Burd, MacDonald and van Roodselaar2010; Jumars et al., Reference Jumars, Dorgan and Lindsay2015). The analysis of the feeding guild composition was very similar to those obtained using the entire macrobenthic community or polychaete community at a species-level resolution. Therefore, we suggest that environmental monitoring could be conducted using polychaetes alone or the feeding guild of polychaetes which would be faster and cost-effective with best equilibrium between the precision of results and a decrease in taxonomic effort.
The high similarity of contiguous sampling months (Table 3) revealed that our sampling frequency may be excessive for the analysis of polychaete communities, and a reduction of sampling frequency could save unnecessary expense and effort.
Although the feeding guild has been used worldwide for environmental monitoring, studies are still deficient in the classification of the real feeding habit of each group in the natural environment (Fauchald & Jumars, Reference Fauchald and Jumars1979; MacDonald et al., Reference MacDonald, Burd, MacDonald and van Roodselaar2010). Any erroneous assignment may mislead us easily with inaccurate information or conclusions, especially for these ubiquitously dominant taxa. Caution is required when determining the real feeding habits of every group and assigning the species or families into the correct feeding guilds. Moreover, the morphological features of feeding structures may not correspond to the differences in the utilized resource (Jaksic, Reference Jaksic1981; MacDonald et al., Reference MacDonald, Burd, MacDonald and van Roodselaar2010) because the same anatomical structures for food gathering could have a wide variety of feeding behaviours (Dauer et al., Reference Dauer, Maybury and Ewing1981). Some spionid polychaetes are capable of both suspension feeding and deposit feeding and switch between these feeding habits, depending on density-related interactions, the near-bottom flux of suspended matter and varying boundary flow rates (Pearson & Rosenberg, Reference Pearson and Rosenberg1978; Taghon et al., Reference Taghon, Nowell and Jumars1980, Reference Taghon1982, Reference Taghon1992; Dauer et al., Reference Dauer, Maybury and Ewing1981; Snelgrove & Butman, Reference Snelgrove and Butman1994; Shimeta, Reference Shimeta1996; Gaudêncio & Cabral, Reference Gaudêncio and Cabral2007; Manokaran et al., Reference Manokaran, Khan, Lyla, Raja and Ansari2013).
In this research, the four most abundant species accounted for 67.04% of the total individuals, and the dominant species were L. latreilli, H. filiformis, P. cristata and N. polybranchia. Nevertheless, most polychaetes seemed to adopt constant feeding behaviours in a relatively stabilized substrate and benthic community (Manokaran et al., Reference Manokaran, Khan, Lyla, Raja and Ansari2013). In this study, all of the species of the family Spionidae were assigned as SDT, regardless of the possible feeding habit switching, because they contributed a negligible portion (2.57%) of the total abundance of surface deposit feeders and could have had a weak influence on our final results.
The environmental data, such as grain size and other covarying environmental factors, should be of great importance for the composition and distribution of both the polychaete community and feeding guilds (Snelgrove & Butman, Reference Snelgrove and Butman1994; García-Arberas & Rallo, Reference García-Arberas, Rallo, Orive, Elliott and Jonge2002; Gaudêncio & Cabral, Reference Gaudêncio and Cabral2007; Manokaran et al., Reference Manokaran, Khan, Lyla, Raja and Ansari2013; Mattos et al., Reference Mattos, Cardoso and Santos2013). In addition to these causative environmental factors, the population dynamics of different feeding guilds may be strongly affected by the food supply, interspecies interactions and larval supply (Levinton, Reference Levinton1972; Snelgrove & Butman, Reference Snelgrove and Butman1994). In this study, the abundance of almost all of the feeding guilds (except for filter feeders) was influenced by the texture of the sediments. Burrowers were the most abundant feeding guilds (61.48%), followed by surface deposit feeders (21.20%) and carnivores (16.80%). Surface-deposit feeders usually dominate the soft bottom habitats (Gaston, Reference Gaston1987), but in this study, burrowers were the predominant feeding guild. The abundance of burrowers and filter feeders did not seem to be influenced by any of the environmental factors studied here, with the exception of per cent of coarse silt, whereas the carnivores and surface deposit feeders were affected by most of these environmental factors. The proportion of burrowers was greatest in fine-sediment habitats and positively correlated with the percentage of coarse silt. Deposit-feeders, including burrowers and surface deposit feeders, are usually more abundant in muddy habitats, whereas suspension feeders prevail in sediments with a low content of fine fractions (Snelgrove & Butman, Reference Snelgrove and Butman1994; García-Arberas & Rallo, Reference García-Arberas, Rallo, Orive, Elliott and Jonge2002; Gaudêncio & Cabral, Reference Gaudêncio and Cabral2007; Manokaran et al., Reference Manokaran, Khan, Lyla, Raja and Ansari2013). The high abundance of carnivores in this area was most likely attributed to the high abundance of the prey organisms that occurred in the interstitial spaces (Muniz & Pires, Reference Muniz and Pires1999) and was related to coarse silt instead of sand. Surface deposit feeders and carnivores appeared to be more vulnerable to the variation of environmental conditions, including TN and TS content, percentage of different fractions of clay, fine silt and coarse silt. The substrate provides diverse habitats, sufficient dissolved oxygen and abundant food resources for surface deposit feeders and preys of carnivores, and so slight change of chemical and physical traits of substrates can result in the abundance variation of these taxa. However, burrowers and filter feeders are usually not susceptible to natural or anthropogenic disturbances, with the exception of composition of grain size. The high current and tidal velocity (average 14.8 cm s−1, maximum to 30 cm s−1) may be responsible for the low abundance of filter feeders (0.16% of the total abundance) by inhibiting their settlement or even survival. In the present study, we attempted to obtain a general pattern of feeding guilds structure to establish relationships between their distribution and environmental variables, such as TN, TOC, TS, depth, sediment composition or even hydrodynamics. It must be noted that factors not directly related to the feeding guilds were most likely just as important in understanding the observed distributional patterns (Dauer et al., Reference Dauer, Maybury and Ewing1981), such as tidal, diurnal, illumination changes or other regular environmental variability (Levinton, Reference Levinton1972).
ACKNOWLEDGEMENTS
We would like to express our gratitude to Jin Zhou, Wenliang Liu and Wenqian Cai for their kind help for some species identification. We also thank Yong Zhang, Yueqi Wang, Quanchao Wang, Chao Wang, Junkai Han and Bingjian Chen for their kind help during our field investigation, and Wentao Song for the measurement of sediments.
FINANCIAL SUPPORT
This research project was funded by a granted from the National Natural Science Foundation of China (No. 41006076), Open Fund of Zhejiang Provincial Top Key Discipline of Aquaculture in Ningbo University (xkzsc1407) and K.C. Wong Magna Fund in Ningbo University.











