Introduction
Studies over more than two decades indicate that peak bone mass is partly inheritedReference Ralston1 and that the peak bone mass of an individual in later life depends upon the peak bone mass obtained during skeletal growth and the subsequent rate of bone loss. We have demonstratedReference Cooper, Cawley and Bhalla2–Reference Fall, Hindmarsh and Dennison5 that poor growth during foetal life, infancy and childhood is associated with decreased bone mass in adulthood and an increased risk of fracture. However, to date, little is known of the relationship of the cellular and molecular mechanisms whereby environmental modulation in utero Reference Godfrey and Barker6, Reference Barker7 may lead to an altered skeletal growth trajectory and susceptibility to later fracture.
It is known that during pregnancy and lactation, foetal calcium is derived from the mother. As adult calcium levels and bone metabolism are tightly regulated by vitamin D, then sufficient maternal vitamin D would appear to be especially critical during pregnancy and lactation. Maternal adaptations during pregnancy, lactation and foetal development provide the necessary calcium for the offspring relatively independently of vitamin D status.Reference Kovacs8 Vitamin D has been shown to be necessary and important for the offspring after birth, at least with respect to calcium metabolism and skeletal health. However, maternal hypovitaminosis D during pregnancy could have detrimental effects on the skeleton of the foetus, as well as the mother, with regard to calcium availability.
Animal studies suggest such a pivotal role of vitamin D in skeletal development. Using vitamin D-deficient ratsReference Miller, Halloran, DeLuca and Jee9 and vitamin D receptor-null miceReference Li, Amling and Pirro10 it has been demonstrated that while skeletal mineral content appears normal at birth and during the first 2 weeks of life, animals subsequently developed progressive hypocalcaemia, hypophosphataemia, as well as evidence of rickets. This sequence of events parallels the maturation of intestinal calcium absorption postnatally, with a transition from a passive absorption facilitated by lactose to an active process that depends on 1,25 dihydroxyvitamin D3 [1,25(OH)2D3]. However, we have shown in a longitudinal human studyReference Javaid, Crozier and Harvey11 a significant correlation between reduced maternal 25(OH)D concentrations and reduced calcium transfer during pregnancy together with decreased bone mineral density (BMD) and bone mineral content (BMC) in related offspring at 9 years of age. Rummens et al.Reference Rummens, van Bree and Van Herck12 made similar observations in a guinea pig study. Furthermore, Namgung et al.Reference Namgung, Tsang and Lee14 showed seasonal variation in the newborn weight of Korean winter-born v. summer-born newborn infantsReference McGrath, Barnett and Eyles13 and their BMC. These data suggest maternal vitamin D levels may influence foetal bone parameters.
The purpose of the study presented here was to determine in a rat model if vitamin D deficiency in the mother during pregnancy had a deleterious effect on bone structure, composition and development in the offspring at skeletal maturity.
Methods and materials
Experimental design and animal care
Female Sprague-Dawley rats were housed in a temperature controlled room (20–22°C) on a 12 h light–dark cycle. Food and water were provided ad libitum. Vitamin D deficiency was induced by feeding female rats with a formulated diet (AIN-93G + AIN93-G mineral mix and AIN93-VX vitamin mix) deficient in vitamin D (0 IU/kg, containing normal calcium and phosphorus, Dyets Inc., PA, USA) as well as placement and housing under incandescent lighting (absence of ultraviolet radiation in the vitamin D action spectrum, 290–315 nm). Control animals were fed a matched formulated diet containing vitamin D (1000 IU/kg, Dyets Inc.) and housed in the same room. Control and deplete female rats were housed under these respective environments from 4 weeks of age. Females were mated at 10 weeks of age and housed under these conditions until the birth of the pups. Previous studiesReference Eyles, Brown, kay-Sim, McGrath and Feron15, Reference Burne, O'Loan and Splatt16 showed dams and neonates kept under vitamin D deplete conditions were severely depleted of both 25-hydroxy and 1,25-dihydroxy vitamin D. At birth the vitamin deplete dams were switched to control diet. Offspring were weaned at 3 weeks. At 5 weeks of age all offspring were transferred to normal lighting conditions.
Offspring were harvested at 0 and 140 days of age. By 140 days of age, bone growth rate is minimal and BMD is beginning to plateau.Reference Horton, Bariteau, Loomis, Strauss and Damron17 All housing and behavioural assessment procedures were performed with approval from the University of Queensland Animal Ethics Committee, under the guidelines of the National Health and Medical Research Council of Australia.
Collection and preparation of bone specimens
Femora were dissected out, stripped of soft tissue using a scalpel and immediately frozen for subsequent micro-computed tomography (CT) and 3-point bend testing.
Histology
The left femora were fixed in 4% formaldehyde in PBS and decalcified in a solution of 0.1 M TRIS and fresh 5% EDTA (pH 7.2) solution at 4°C. Decalcification was confirmed using a Faxitron MX-20 (Faxitron X-ray, Wheeling, Illinois, USA). Seven micrometre sections through the centre of the femoral head were stained with Weigert's Haematoxylin, alcian blue and sirius red. Images from each section were captured using an AxioCamHR camera mounted on a Zeiss Axiovert microscope and Axiovision software at a 1300 × 1300 resolution.
3D CT
Right femora from 0 and 140-day-old offspring were scanned using an Xtek Benchtop 160Xi scanner (Nikon Metrology, Tring, Hertfordshire, UK) equipped with a Hamamatsu C7943 X-ray flat panel sensor (Hamamatsu Photonics, Welwyn Garden City, Hertfordshire, UK). All scans were taken at 150 kV, 60 μA using a molybdenum target with an exposure time of 534 ms and 4× digital gain. Voxel size was 17 μm for 140 days samples and 18.6 μm for samples at birth. Reconstructed volume images were analyzed using VGStudio Max 1.2.1 software (Volume Graphics GmbH, Heidelberg, Germany) to give values for length, diameter, bone volume to total volume ratio (BV/TV), bone surface to bone volume ratio (BS/BV), trabecular thickness and spacing. Additional calculations were made of structural model index (SMI, a measure of surface convexity where an ideal plate, cylinder and sphere have SMI values of 0, 3 and 4, respectively) using a custom written package and the Visilog Quantification + package (both Noesis, Crolles, France) within the Amira 4.1.2 package (Mercury Computer System Inc., Chelmsford, USA). The volumes of interest for the data analysis of 140-day-old samples were proximal femur (purely trabecular region of the metaphyseal area of the femoral head) and midshaft of the femur. None of the regions included the growth plate. Cross-sectional moment of inertia (CSMI) was calculated as CSMI = (π/4) × (r 14−r 24), where r 1 is the mean radius of the midshaft and r 2 is the mean radius of the lumen at the same position. Using phantoms of known density (Skyscan, Kontich, Belgium), all the voxels that formed the structure were automatically assigned BMD in g/cm3. To analyze the degree of mineralization in the secondary ossification centre in the femoral head, the complete femoral head epiphyseal region was selected as a volume of interest. The proportion of bone in relation to the total volume (BV/TV) was then determined.
Bone composition and elemental analysis
Sections of femoral heads and midshaft cortical bone were dissolved in 3 M sub-boiled HNO3. An aliquot was removed and diluted with 2% sub-boiled HNO3 to give a calcium concentration of ∼150 ppm, final solutions contained 10 ppb indium and rhenium and 20 ppb beryllium as internal standards. Concentrations of calcium, strontium and barium were determined using a Thermo Instruments X-series 2 ICP-MS (Thermo Scientific, Hemel Hempstead, UK) operating in solution mode. External calibration was performed using a custom multi-element calibration series made from single element ICP-MS standards. Element concentrations are reported as element/calcium ratios to control for variations in mineralization between samples.
Mechanical bone strength testing
All testing was performed on a Bose Electroforce 3200 electromagnetic test instrument (Bose Corporation, Eden Prairie, Minnesota, USA). The midshaft strength of the right femur from 140-day-old offspring was tested using a three-point bend test. Bones were placed anterior surface down on two supports equidistant from the ends and 10 mm apart. Samples were centrally loaded at a constant rate (6 mm/min) up to fracture. Load-displacement curves were used to calculate maximum load, maximum deflection and stiffness. Stiffness was calculated as the slope of the linear portion of the load-displacement curve. CSMI was calculated as CSMI = (π/4) × (r 14−r 24), where r 1 is the mean radius of the midshaft and r 2 is the mean radius of the lumen at the same position.
Statistics
The effects of developmental vitamin D deficiency were determined by two-way analysis of variance (ANOVA) with diet and gender as factors, followed by the Tukey's post-hoc analysis test using SigmaStat 3.5 for Windows (Systat Software Inc., Hounslow, London, UK). Analysis of the degree of mineralization of the femoral head secondary ossification centre was performed using Mann–Whitney U-test for diet differences and two-way ANOVA using sex and diet as factors. For element analysis three-way ANOVA was used with age, diet and site as factors. Data are presented as mean ± s.d. unless otherwise shown; significance was determined with a P-level of 0.05 or lower.
Results
Bone structure analysis
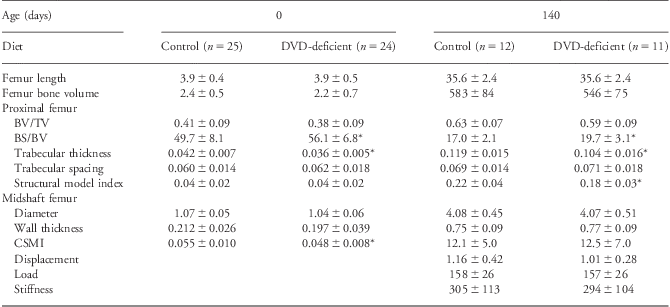
At birth differences were seen in the proximal femur in the dietary vitamin D (DVD)-deficient group, which showed higher BS/BV and reduced trabecular thickness (Table 1). In addition, the midshaft of the same animals showed a reduced CSMI (CSMI is a biomechanical indicator of the structural distribution of bone mass from the neutral bending axis). The same differences were seen in female DVD-deficient offspring, but not in males (data not shown). No significant differences were found for bone lengths or volumes for the bones studied in the DVD-deficient group compared with controls. In addition, no differences were found in BMD between the diet offspring (data not shown).
Table 1 Bone structural analysis at birth and 140 days of age

DVD, dietary vitamin D; BV/TV, bone volume to total volume ratio; BS/BV, bone surface to bone volume ratio; CSMI, cross-sectional moment of inertia.
Structural data shown are for bone length (mm), bone volume (mm3), BV/TV, BS/BV, trabecular thickness (mm), trabecular spacing (mm), structural model index, cortical thickness (mm), midshaft diameter (mm), CSMI (mm4), displacement (mm), load (N) and stiffness (N/mm).
*P < 0.05 compared with controls at same age.
All values shown are mean ± s.d.
At 140 days of age, the DVD-deficient group still showed increased BS/BV, and reduced trabecular thickness in the proximal femur (Table 1). Also, the SMI was significantly reduced in these animals indicating a more plate-like structure. These differences were seen in female DVD-deficient offspring, but not in males (data not shown). Again, there were no significant differences in bone length or volumes for the bones studied in the DVD-deficient group compared with controls. No differences were found in BMD between the diet offspring at 140 days of age (data not shown).
Bone mineralization
At 140 days, histological analysis of the femoral head showed that complete mineralization of the secondary ossification centre was observed in 9 of 11 DVD-deficient samples, whereas this was only seen in 4 of 12 controls (Fig. 1a). In order to determine if this observation extended to the entire region of the secondary ossification within the femoral head, this region was extracted in the imaging software and the level of mineralization (BV/TV) within the region was determined. No significant difference was found in the total volume of the secondary ossification centre (P-value 0.7, data not shown). However, the level of mineralization of the secondary ossification centre was found to be significantly raised in the DVD-deficient group (Fig. 1b). Figure 1c shows examples of low and high levels of mineralization of the femoral head secondary ossification centre. No significant differences were found when the effect of sex was determined; however, the mean level of mineralization in the ossification centre was higher in the DVD-deficient males and females (data not shown).

Fig. 1 Secondary ossification in 140-day-old offspring. (a) Representative images of femoral head sections stained with alcian blue and sirius red to visualize cartilage and bone, respectively. Developmentally vitamin D-deficient group sample suggesting absence of unmineralized bone in secondary ossification area and control group sample suggesting presence of unmineralized bone in secondary ossification area (arrows). (b) BV/TV between diet groups within the whole femoral secondary ossification centre as determined by computed tomography analysis. (c) 3D tomography images of examples of low and high levels of mineralization with the femoral head secondary ossification centre. The centres were extracted from the femoral head at the level of the growth plate and are viewed from below. Graph shows mean plus 95% confidence limits. DVD, dietary vitamin D; BV/TV, bone volume to total volume ratio.
Bone composition
At 140 days, Sr/Ca ratios were higher in DVD-deficient rats, in both the femoral head (P-value 0.009) and the midshaft (P-value 0.05) (Fig. 2a). No differences were found in Ba/Ca ratios in femoral head or midshaft between the diet groups (Fig. 2b).

Fig. 2 (a and b) Elemental analysis of bone composition in 140-day-old offspring. Graphs show strontium to calcium ratio (Sr/Ca; mg/g) and barium to calcium ratio (Ba/Ca; μg/g) in the femoral head and midshaft regions between the dietary vitamin D (DVD)-deficient group and controls. P-values between groups are shown, ns – not significant. Graphs show mean plus 95% confidence limits.
No differences were found in the calcium concentration between the DVD-deficient group and controls in the femoral head or the midshaft 140 days of age (Fig. 3).

Fig. 3 Bone calcium (Ca) content analysis in 140-day-old offspring. Graphs show Ca concentration in the femoral head and midshaft regions between the dietary vitamin D (DVD)-deficient group and controls. ns – not significant. Graph shows mean plus 95% confidence limits.
Discussion
We show here that vitamin D deficiency in the mother during pregnancy alters the bone structure and elemental composition in the offspring. In addition, there was evidence of mineralization of secondary ossification centres in these offspring, which was not observed in controls. The current studies indicate vitamin D depletion during in utero development in the offspring has a long-lasting effect, on structure and bone content, despite the repletion of vitamin D from birth, particularly in female offspring. It is not known why females appear more susceptible, however, female rat offspring show long-term alterations to bone structure when exposed to a maternal low-protein diet, whereas male offspring do not.Reference Lanham, Bertram, Cooper and Oreffo18 Our current theory is that this is due to different responses to in utero programming, whereby males try to maintain reproductive fitness by large body size at the expense of life expectancy, whereas female offspring try to maintain life expectancy for rearing more litters at the expense of body size. We are currently working to address this issue.
In previous studiesReference Eyles, Brown, kay-Sim, McGrath and Feron15, Reference Burne, O'Loan and Splatt16 we have shown the non-UV light and vitamin D deplete diet protocol used here produces dams and neonates that are severely depleted of both 25-hydroxy and 1,25-dihydroxy vitamin D. Maternal vitamin D depletion combined with normal dietary calcium and phosphate intake did not lead to reduced serum Ca2+ in neonates at birth, but did produce lower serum phosphate levels.Reference Burne, O'Loan and Splatt16 However, these were within the normal range, hence the offspring did not have hypophosphataemia'. In addition, prenatal vitamin D deficiency resulted in elevated levels of parathyroid hormone (PTH) in the dam and offspring. Gestational times were normal in the developmentally vitamin D (DVD)-deficient group.Reference O'Loan, Eyles and Kesby19 Vitamin D deficiency had no apparent effect on dam or pup general health and well-being. The DVD-deficient group was the same weight at delivery as control dams. Pups developed at the same rate as controls in respect to weight gain, signs of physical maturity, posture, gait and other behaviour. Similarly, litter size and pup mortality were also unaffected by vitamin D deficiency.
In the rat pup, vitamin D originates primarily from the dam in utero during the first postnatal week.Reference Clements and Fraser20 Vitamin D levels are low in rat milkReference Clements and Fraser20 and so the DVD-deficient group pups would be expected to have low vitamin D levels during gestation and at least the first postnatal week. Vitamin D levels increase in the dam's milk from the second week, thus DVD-deficient group pups may not be fully replete during the first 2 weeks of postnatal life.Reference Clements and Fraser20 One caveat, however, is that we cannot definitely conclude that the resolution of the bone differences observed is a consequence of vitamin D repletion as we did not have a study group that were maintained under vitamin D deplete conditions after birth. However, given the importance of vitamin D in postnatal bone development, we would expect this group to have severe bone deformities as seen in vitamin D-deficient ratsReference Miller, Halloran, DeLuca and Jee9 and vitamin D receptor-null mice.Reference Li, Amling and Pirro10 In addition, as these animals would have died from cardiovascular abnormalities before 140 days of age, we felt it unethical to include this group for this study of long-term bone alterations.
Vitamin D is not limited to calcium homeostasis, but appears to have an increasing number of important roles in other systems such as the immune system and cell growth.Reference Morris and Anderson21 Foetal cells expressing vitamin D receptors or genes with vitamin D response elements will also be affected by vitamin D deficiency, and this may be an explanation for the long-term differences seen in bone structure in the DVD-deficient offspring. However, it is important to remember that humans have more vitamin D dependant systems than rodents, for example, induction of the innate immune system is vitamin D dependant in humans, yet nitric oxide dependant in rats.Reference Gombart, Borregaard and Koeffler22, Reference Thoma-Uszynski, Stenger and Takeuchi23 Hence, maternal vitamin D deficiency in humans may have more profound long-term effects.
It is currently believed that vitamin D does not play a significant part in skeletal development until after birth. Indeed, in vitamin D receptor knockout mice the near-term foetal offspring appear normal in regard to skeletal morphology and mineral content.Reference Kovacs, Woodland, Fudge and Friel24 In a guinea pig model of DVD deficiency, Rummens et al.Reference Rummens, van Bree and Van Herck12 noted increased osteoid surface and thickness in the deplete near-term foetal offspring, but again, no subsequent studies were performed on older offspring to determine the permanency of these defects. However, in both studies it was stated that the results suggested vitamin D was not necessary for foetal growth and mineralization. It is possible that all the alterations seen in this study are simply due to low vitamin D levels during the first 2 weeks of postnatal life. Using a guinea pig model, Finch et al.Reference Finch, Rauch and Weiler25 found lower BMC and tibial bone strength at birth and 21 days of age in DVD-deficient offspring regardless of vitamin D supplementation at birth. On the basis of their data and those of others, the authors suggested that postnatal supplementation did not have a significant effect on bone mass or markers of bone modelling in the offspring, and that maternal vitamin D levels during pregnancy were most important.
Despite the DVD deficiency during gestation and potentially the first 2 weeks of postnatal life, the calcium composition of the femoral head and midshaft bone was normal at 140 days of age. However, by 140 days of age the DVD-deficient group appear to have specifically increased the deposition of strontium within the bones (ratios for barium, magnesium, manganese, copper and lead were unaltered, data not shown). Interestingly, the strontium to calcium ratio was the same in the midshaft and femoral head regions at 140 days of age regardless of diet group (also seen with magnesium, manganese, copper and lead, data not shown), whereas the barium to calcium ratio was significantly higher in the femoral head region at this age in both diet groups. This suggests strontium was incorporated into bone with calcium, whereas barium was preferentially incorporated into higher turn-over trabecular bone rather than cortical bone. Another possible explanation is that barium preferentially binds to the surface of bone, and hence it would have a higher concentration in trabecular bone. These differences were also seen when males and females were analyzed separately, although not to a statistically significant level.
Hypovitaminosis D is highly prevalent during pregnancy even in apparently otherwise healthy mothers.Reference Dawodu and Wagner26 A recent study in human by Morley et al.Reference Morley, Carlin, Pasco and Wark27 found lower long bone length in babies born to mothers with low vitamin D levels. Although this was partly explained by a shorter gestation, there was still evidence of altered bone growth. Mahon et al.Reference Mahon, Harvey and Crozier28 discovered femoral splaying, but not altered femoral length at 19 and 34 weeks gestation in babies of low vitamin D mothers. No evidence of altered bone longitudinal growth or splaying was found in the study presented here, however, the percentage differences seen by Morley and Mahon would not be detected with the sample number of rats used. Hence, altered bone growth differences cannot be ruled out in our study. There have been many science-based calls for supplementation,Reference Williams29 but few have been implemented. The evidence presented here shows that supplementation of the offspring from a deficient mother may still lead to skeletal problems in later life, and the importance of appropriate maternal vitamin D sufficiency before and during pregnancy, and while breastfeeding to ensure appropriate skeletal development throughout the lifecourse.
Acknowledgements
This work was supported by the following grant: Research into Ageing [grant number 253].