Introduction
Antisocial personality disorder (ASPD) is temporally contiguous with severe and persistent delinquent behaviors from childhood through adulthood. Estimates suggest prevalence rates being approximatively 5% at a population level but up to 50% in prison settings (Black, Gunter, Loveless, Allen, & Sieleni, Reference Black, Gunter, Loveless, Allen and Sieleni2010; Fazel & Danesh, Reference Fazel and Danesh2002; Pondé, Freire, & Mendonça, Reference Pondé, Freire and Mendonça2011). Thus, it is not surprising to observe that early conduct disorder (CD) is an inherent criterion for adult ASPD and is an important risk factor for criminal conviction, incarceration, and a wide range of health and psychosocial problems (Bevilacqua, Hale, Barker, & Viner, Reference Bevilacqua, Hale, Barker and Viner2018; Colman et al., Reference Colman, Murray, Abbott, Maughan, Kuh, Croudace and Jones2009; Erskine et al., Reference Erskine, Norman, Ferrari, Chan, Copeland, Whiteford and Scott2016; Moffitt, Reference Moffitt, Lahey, Moffitt and Caspi2003, Reference Moffitt, Cicchetti and Cohen2006). Although social science research has substantially increased our understanding of risk factors and outcomes regarding antisocial behaviors, the pathophysiological mechanisms underlying the antisocial spectrum have yet to be elucidated.
In the last decades, researchers have attempted to unveil the neurobiological correlates underlying the antisocial spectrum [i.e. conduct problems-to-antisocial personality disorder spectrum (CP/ASPD)] by using a variety of magnetic resonance imaging (MRI) methods. For instance, studies have mainly focused on structural deficits in CP/ASPD individuals (Aoki, Inokuchi, Nakao, & Yamasue, Reference Aoki, Inokuchi, Nakao and Yamasue2013; Rogers & De Brito, Reference Rogers and De Brito2016) and task-based functional abnormalities (Deming & Koenigs, Reference Deming and Koenigs2020; Dugré et al., Reference Dugré, Radua, Carignan-Allard, Dumais, Rubia and Potvin2020; Poeppl et al., Reference Poeppl, Donges, Mokros, Rupprecht, Fox, Laird and Eickhoff2019). More precisely, results from two voxel-based morphometry meta-analyses (most studies controlling for total intracranial/grey matter volume) revealed that CP/ASPD subjects showed grey matter volume abnormalities in the anterior insula, the amygdala, the ventrolateral (vlPFC) and dorsomedial prefrontal cortex (dmPFC), and the fusiform gyrus (Aoki et al., Reference Aoki, Inokuchi, Nakao and Yamasue2013; Rogers & De Brito, Reference Rogers and De Brito2016). Likewise, two recent meta-analyses of task-based functional MRI (fMRI) studies indicated that across tasks, psychopathic individuals exhibit significant alterations in the dmPFC, lateral PFC and amygdala (Deming & Koenigs, Reference Deming and Koenigs2020; Poeppl et al., Reference Poeppl, Donges, Mokros, Rupprecht, Fox, Laird and Eickhoff2019) ACC, PCC-precuneus (Deming & Koenigs, Reference Deming and Koenigs2020), and fronto-insular cortex (Poeppl et al., Reference Poeppl, Donges, Mokros, Rupprecht, Fox, Laird and Eickhoff2019). Furthermore, Dugré et al. (Reference Dugré, Radua, Carignan-Allard, Dumais, Rubia and Potvin2020) have recently observed, through a large meta-analysis of task-based fMRI studies, that the antisocial spectrum (i.e. CP/ASPD) is mainly characterized by brain alterations during acute threat response tasks, but also across social cognition and cognitive control neurocognitive domains. More precisely, during social cognition tasks (e.g. theory of mind, empathic decision making), CP/ASPD individuals exhibited aberrant activation in the putamen, precuneus, medial and dorsolateral PFC, fusiform gyrus, midcingulate cortex (MCC), and the hippocampus. Moreover, in response to threatening stimuli (e.g. negative emotional faces), the authors observed decreased activation in CP/ASPD in the pregenual anterior cingulate cortex, lateral PFC, anterior insula, and inferior parietal lobule, whereas decreased activations in the premotor, anterior insula, and vlPFC were mainly observed during cognitive control tasks (e.g. Stroop, Go-No/Go) (Dugré et al., Reference Dugré, Radua, Carignan-Allard, Dumais, Rubia and Potvin2020). Despite that evidence suggests task-based and structural abnormalities, the disrupted functional connectivity patterns that may characterize CP/ASPD subjects remain largely unknown.
Recently, resting-state fMRI (rs-fMRI) has gained considerable attention in research on the neurobiological correlates of CP/ASPD. Rs-fMRI modality permits us to examine brain activity at rest without an explicit fMRI task, but also to characterize brain regions that communicate with each other (i.e. functional connectivity) during low-frequency spontaneous brain fluctuations. To examine resting-state connectivity, several methods have been developed spanning the network-level, seed-based, and non-seed-based approaches. The former method reduces brain features to a macroscopic organization of voxels showing strong temporal coherence. These usually include the medial fronto-parietal (e.g. default-mode network), occipital (e.g. medial and lateral visual), pericentral network (e.g. sensorimotor, somatomotor), dorsal fronto-parietal (e.g. dorsal attention), lateral fronto-parietal (e.g. cognitive control), midcingulo-insular (e.g. salience, ventral attention, cingulo-opercular) (Gordon et al., Reference Gordon, Laumann, Adeyemo, Huckins, Kelley and Petersen2016; Schaefer et al., Reference Schaefer, Kong, Gordon, Laumann, Zuo, Holmes and Yeo2018; Uddin, Yeo, & Spreng, Reference Uddin, Yeo and Spreng2019; Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Polimeni2011). Recently, several studies have adopted a large-scale network meta-analytical approach (i.e. inferring large-scale networks from studies’ peak coordinates) (Dong, Wang, Chang, Luo, & Yao, Reference Dong, Wang, Chang, Luo and Yao2018; Li et al., Reference Li, Hu, Zhang, Tao, Dai, Gong and Lui2019; Sutcubasi et al., Reference Sutcubasi, Metin, Kurban, Metin, Beser and Sonuga-Barke2020; Xu et al., Reference Xu, Van Dam, Feng, Luo, Ai, Gu and Xu2019). However, this method inherently reduces the spatial preciseness when investigating the deficits of a particular disorder. Thus, other techniques such as seed-based approaches have the advantage of the straightforward interpretability of results, but the limitations of being influenced by spatial confounds and noises (Cole, Smith, & Beckmann, Reference Cole, Smith and Beckmann2010). Therefore, examining the spatial convergence across studies (Cortese, Aoki, Itahashi, Castellanos, & Eickhoff, Reference Cortese, Aoki, Itahashi, Castellanos and Eickhoff2020) is one meta-analytical approach that addresses such limitations.
Concerning individuals from the antisocial spectrum, early evidence by Motzkin, Newman, Kiehl, and Koenigs (Reference Motzkin, Newman, Kiehl and Koenigs2011) showed that psychopathic criminals exhibited a reduced amygdala-vmPFC connectivity during resting-state. Although this may be in line with affective deficits observed in psychopathy, the two groups (psychopathic and non-psychopathic offenders) substantially differed on both Factor 1 (i.e. affective/interpersonal factor) and Factor 2 (lifestyle/antisocial behaviors) of the psychopathy checklist [PCL-R (Hare, Reference Hare2003)]. Thus it remains unclear whether this dysconnectivity was driven by the severity of antisocial behaviors. This reduced connectivity was also observed in pedophilia with child sexual offending (Kärgel et al., Reference Kärgel, Massau, Weiß, Walter, Kruger and Schiffer2015), whereas increased connectivity characterized violent offenders and positively correlated with the severity of reactive and proactive aggression (Siep et al., Reference Siep, Tonnaer, van de Ven, Arntz, Raine and Cima2019). Furthermore, task-based connectivity studies also reported a reduced amygdala-vmPFC connectivity when using fearful facial expressions (Marsh et al., Reference Marsh, Finger, Mitchell, Reid, Sims, Kosson and Blair2008) and moral judgments (Marsh et al., Reference Marsh, Finger, Fowler, Jurkowitz, Schechter, Henry and Blair2011; Yoder, Harenski, Kiehl, & Decety, Reference Yoder, Harenski, Kiehl and Decety2015) stimuli in CP/ASPD. Additionally, using non-seed-based approaches [e.g. amplitude of low-frequency fluctuations (ALFF), regional homogeneity (ReHo)], decreased connectivity in the precuneus was associated with both early-onset and late-onset CD (Cao et al., Reference Cao, Li, Zhang, Dong, Sun, Yao and Liu2019) and adolescent violent offenders (Chen et al., Reference Chen, Zhou, Liu, Witt, Zhang, Jing and Li2015), whereas school bullies with severe delinquent behaviors exhibit a significant increased connectivity in this (Kim et al., Reference Kim, Kang, Lee, Cha, Park, Kweon and Kim2018). Given the current state of literature which suggests that subjects with antisocial behaviors may be associated with socio-affective deficits, results on rs-fMRI vary significantly, particularly in the use of seed v. non-seed-based approaches as well as in the selection of seed regions. Therefore, brain regions and underlying the dysconnected networks remain to be determined. Meta-analytical findings are thus crucial for a better understanding of the connectivity patterns characterizing this population.
The current study aims to investigate functional connectivity alterations in CP/ASPD through a spatial convergence meta-analysis of rs-fMRI studies. More specifically, we meta-analyzed studies employing a seed-based connectivity (SBC) approach, studies using non-SBC method and studies that reported an association between functional connectivity and dimensional constructs related to the antisocial spectrum. According to previous structural and task-based MRI meta-analyses, we hypothesized that CP/ASPD subjects would show disrupted functional connectivity in brain regions such as the amygdala, anterior insula, medial PFC, and the PCC/precuneus.
Method
Selection procedures
Search strategies
A systematic search strategy, using three search engines (Google Scholar, PubMed, and EMBASE), was performed independently by two researchers (JRD and SP) up to October 2020 to identify relevant studies. The following search terms were used: (‘conduct problems’ or ‘conduct disorder’ or ‘disruptive behaviors’ or ‘antisocial personality disorder’ or ‘antisocial behaviors’ or ‘psychopathy’ or ‘sociopathy’ or ‘aggression’ or ‘delinquency’ or ‘inmates’) AND (‘functional connectivity’ or ‘resting state’) AND (‘neuroimaging’). Additional search was executed by cross-referencing the reference lists of the included articles.
Selection criteria
Flow-chart and reasons for study exclusion can be retrieved in online Supplementary Fig. S1. Articles were included if they met the following criteria: (1) original paper from a peer-reviewed journal, (2) inclusion of individuals with antisocial behaviors without a comorbid major mental illness or organic impairment, (3) use of rs-fMRI method, (4) employed a case–control SBC, a case–control non-SBC or a dimensional analysis (case–control or one-sample) between rsFC results and constructs closely related to this population: impulsivity/hyperactivity dimension (e.g. ADHD symptoms), affective/interpersonal factor (e.g. F1 score, callousness), and/or lifestyle/antisocial behaviors (e.g. CP or ASPD symptoms, F2 score). Furthermore, when papers did not report peak coordinates for seed or targets, authors were contacted. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche, Ioannidis and Moher2009) and the 10 rules for neuroimaging meta-analysis (Müller et al., Reference Müller, Cieslik, Laird, Fox, Radua, Mataix-Cols and Turkeltaub2018) were followed across the meta-analysis steps.
Activation Likelihood Estimate method
We used the Activation Likelihood Estimate (ALE) approach for coordinate-based meta-analysis to extract significant spatially convergent peaks that differ between our population of interest and controls (GingerALE version 3.0.2, http://www.brainmap.org/ale/). Experiments reporting Talairach coordinates were further converted into MNI (Montreal Neurologic Institute) space before using them in analyses. Briefly, for each experiment, a modeled activation (MA) map was created by modeling coordinate foci with a spherical Gaussian probability distribution, weighted by the number of subjects in each experiment. This is performed in order to account for spatial uncertainty due to template and between-subject variance (Eickhoff et al., Reference Eickhoff, Laird, Grefkes, Wang, Zilles and Fox2009), and ensure that multiple coordinates from a single experiment do not jointly influence the MA value of a single voxel. Voxel-wise ALE scores were then computed as the union of MA maps, which provide a quantitative assessment of convergence between brain activation across experiments. The size of the supra-threshold clusters was compared against a null distribution of cluster sizes derived from artificially created datasets in which foci were shuffled across experiments, but the other properties of the original experiment (e.g. number of foci, uncertainty) were kept. We used the following statistical threshold: p < 0.001 at voxel-level and FWE-p < 0.05 at a cluster-level with 5000 permutations.
Seed-based connectivity
In this meta-analysis, we focused on spatial convergence rather than a large-scale network approach. Indeed, we used the ALE method to examine voxels that show spatial convergence across studies, irrespective of the connections between voxels, as used recently (Cortese et al., Reference Cortese, Aoki, Itahashi, Castellanos and Eickhoff2020). Since the main results are presented as peaks that show spatial convergence across connectivity studies (and not dysconnectivity between two brain regions), further analyses were carried out which specifically aimed to examine the connections between a seed-of-interest (SOI) and voxels regions (target). SOIs were defined as the results of the main seed-based meta-analysis (peaks where there was significant spatial convergence across studies). Next, we assumed that functional connectivity analyses should be reciprocal (i.e. seed A is connected to target B to the same extent that B should also be connected to A). Concretely, we extracted connectivity experiments from studies that have reported at least one coordinate within the SOI. Independent connectivity experiments were defined as the following: In each connectivity result for every original study, (A) if the peak coordinate falling within the SOI was a seed in the original study, every target was included as connecting together in an experiment; (B) if the peak coordinate was a target, only the target with its respective seed were entered in the experiment. This method addressed the between-study variability regarding seed selection since the functional connectivity between seeds and targets should be reciprocal. For each SOI, significant results therefore suggest that clusters are coactivating (i.e. functionally connected) with their respective SOI across studies. For each SOI, an SBC coactivation meta-analysis was executed on independent connectivity experiments that meet the inclusion criteria. Meta-analyses were done separately for increased (CP/ASPD > HC) and decreased (HC > CP/ASPD) connectivity since ALE method does not inherently use effect sizes.
Non-seed-based connectivity
We also meta-analyzed non-SBC studies. These included every other rsFC method (than SBC) such as ReHo, fALFF and ALFF, short/long-range functional connectivity density, and voxel-mirrored homotopic connectivity. Despite that ReHo and ALFF are both voxel-wise data-driven methods to examine rsFC in local brain regions, ReHo measures the synchronization between the time series of a given voxel and its neighbors (Zang, Jiang, Lu, He, & Tian, Reference Zang, Jiang, Lu, He and Tian2004), whereas the ALFF estimates the amplitude of fluctuations of individual voxels (Yu-Feng et al., Reference Yu-Feng, Yong, Chao-Zhe, Qing-Jiu, Man-Qiu, Meng and Yu-Feng2007). Also, studies using large-scale network analyses (e.g. independent component analysis) were excluded. This was done to avoid contaminating our results with studies using a different approach (peak convergence v. large-scale network) than the one used in this meta-analysis. However, these studies were added only in cases where studies reported voxel-wise group effects within specific networks (e.g. within-DMN), since these studies report significant peak coordinates rather than large-scale network statistics.
Subanalyses
Several sensitivity analyses were performed to assess potential moderators of our results. First, since SBC results may have been driven by preference in seed selection (e.g. the amygdala), we ran the meta-analysis by removing every seed from seed-to-voxel studies (i.e. correlation between the time-series of a seed with the time-series of every voxel across the brain) and kept only the target cluster coordinates. Similarly, to address the potential effect of studies investigating only a small number of connections, we have performed a second subanalysis by setting a minimum threshold of five seeds in seed-to-voxels studies and 10 seeds in studies using seed-to-ROI (i.e. correlation between time-series of two ROIs) (i.e. resulting in a minimum of 45 connections pairs). In meta-analyses with less than 10 experiments, we carried out jack-knife analyses (i.e. recomputing ALE while leaving one experiment out of the dataset), to examine whether the results may be driven by a single experiment. However, it should be noted that this method is very conservative in small datasets and is unnecessary when meta-analyzing a considerable number of experiments since it is unlikely that results may have been driven by a single experiment (Eickhoff et al., Reference Eickhoff, Nichols, Laird, Hoffstaedter, Amunts, Fox and Eickhoff2016). Also, given the body of research showing that callous-unemotional traits and age-of-onset may be sources of clinical heterogeneity, further subanalyses were carried out (online Supplementary Material).
Dimensional associations with psychopathologies
Meta-analyses were also performed to investigate relationships between connectivity results and psychopathological dimensions associated with CP/ASPD subjects, namely the Hyperactivity/Impulsivity, Affective/Interpersonal of psychopathic traits and Antisocial Behaviors/Lifestyle dimensions. These meta-analyses were performed across SBC and non-SBC studies, for positive and negative rsFC-dimensions relationships, separately.
Exploratory analyses
To better interpret results, meta-analytical connectivity modeling (MACM) and functional characterization analyses were realized. First, we assessed which paradigm and task domains significantly characterize our results, using the BrainMap Database. More precisely, we examined large-scale and brain-wide co-activation profile for each brain region disrupted (ROI) in CP/ASPD subjects. This was done to investigate whether our results mutually co-activate across tasks (BrainMap Database). Thus, we first examined how strongly the ROI-based MACM maps correlated with each other as an indication being part of similar network. Following this, these maps were correlated with well-defined seven canonical networks for interpretability purpose concerning large-scale networks (Schaefer et al., Reference Schaefer, Kong, Gordon, Laumann, Zuo, Holmes and Yeo2018).
Results
Case–control meta-analyses
Seed-based connectivity
A total of 19 samples derived from 18 studies (see online Supplementary Table S1) were included for the SBC meta-analysis. A total of 513 cases was compared to 488 control subjects (mean age = 28.34, seven samples reporting that all participants met the diagnostic criteria for CD/ASPD).
Only seven studies have reported increased connectivity results in CP/ASPD subjects (42 foci including seeds). We observed significant spatial convergence in the anterior MCC [extending to the pre-supplementary motor area (pre-SMA)] and the left amygdala. Jackknife analyses replicated results in five out of seven iterations, indicating that the spatial convergence between both regions might have been driven by two studies. Additionally, removing seed coordinates from studies yielded no significant results, suggesting that results may have been driven by seed selection. Finally, no additional analyses were performed since they comprised too few experiments to achieve acceptable statistical power (⩽8 experiments).
Meta-analysis on 15 experiments reporting decreased connectivity was performed (13 studies, 110 foci including seeds), revealing spatial convergence in six clusters: left ventral PCC/precuneus (vPCC/PCUN), left vmPFC, left premotor cortex overlapping on the frontal eye field, left amygdala, left and right dmPFC (Table 1). The left vPCC/PCUN, left amygdala, and left premotor cortex remained statistically significant after excluding children's samples from the main analysis (k = 11, 99 foci). When adopting a more restricting inclusion approach (i.e. minimum of five seed-to-voxel maps, 10 seed-to-ROI) (k = 8, 64 foci), only the vPCC/PCUN and the right dmPFC were replicated, suggesting that these clusters were not driven by focused studies. Finally, removing seed coordinates from seed-to-voxel experiments (k = 15, 96 foci) revealed that the left premotor cortex and both left and right dmPFC were not driven by seed selection.
After having identified the convergent peaks across experiments (SOI), we were interested in examining their connectivity targets. Indeed, we meta-analyzed experiments reporting at least one peak coordinate located within the SOI, to extract brain regions that significantly coactivate with the SOI. For the left vPCC/PCUN, 11 independent connectivity experiments were included, comprising 32 foci. However, no significant dysconnectivity target was found, even when removing adolescent samples (nine experiments, 28 foci). When examining nodes that were dysconnected with bilateral dmPFC cluster (eight experiments, 19 foci), we observed significantly decreased connectivity with the left superior parietal lobule (x = −23, y = −60, z = 59, ALE = 0.026, 136 voxels) (see Fig. 1c). Jackknife analyses replicated results in six out of eight iterations, suggesting that the reduced dmPFC-SPL connectivity might have been driven by two studies out of eight. No additional subanalysis was performed on the dmPFC due to an insufficient number of experiments. Since the other SOIs (i.e. left vmPFC, left premotor cortex, left amygdala, and bilateral dmPFC) comprised too few experiments to achieve acceptable statistical power (<8 experiments), we did not perform SBC meta-analyses (Table 1) .

Fig. 1. Seed-based connectivity results: (a) convergent peaks across increased SBC studies: left mid-cingulate cortex and left amygdala; (b) convergent peaks across decreased SBC studies: left amygdala, left-right dorsomedial prefrontal cortex, left posterior cingulate cortex/precuneus, left vmPFC and left premotor cortex. (c) Significant reduced connectivity (blue edge) between bilateral dorsomedial prefrontal cortex (yellow: seed) and superior parietal lobule (red: target). CP/ASPD, conduct problems-to-antisocial personality disorder spectrum; HC, healthy controls.
Table 1. Main results of the meta-analyses

CP/ASPD, conduct problems to antisocial personality disorder spectrum; HC, healthy controls; aMCC, anterior mid-cingulate cortex; pre-SMA, pre-supplementary motor area; vPCC, ventral posterior cingulate cortex; PCUN, precuneus; vmPFC, ventromedial prefrontal cortex; PMC, premotor cortex; dmPFC, dorsomedial prefrontal cortex; CAL, calcarine cortex; OP4, operculum parietal (area 4); CUN, cuneus.
Non-seed-based connectivity
Twenty studies were included in the non-SBC meta-analyses (see online Supplementary Table S2). However, one study was removed since the authors failed to provide us their peak coordinates. A total of 453 cases were compared to 460 control subjects (mean age = 19.61, 15 samples reporting that all participants met the diagnostic criteria for CD/ASPD).
Non-SBC meta-analyses were performed for both increased (CP/ASPD > HC: 14 experiments, 42 foci) and decreased rsFC contrasts (HC > CP/ASPD: 18 experiments, 88 foci) (Table 1) . ALE meta-analyses revealed significant peak convergence in the right vPCC/PCUN (in studies reporting increased connectivity) as well as in the parietal operculum encompassing the posterior insula, in the right intracalcarine and the left cuneus (in studies reporting decreased connectivity). When removing experiments using adult samples, meta-analysis on increased rsFC studies replicated the vPCC/PCUN (k = 10, 29 foci), while meta-analysis on decreased rsFC studies (k = 14, 71 foci) replicated results in the intracalcarine and cuneus (Fig. 2).

Fig. 2. Significant peak convergence across: (a) non-seed-based connectivity studies reporting increased connectivity (i.e. ventral posterior cingulate cortex/precuneus), (b) non-seed-based connectivity studies reporting decreased connectivity (i.e. parietal operculum, cuneus, intracalcarine), (c) negative relationship between resting-state connectivity of the ventromedial prefrontal cortex and the severity of lifestyle/antisocial behaviors. CP/ASPD, conduct problems-to-antisocial personality disorder spectrum; HC, healthy controls.
Dimensional meta-analyses
A total of 32 studies were included in dimensional meta-analyses (n = 3787, mean age = 22.87), of which 10 included a measure related to Hyperactivity/Impulsivity dimension (n = 263, mean age = 19.15, four SBC studies), 15 to Affective/Interpersonal psychopathic traits (n = 2863, mean age = 23.77, nine SBC studies) and 20 to Lifestyle/Antisocial behaviors dimension (n = 3462, mean age = 25.25, 14 SBC studies) (see online Supplementary Table S3).
Concerning the Lifestyle/Antisocial dimension (k = 20), 11 studies found positive rsFC associations (62 foci), 10 observed negative rsFC correlations (45 foci) and four yield no significant result. ALE meta-analyses revealed that the Lifestyle/Antisocial dimension was not positively associated with any rsFC but showed a significant peak convergence in the right vmPFC across studies (negative relationship). This result was replicated when removing seed coordinates from SBC experiments, indicating that the result was not driven by seed selection. No additional subanalyses were performed due to the insufficient number of experiments (<8 experiments).
No analyses were performed on the Affective/Interpersonal component of psychopathic traits, nor on Hyperactivity/Impulsivity dimension since they comprised too few experiments for positive (k = 6, k = 5, respectively) and negative (k = 6 and k = 3, respectively) relationships.
Additional analyses
Of the 12 studies that have reported the severity of callous-unemotional traits, only six showed severe levels (see online Supplementary Material). Thus, we did not perform any meta-analysis. Also, 21 experiments (485 cases, 186 foci) reported a sample comprising at least 50% of early-onset disordered subjects (average 93%, s.d. = 16.8%), whereas nine experiments (296 subjects, 55 foci) reported other samples (adolescent-onset disorder or <50% of subjects with early-onset disorder). Meta-analysis on early-onset disorder replicated results found in the vPCC, the right parietal operculum/posterior insula, and right dmPFC. A more detailed description of the results can be found in online Supplementary Material.
Exploratory analyses
To better interpret our results, we extracted significant region-of-interest and performed functional characterization and MACM analyses. Pairwise spearman ρ was carried out between the MACM map for each ROI. Then overlaps between MACM maps of our ROIs were examined using a dendrogram plot (Fig. 3). Further analyses were made to examine MACM correspondence with canonical networks. By investigating brain-wide coactivation map of each ROI, we observed shared similarities in MACM maps between the PCC, vmPFC, dmPFC, and amygdala, which were mainly associated with the DMN. Furthermore, we showed that the MACM maps of the aMCC/pre-SMA, SPL, PMC, and CUN were closely related altogether, spanning both the ventral and dorsal attention networks. Summary of the MACM analyses is displayed in Fig. 3. See online Supplementary Fig. 2A–H and Table S2 for detailed information.

Fig. 3. Hierarchical representation of spatial correlations between ROI-based MACM maps and their relationship with Schaefer's seven intrinsic functional networks. Dendrogram revealed that the ROI-based MACM maps were distributed in three groups: Group 1 (DMN: PCC, vmPFC, dmPFC, AMY), Group 2 (Sommot: OP4), and Group 3 (VentAttn, DorsAttn, and Visual: MCC, PMC, SPL, CUN, CAL). The CAL and CUN are shown in a different radar chart (than the MCC, PMC, and SPL) for display purpose. PCC, posterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; AMY, amygdala; OP4, operculum parietal (area 4); CAL, calcarine cortex; CUN, cuneus; MCC, mid-cingulate cortex; PMC, premotor cortex; SPL, superior parietal lobule. VentAttn, ventral attention; Sommot, somato-motor; DMN, default mode network; DorsAttn, dorsal attention.
Discussion
In this systematic review and meta-analysis of rsFC studies, we aimed to investigate SBC and non-SBC deficits in individuals with CP/ASPD, as well as connectivity correlates of psychopathological dimensions characterizing CP/ASPD. To our knowledge, this is the first meta-analysis to investigate resting-state functional connectivity patterns in this population. In the SBC meta-analysis, comprising 513 cases (18 studies), spatial convergence was observed in amygdala, the MCC, the vPCC/precuneus, the vmPFC and dmPFC, and the premotor cortex. Further analyses were carried out to examine dysconnectivity between two nodes. We observed that CP/ASPD subjects showed significant reduced dmPFC-SPL connectivity. Additionally, the meta-analysis on 20 non-SBC studies revealed aberrant peaks in the vPCC/precuneus (increased connectivity), the parietal operculum, intracalcarine, and the cuneus (decreased connectivity) in cases compared to controls. Finally, in studies investigating the association between the severity of lifestyle/antisocial behavior dimension and connectivity patterns (3462 subjects), significant peak convergence across studies was observed in the vmPFC (negative correlations).
Here, we used reverse-inference method to examine the relationship between the dysconnected nodes found in the current meta-analysis and large-scale canonical networks. More precisely, the co-activation profiles of our findings, provided by the MACM analyses, suggested that our results were mainly characterized by networks spanning the DMN and ventral/dorsal attentional networks (see Figs 3 and 4). Interestingly, this concurs with the results of recent studies showing deficits in large-scale networks (not included in this meta-analysis). Indeed, data from the ABCD study (n = 9636) indicated that CU traits were negatively associated with within-network connectivity in the DMN, whereas the severity of aggressive and delinquent behaviors was rather associated with connectivity within the Dorsal Attention Network (Umbach & Tottenham, Reference Umbach and Tottenham2020). Likewise, results from a recent study revealed negative relationships between physical aggression and within-network connectivity in the DMN and the Dorsal Attention Network (Weathersby, King, Fox, Loret, & Anderson, Reference Weathersby, King, Fox, Loret and Anderson2019). This concurs with our exploratory analyses (ROI-based MACM) indicating that the clusters observed in the main meta-analysis showed prominent associations with the DMN and attentional networks. Furthermore, we found reduced dmPFC-SPL connectivity suggesting that CP/ASPD may display disrupted connectivity between these networks. Indeed, reduced anti-correlation between the DMN and the Fronto-Parietal Network (which spanned both dorsal and ventral attentional networks) has been observed in CD and was significantly associated with the severity of CU traits (Pu et al., Reference Pu, Luo, Jiang, Gao, Ming and Yao2017). Past results also demonstrated that physical aggression was negatively associated with a DMN-Ventral Attention anticorrelation (Weathersby et al., Reference Weathersby, King, Fox, Loret and Anderson2019). Finally, altered connectivity between DMN and brain regions involved in dorsal attention network (i.e. SPL) was also identified in pedophilic offenders (Cantor et al., Reference Cantor, Lafaille, Hannah, Kucyi, Soh, Girard and Mikulis2016) and correlated with the severity of psychopathic traits (Dotterer et al., Reference Dotterer, Hyde, Shaw, Rodgers, Forbes and Beltz2020). Hence, through a complementary perspective to our meta-analysis, these studies also highlighted the crucial role of brain regions involved in DMN and attentional canonical networks in the connectivity deficits characterizing CP/ASPD subjects (Hamilton, Hiatt Racer, & Newman, Reference Hamilton, Hiatt Racer and Newman2015).
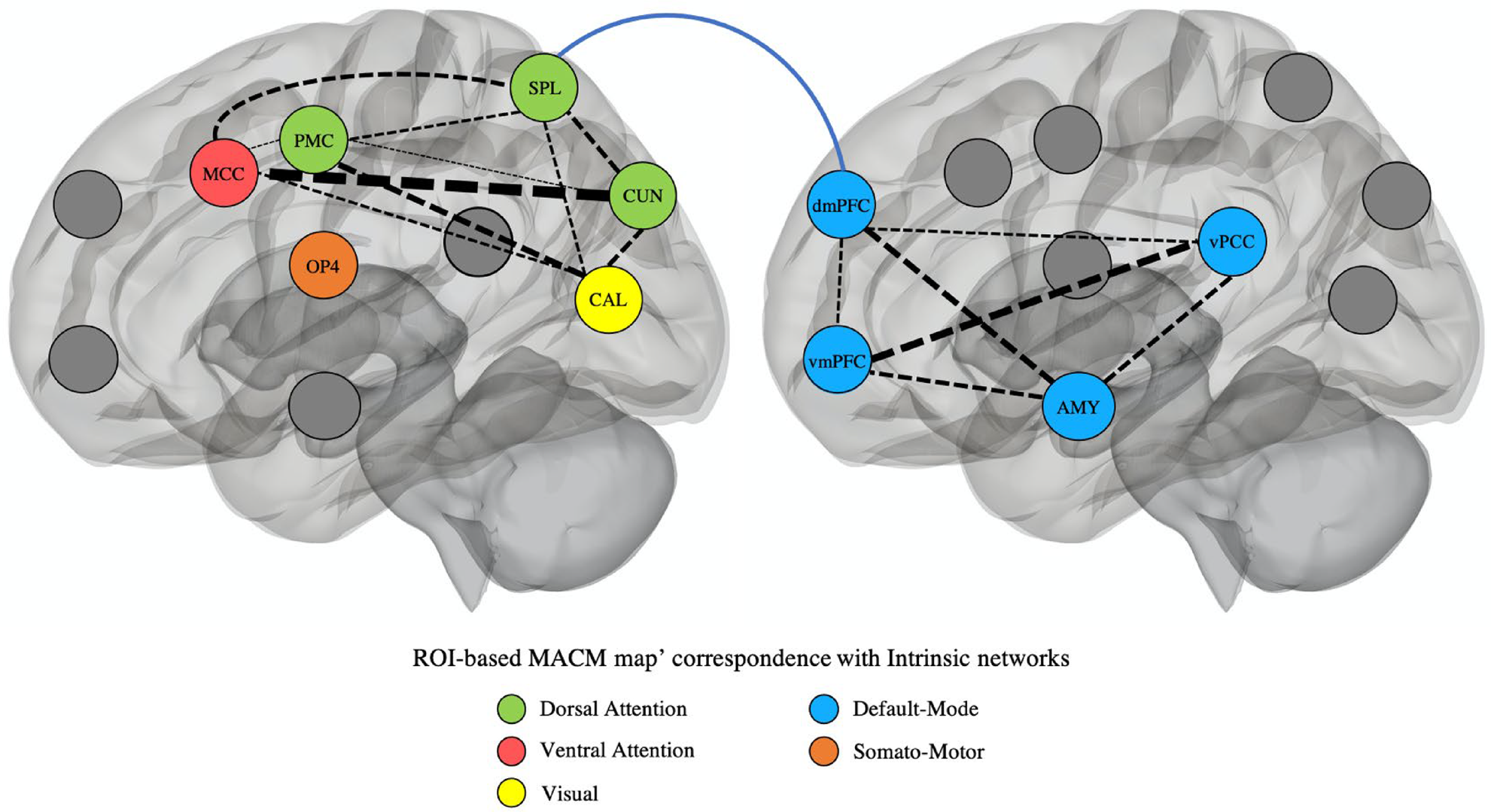
Fig. 4. Summary of the results identified in the current meta-analysis in CP/ASPD subjects. 3D Visualization can be found here: https://juldugre.github.io/3D_Neuroscience/Connect.html. Dashed arrows represent correlation between ROI-based MACM maps [weighted by correlation coefficient: thickness (pt) from 1 pt (r = 0.10–0.19) to 7 pt (r = 0.70–0.79)]. Higher values reflect closer spatial correlation. Solid arrow represents the reduced dmPFC-SPL connectivity observed in the meta-analysis on seed-based connectivity studies. Colors in nodes represent the intrinsic functional networks that showed the highest correlation coefficient with each ROI-based MACM map. Intrinsic networks were defined by Schaefer et al. (Reference Schaefer, Kong, Gordon, Laumann, Zuo, Holmes and Yeo2018)'s seven networks. AMY, amygdala; MCC, middle cingulate cortex; vmPFC, ventromedial prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; vPCC, posterior cingulate cortex that includes also the precuneus; PMC, premotor cortex; OP4, operculum parietal (area 4); SPL, superior parietal lobule; CUN, cuneus; CAL, calcarine cortex; ROI, region-of-interest; MACM, meta-analytical connectivity modeling.
In addition, we found spatial convergence in the amygdala across both increased and decreased connectivity experiments, indicating that this particular region may be a crucial node in subjects with CP/ASPD. Despite that the amygdala is not directly involved in canonical networks, some evidence suggests its association with brain regions subserving the DMN such as the vmPFC, PCC, dmPFC, and the temporo-parietal junction during resting-state (Amft et al., Reference Amft, Bzdok, Laird, Fox, Schilbach and Eickhoff2015; Sylvester et al., Reference Sylvester, Yu, Srivastava, Marek, Zheng, Alexopoulos and Dierker2020). These observations concur with our MACM exploratory analyses indicating overlaps in co-activating network (DMN) across the dmPFC, vmPFC, vPCC, and the amygdala. Moreover, the amygdala and DMN regions are frequently co-activated in fMRI tasks eliciting empathy and morality processes (Bzdok et al., Reference Bzdok, Schilbach, Vogeley, Schneider, Laird, Langner and Eickhoff2012). Interestingly, in such tasks, subjects with CP/ASPD exhibit numerous dysconnectivities between the amygdala and DMN regions. Indeed, when viewing facial expressions of negative emotions (e.g. fear), Marsh et al. (Reference Marsh, Finger, Mitchell, Reid, Sims, Kosson and Blair2008) observed that youths with disruptive behaviors and high psychopathic traits exhibited reduced connectivity between the amygdala and the vmPFC and PCC. Likewise, reduced connectivity between the amygdala and regions subserving the DMN were also noticed in moral judgments tasks (Marsh et al., Reference Marsh, Finger, Fowler, Jurkowitz, Schechter, Henry and Blair2011: vmPFC; Yoder et al., Reference Yoder, Harenski, Kiehl and Decety2015: vmPFC, dmPFC, PCC) as well as in passive viewing of painful situations caused by others (dmPFC: Decety et al., Reference Decety, Michalska, Akitsuki and Lahey2009). Hence, these results highlight coupling between the amygdala and DMN regions as a crucial socio-affective network in the understanding of connectivity functioning in CP/ASPD subjects.
In our meta-analysis, we also found spatial convergence in the aMCC/pre-SMA, SPL, premotor (frontal eye field), and the cuneus. These brain regions were further characterized as belonging to attention canonical networks (ventral/dorsal) in our MACM analyses. Indeed, the aMCC/pre-SMA mostly corresponded to the ventral network, whereas the SPL and premotor cortex were associated with the dorsal network. Their relationships are unsurprising given that the aMCC/pre-SMA, SPL, and the premotor cortex (frontal eye field) are frequently co-activated in tasks requiring attentional processes [e.g. meta-analytical finding: shifting: (Wager, Jonides, & Reading, Reference Wager, Jonides and Reading2004); sustained: (Langner & Eickhoff, Reference Langner and Eickhoff2013); oddball: (Kim, Reference Kim2014); working memory: (Rottschy et al., Reference Rottschy, Langner, Dogan, Reetz, Laird, Schulz and Eickhoff2012)]. More precisely, evidence suggests that ventral network regions (e.g. aMCC/pre-SMA) may correspond to a stimulus-driven attentional system, whereas regions subserving the dorsal attentional network (e.g. SPL, premotor cortex) may play a crucial role in top-down or goal-driven attentional control (Corbetta, Patel, & Shulman, Reference Corbetta, Patel and Shulman2008; Kim, Reference Kim2014; Vossel, Geng, & Fink, Reference Vossel, Geng and Fink2014). Despite the overutilization of the amygdala as a seed of interest in CP/ASPD subjects, task-based connectivity studies nonetheless revealed relevant dysconnectivity with the aMCC and the SPL. Indeed, past research has shown increased amygdala-aMCC connectivity when CP/ASPD subjects were confronted with negative emotional stimuli such as in moral judgment tasks (Yoder et al., Reference Yoder, Harenski, Kiehl and Decety2015), facial expressions of negative emotions (e.g. fear) (Marsh et al., Reference Marsh, Finger, Mitchell, Reid, Sims, Kosson and Blair2008), or painful situations caused by others (Decety et al., 2009). Researchers have also observed a reduced amygdala-SPL connectivity in antisocial subjects with psychopathic traits during moral judgments tasks (Marsh et al., Reference Marsh, Finger, Fowler, Jurkowitz, Schechter, Henry and Blair2011; Yoder et al., Reference Yoder, Harenski, Kiehl and Decety2015). Hence, these results also highlighted deficits in brain regions involved in attentional processes, which deserve extensive research.
Recently, researchers have shown that some dysconnectivities within and between large-scale networks may be transdiagnostic features. For instance, results from meta-analyses suggest that DMN dysconnectivity is found in patients with schizophrenia (Dong et al., Reference Dong, Wang, Chang, Luo and Yao2018), MDD (Kaiser, Andrews-Hanna, Wager, & Pizzagalli, Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015), obsessive-compulsive disorder (Gürsel, Avram, Sorg, Brandl, & Koch, Reference Gürsel, Avram, Sorg, Brandl and Koch2018), and attention-deficit/hyperactivity disorder (Sutcubasi et al., Reference Sutcubasi, Metin, Kurban, Metin, Beser and Sonuga-Barke2020). However, psychiatric disorders may actually differ when examining these abnormalities at a seed level (Doucet et al., Reference Doucet, Janiri, Howard, O'Brien, Andrews-Hanna and Frangou2020). Indeed, in a recent transdiagnostic meta-analysis of resting-state connectivity in the DMN, researchers have found that deficits in the dmPFC were mainly characterized by hyperconnectivity in MDD, whereas deficits in the precuneus were mostly associated with hypoconnectivity in schizophrenia (Doucet et al., Reference Doucet, Janiri, Howard, O'Brien, Andrews-Hanna and Frangou2020). In our meta-analysis, we found that CP/ASPD subjects rather exhibited hypoconnectivity in the dmPFC, in the most ventral part of the PCC (compared to the precuneus transdiagnostic cluster) but also in the vmPFC which negatively correlated with the severity of antisocial behaviors. Given that most meta-analyses on resting-state connectivity used large-scale network approaches, which reduces spatial preciseness, the comparison of deficits in connectivity found in our study and other mental illnesses remain challenging. Nonetheless, several differences are perceivable when comparing deficits in fMRI co-activation patterns between CP/ASPD subjects (Dugré et al., Reference Dugré, Radua, Carignan-Allard, Dumais, Rubia and Potvin2020) and transdiagnostic maps (Janiri et al., Reference Janiri, Moser, Doucet, Luber, Rasgon, Lee and Frangou2020; McTeague et al., Reference McTeague, Huemer, Carreon, Jiang, Eickhoff and Etkin2017, Reference McTeague, Rosenberg, Lopez, Carreon, Huemer, Jiang and Etkin2020; Sprooten et al., Reference Sprooten, Rasgon, Goodman, Carlin, Leibu, Lee and Frangou2017). For instance, across emotion processing tasks, transdiagnostic features mainly involved hyperactivations of subcortical regions (i.e. amygdala, hippocampus, parahippocampal gyrus) as well as hypoactivation of vlPFC (McTeague et al., 2020), whereas CP/ASPD subjects were rather characterized by reduced activation of the anterior insula and dlPFC (Dugré et al., Reference Dugré, Radua, Carignan-Allard, Dumais, Rubia and Potvin2020). Dugré et al. (Reference Dugré, Radua, Carignan-Allard, Dumais, Rubia and Potvin2020) also showed that the activity in the amygdala was negatively associated with the severity of antisocial behaviors and callous-unemotional traits. Concerning cognitive control tasks, despite that CP/ASPD subjects and major mental disorders both similarly show hypoactivation in the vlPFC (Dugré et al., Reference Dugré, Radua, Carignan-Allard, Dumais, Rubia and Potvin2020; McTeague et al., Reference McTeague, Huemer, Carreon, Jiang, Eickhoff and Etkin2017), antisocial subjects also display co-occurrent decreased activity within the premotor cortex and the inferior parietal lobule, which were not reported as transdiagnostic features (McTeague et al., Reference McTeague, Huemer, Carreon, Jiang, Eickhoff and Etkin2017). Considering these results, although CP/ASPD subjects may exhibit differences in neural processing compared to other mental illnesses, more research is needed to better understand their specific or shared deficits.
In sum, various evidence including our functional characterization analyses and our exploratory MACM but also past meta-analyses of fMRI tasks in healthy subjects and task-based connectivity studies in CP/ASPD subjects showed the involvement of the amygdala, the dmPFC, vPCC, and vmPFC in socio-affective processes, whereas the aMCC/pre-SMA, SPL, premotor cortex (frontal eye field), in attention-related processes. Furthermore, the reduced dmPFC-SPL connectivity found in our meta-analysis, alongside studies examining between-network (Cantor et al., Reference Cantor, Lafaille, Hannah, Kucyi, Soh, Girard and Mikulis2016; Dotterer et al., Reference Dotterer, Hyde, Shaw, Rodgers, Forbes and Beltz2020; Pu et al., Reference Pu, Luo, Jiang, Gao, Ming and Yao2017; Weathersby et al., Reference Weathersby, King, Fox, Loret and Anderson2019) or task-based connectivity (Decety et al., 2009; Marsh et al., Reference Marsh, Finger, Mitchell, Reid, Sims, Kosson and Blair2008, Reference Marsh, Finger, Fowler, Jurkowitz, Schechter, Henry and Blair2011; Yoder et al., Reference Yoder, Harenski, Kiehl and Decety2015), indicated that CP/ASPD may potentially show deficits in the interaction between both processes. It is unequivocal that the interaction between socio-affective and attentional processes in CP/ASPD subjects needs to be tackled in future studies. It would also be crucial to examine their specific association with specific subtypes of behaviors (i.e. reactive/proactive aggression v. rule-breaking behaviors) but also with callous-unemotional traits. Nonetheless, these findings support the view that both processes may be crucial to our understanding of rs-fMRI deficits in individuals at risk for antisocial behaviors (Hamilton et al., Reference Hamilton, Hiatt Racer and Newman2015).
Limitations
First, we performed a coordinate-based meta-analysis rather than a meta-analysis of original statistical brain maps. Although ALE method yields relatively similar results to image-based meta-analysis (Salimi-Khorshidi, Smith, Keltner, Wager, & Nichols, Reference Salimi-Khorshidi, Smith, Keltner, Wager and Nichols2009), this could have reduced results accuracy. Second, to achieve 80% power to detect effects that are present in approximately 1/3 of the population, a sample size of 17 experiments is required when using cluster-level FWE (Eickhoff et al., Reference Eickhoff, Nichols, Laird, Hoffstaedter, Amunts, Fox and Eickhoff2016). Second, in analyses with less than 10 experiments, we performed jackknife reliability analyses. However, these analyses yield very conservative results in small datasets (Eickhoff et al., Reference Eickhoff, Nichols, Laird, Hoffstaedter, Amunts, Fox and Eickhoff2016). Indeed, when spatial convergence is detected in two studies out of 7–8 (such as in this meta-analysis), removing one of the contributing studies undoubtedly yields non-significant findings. Third, there is currently no software nor gold standard to meta-analyze functional connectivity studies. Thus, current meta-analytical methods are not designed to explicitly examine dysconnectivity between two nodes. Despite our attempt to carry out such analyses (i.e. vPCC and dmPFC), larger datasets are required to specifically meta-analyze dysconnectivity between two nodes. Fourth, the meta-analysis on non-SBC connectivity studies included several different methods such as ReHo, ALFF, and voxel-wise group effects within ICA-derived networks. Given that there were too few studies per non-SBC methods, we could not perform a subanalysis on this issue. Fifth, no subanalysis was performed to investigate high v. low callous-unemotional traits due to the small number of studies that have used (or reported) psychometric scales measuring this feature. Likewise, there was only a very limited number of studies that specifically aimed to examine early v. late-onset disorders (or even reported such distinction). Additionally, in adults with ASPD, no study reported the mean age-of-onset of their sample. More importantly, the CD criterion in ASPD diagnosis suggests the occurrence of CD < 15 years old, while early-onset CD is generally defined by the appearance of CD prior to 10 years old (American Psychiatric Association, 2013). We encourage researchers to report the mean age of onset and levels of callous-unemotional traits in the future. Finally, since studies used different psychometric scales to assess dimensional associations with rsFC results, we had to merge them based on three dimensions.
Conclusion
Results from SBC, non-SBC, and dimensional studies indicate resting-state dysconnectivity indicates resting-state dysconnectivity in CD/ASPD in several brain regions such as vPCC/precuneus, vmPFC, dmPFC, premotor cortex, superior parietal lobule, amygdala, MCC, parietal operculum, cuneus, and intracalcarine cortex. Further examining these regions by functional characterization and MACM analyses underscores the importance of socio-affective and attentional processes in characterizing CP/ASPD population. This meta-analysis offers a complementary perspective to the emotion-cognition interaction deficits underlying individuals on the antisocial spectrum. In view of the results obtained in this meta-analysis, there is a crucial need to investigate neural signaling directionality between socio-affective and attentional networks, through effective connectivity methods, to better understand the interaction between both processes in this population. Future studies may seek to examine the specificity of functional connectivity alterations in CP/ASPD subjects compared with patients with major mental disorders.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721001525
Acknowledgments
JD is a holder of a doctoral scholarship from the Fonds de Recherche du Québec en Santé. SP is a holder of the Eli Lilly Canada Chair on schizophrenia research.
Author contributions
Both JRD and SP have made substantial contributions to the conception, design, acquisition, and interpretation of the data. JRD wrote the first draft and SP critically revised it. Both authors approved the final version of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.








