1. Introduction
Communicable livestock disease is costly for livestock-dependent households and communities in the tropics, and can be especially important for the economic well-being of low-income rural households for whom livestock represents a primary household asset. Livestock disease results in loss of wealth and income through livestock mortality and decrease in livestock productivity (Lybbert et al., Reference Lybbert, Barrett, Desta and Layne2004; Marsh et al., Reference Marsh, Yoder, Deboche, McElwain and Palmer2016). It also poses a threat to human health through loss of animal-based protein intake, zoonosis and food-borne illnesses (Narrod et al., Reference Narrod, Zinsstag and Tiongco2012; Mosites et al., Reference Mosites, Rabinowitz, Thumbi, Montgomery, Palmer, Susanne, Rowhani-Rahbar, Marian and Walson2015; Caudell et al., Reference Caudell, Quinlan, Subbiah, Call, Roulette, Roulette, Roth, Matthews and Quinlan2017b).
Livestock disease burden can be mitigated by reducing disease transmission risk, by reducing animal susceptibility, and through treatment. Disease transmission risk is dependent on general animal husbandry such as grazing and feeding practices that affect the frequency and nature of inter-herd contact (Bronsvoort et al., Reference Bronsvoort, Nfon, Hamman, Tanya, Kitching and Morgan2004; Rufael et al., Reference Rufael, Catley, Bogale, Sahle and Shiferaw2008; Schoonman and Swai, Reference Schoonman and Swai2010). Illness in the face of transmission risk can be avoided or mitigated by modern vaccination strategies, antibiotic use, traditional medicine, and other treatment methods. These targeted avoidance and treatment investments by herd owners are mediated through perceptions and understanding of disease transmission risk and the relative benefits and costs of avoidance and treatment options. Thus, general livestock husbandry and targeted disease management decisions can be related to, and through, livestock health.
In East Africa and other parts of the world, there is substantial variation in livestock feeding and grazing practices depending on localized environmental factors, land tenure, and cultural norms (Nugent and Sanchez, Reference Nugent and Sanchez1993; Davies and Hatfield, Reference Davies and Hatfield2007). In areas with sufficient rainfall and forage availability and relatively limited grazing land, fodder is often brought to livestock and grazing is more limited (Keyyu et al., Reference Keyyu, Kassuku, Msalilwa, Monrad and Kyvsgaard2006; Caudell et al., Reference Caudell, Quinlan, Quinlan and Call2017a). In more arid environments, extensive grazing is widely practiced, and in Tanzania in particular, communal and transhumant grazing practices are common. These types of grazing practices may lead to higher rates of inter-herd contact and disease transmission than under other management practices (Hutchings and Harris, Reference Hutchings and Harris1997; Keyyu et al., Reference Keyyu, Kassuku, Msalilwa, Monrad and Kyvsgaard2006; Bohm et al., Reference Bohm, Hutchings and White2009) such as ‘zero grazing’ common among the Chagga and some peri-urban Arusha households (Caudell et al., Reference Caudell, Quinlan, Subbiah, Call, Roulette, Roulette, Roth, Matthews and Quinlan2017b).
Antimicrobials are an important health intervention widely used in livestock and poultry management even in remote, rural communities as a prophylaxis, as treatment for microbial and protozoal infections, and in some (primarily commercial) settings for growth augmentation (Page and Gautier, Reference Page and Gautier2012; Perry et al., Reference Perry, Grace and Sones2013). As with other management inputs, the extent of antimicrobial use is driven in part by the perceived value of the input, and is likely to be used more where the threat and incidence of diseases thought to be treatable with antimicrobials is high (Gustafson and Bowen, Reference Gustafson and Bowen1997). Antimicrobial use can also reduce the extent of pathogen shedding and the likelihood of transmission to other animals, but may also lead to development of antibiotic resistance within the microbiome.
Thus, communal grazing is potentially related to disease risk through higher rates of direct and indirect inter-herd contact than private grazing or zero-grazing. Higher objective risk may then be associated with higher perceived disease risk. Therapeutic antimicrobial use may increase in response to actual incidence of disease (and therefore disease risk), and prophylactic antimicrobial use would be positively correlated with perceived disease risk. The perceived marginal value of antimicrobials could be higher where actual or perceived risk is high, potentially leading to higher antimicrobial use.
The objective of this paper is to examine the relationships between livestock grazing practices, past disease outcomes, and demand for antimicrobials among agropastoralists of northern Tanzania. We develop a theoretical model that elucidates basic connections between grazing practices, past and current disease incidence, and antimicrobial use. We then estimate these relationships by using data from surveys of agricultural households around ecologically heterogeneous regions of Mount Meru and Mount Kilimanjaro. This heterogeneity in ecology leads to widely different grazing practices, from communal grazing to zero-grazing in the region, and allows us to examine how grazing patterns are related to antimicrobial use. There are some changes in the grazing patterns of the Maasai with the seasons, but the inhabitants on the slopes of Mount Meru and Mount Kilimanjaro tend to keep their animals confined, with fodder delivered to the animals. This zero-grazing behavior is relatively stable over all seasons in a year (Caudell et al., Reference Caudell, Quinlan, Subbiah, Call, Roulette, Roulette, Roth, Matthews and Quinlan2017b).
Communal land tenure and use and transhumant grazing can provide vital benefits in spatiotemporally variable climates (Nugent and Sanchez, Reference Nugent and Sanchez1993; Agrawal, Reference Agrawal2001; Davies and Hatfield, Reference Davies and Hatfield2007; Ostrom, Reference Ostrom2015). That said, overgrazing has long been recognized as a potential problem of communal grazing land ownership, although the details of the social contract over communal grazing can be important mitigating factors (Ciriacy-Wantrup and Bishop, Reference Ciriacy-Wantrup and Bishop1975; Runge, Reference Runge1981; Swallow and Bromley, Reference Swallow and DW1995). Additionally, communal grazing and transhumant management practices may increase disease transmission risk (Sanderson et al., Reference Sanderson, Dargatz and Garry2000) and impose disease risk on other grazers that may not be fully accounted for in the private decision calculus of an individual herd owner. The consequence is that disease transmission mitigation practices and safeguards are likely to be under-applied, and disease transmission may be higher than socially optimal (Brito et al., Reference Brito, Sheshinski and Intriligator1991; Philipson, Reference Philipson, Culyer and Newhouse2000; Hennessy et al., Reference Hennessy, Roosen and Jensen2005).
The historic value of antimicrobials for global health outcomes is hard to overstate (Gustafson and Bowen, Reference Gustafson and Bowen1997; Kingston, Reference Kingston2000). But antimicrobial resistance is becoming a major public health concern globally, and the use of veterinary antimicrobials in agriculture sectors may be an important contributor (Carlet et al., Reference Carlet, Jarlier, Harbeth, Voss, Goossens and Pittet2012; Van Boeckel et al., Reference Van Boeckel, Brower, Gilbert, Grenfell, Levin, Robinson, Teillant and Laxminarayan2015). To the extent that antimicrobial use or misuse can impose external costs on other herd owners through antimicrobial resistance, herd owners may tend to overuse or misuse antimicrobials from a social economic efficiency perspective (Althouse et al., Reference Althouse, Bergstrom and Bergstrom2010), which may exacerbate the emergence and prevalence of antimicrobial resistance (Laximinarayan and Brown, Reference Laximinarayan and Brown2001; Secchi and Babcock, Reference Secchi and Babcock2002).
The externalities described above–higher potential inter-herd disease transmission from communal grazing, reduced pathogen shedding due to effective antimicrobials and reduced effectiveness from antimicrobial resistance–interact in complex ways. While our data do not allow us to tease out the externalities associated with these dimensions of grazing and antimicrobial use, we are able to examine the relationships between communal grazing, reported livestock illness, and antimicrobial use, and therefore contribute to an understanding of the incentives surrounding antimicrobial use for livestock in agropastoral settings.
We contribute to the literature in several ways. We extend the analysis of Caudell et al. (Reference Caudell, Quinlan, Subbiah, Call, Roulette, Roulette, Roth, Matthews and Quinlan2017b) who treat communal grazing as a component of Maasai ethnicity, and account for the fact that grazing decisions of households may be jointly (endogenously) determined along with antimicrobial use in response to disease risk. Moreover, we extend Caudell et al. (Reference Caudell, Quinlan, Subbiah, Call, Roulette, Roulette, Roth, Matthews and Quinlan2017b) by conceptualizing how past disease incidence contributes to current antimicrobial use, perhaps through its impact on perceived risk. In doing so, we also contribute to the literature on subjective risk assessment generally. Subjective inference about disease risk is often based on sparse information from direct observation, indirect covariates, and broader belief contexts, and plays an important role in the perceived marginal value of risk-reducing management practices (Tversky and Kahneman, Reference Tversky and Kahneman1973; Johnson et al., Reference Johnson, Hershey, Meszaros and Kunreuther1993; Mittal and Ross, Reference Mittal and Ross1998; McNamara et al., Reference McNamara, Green and Olsson2006; Clark, Reference Clark2013; Cole et al., Reference Cole, Gine, Tobacman, Topalova, Townsend and Vickery2013). Although the role of perceptions in avoidance behavior has been well documented in economics (Courant and Porter, Reference Courant and Porter1981; Crocker et al., Reference Crocker, Forster and Shogren1991; Dickie and Gerking, Reference Dickie and Gerking1996; Ahamad, Reference Ahamad2016), the evidence of the impact of disease risk perceptions on disease mitigation and control strategies such as vaccination and antimicrobial use is scant.
2. Theoretical model
We examine how grazing patterns and past disease history relate to antimicrobial use. Grazing patterns and fodder collection practices chosen by livestock owners depend on relative forage availability, water availability, and land tenure characteristics, and other factors (Coppolillo, Reference Coppolillo2000; Pringle and Landsberg, Reference Pringle and Landsberg2004; Caudell et al., Reference Caudell, Quinlan, Subbiah, Call, Roulette, Roulette, Roth, Matthews and Quinlan2017b).Footnote 1 While grazing practices change somewhat over grazing seasons, the basic pattern of less travel and herd interaction in higher rainfall regions versus more travel and more herd interaction with more arid conditions is a relatively stable, long-term phenomenon (Bollig, Reference Bollig2006). In contrast, decisions about and variation in antimicrobial use can likely be more easily altered in the short-run, depending on the real and perceived disease risk a herd owner faces. These differences allow us to divide the decision process into two stages; the communal grazing decision as a stable, quasi-fixed management practice, and antimicrobial use as a variable input with more flexibility in response to disease risk and outcomes.
Based on this decision environment, we consider a two-stage expected profit (net income) maximization model, with stages distinguished by a long-term grazing decision and a short-term antimicrobial use decision.Footnote 2 In the first stage, the farmer chooses the proportion of the household herd to graze on common grazing land (the grazing rate). In the second stage, the farmer chooses antimicrobial use to maximize expected short-run profits based on preventive and therapeutic antimicrobial goals, the disease environment, and grazing practices. Expected profit to the household from livestock is
The function
![]() $v({g;\tilde{g}})$
is the potential value to a household of livestock production in the absence of disease. The household communal grazing rate is g, and communal grazing by other households is
$v({g;\tilde{g}})$
is the potential value to a household of livestock production in the absence of disease. The household communal grazing rate is g, and communal grazing by other households is
![]() $\tilde{g}. v({g;\tilde{g}})$
increases at a decreasing rate with the communal grazing rate g and decreases with the communal grazing rate of other households (
$\tilde{g}. v({g;\tilde{g}})$
increases at a decreasing rate with the communal grazing rate g and decreases with the communal grazing rate of other households (
![]() $v_{g} >0,\break v_{gg} <0$
,
$v_{g} >0,\break v_{gg} <0$
,
![]() $v_{g\tilde{g}} <0$
, where subscripts represent partial derivatives throughout).
$v_{g\tilde{g}} <0$
, where subscripts represent partial derivatives throughout).
The term
![]() $(1-\gamma ({g;\tilde{g},\tilde{a},\rho})\alpha(a;\tilde{a}))$
is the fraction of potential livestock value realized given disease losses. The function
$(1-\gamma ({g;\tilde{g},\tilde{a},\rho})\alpha(a;\tilde{a}))$
is the fraction of potential livestock value realized given disease losses. The function
![]() $\gamma({g;\tilde{g}},\tilde{a},\rho) \in (0, 1)$
is the fraction of livestock value lost to disease in the absence of private (own-herd) antimicrobial use, where ρ is the background (environmental) disease prevalence. Regional antimicrobial use by others (ã) can reduce private infection risk to the herd, and communal grazing rates by others (
$\gamma({g;\tilde{g}},\tilde{a},\rho) \in (0, 1)$
is the fraction of livestock value lost to disease in the absence of private (own-herd) antimicrobial use, where ρ is the background (environmental) disease prevalence. Regional antimicrobial use by others (ã) can reduce private infection risk to the herd, and communal grazing rates by others (
![]() $\tilde{g}$
) can increase infection risk
$\tilde{g}$
) can increase infection risk
![]() $({\gamma _{g} >0,\gamma_{\tilde{g}} >0,\gamma _{\tilde{a}} <0,\gamma _{\rho} >0})$
. In addition, the marginal losses from grazing increase with the grazing rates of other households, and background disease prevalence
$({\gamma _{g} >0,\gamma_{\tilde{g}} >0,\gamma _{\tilde{a}} <0,\gamma _{\rho} >0})$
. In addition, the marginal losses from grazing increase with the grazing rates of other households, and background disease prevalence
![]() $({\gamma _{g\tilde{g}} >0,\gamma _{g\rho} >0})$
. The function
$({\gamma _{g\tilde{g}} >0,\gamma _{g\rho} >0})$
. The function
![]() $\alpha ( {a;\tilde{a}})\in ({0,1})$
is the reduction in the loss rate from private antimicrobial use. Antimicrobial use reduces losses at a decreasing rate
$\alpha ( {a;\tilde{a}})\in ({0,1})$
is the reduction in the loss rate from private antimicrobial use. Antimicrobial use reduces losses at a decreasing rate
![]() $( {\alpha _{a} <0,\alpha _{aa} >0})$
, and the marginal effectiveness of a declines with regional antimicrobial use, ã due to its impact on antimicrobial resistance
$( {\alpha _{a} <0,\alpha _{aa} >0})$
, and the marginal effectiveness of a declines with regional antimicrobial use, ã due to its impact on antimicrobial resistance
![]() $({\alpha _{a} <0,\alpha _{aa} >0, \alpha _{a\tilde{a}} >0})$
. Thus, regional antimicrobial use has two competing impacts: reductions in disease transmission due to its effect of reducing transmission of antimicrobial-susceptible pathogens, and increases in losses from the transmission of antimicrobial resistant pathogens.
$({\alpha _{a} <0,\alpha _{aa} >0, \alpha _{a\tilde{a}} >0})$
. Thus, regional antimicrobial use has two competing impacts: reductions in disease transmission due to its effect of reducing transmission of antimicrobial-susceptible pathogens, and increases in losses from the transmission of antimicrobial resistant pathogens.
The marginal cost of antimicrobial use is c. Additional grazing costs are suppressed for clarity. Other exogenous factors may drive the value of production, e.g., market prices, livestock characteristics, total forage usage, and other inputs, grazing impacts on disease, and antimicrobial use effectiveness. These are omitted above for clarity but discussed below as they apply to the empirical analysis.
In summary, net returns from livestock ownership are v(1 – γ α) minus private antimicrobial costs ca. The function γ embodies the harm from disease and is a function of grazing and antimicrobial use. Communal grazing has two effects: it increases the value of livestock by providing food for the animals, but may decrease the value of livestock through disease morbidity and mortality. Regional and private antimicrobial use mitigates disease losses, but antimicrobial use also may reduce its effectiveness through resistance.
Expected net returns are solved by backward induction by choosing antimicrobial use subject to grazing practices, and grazing practices conditional on expected optimal antimicrobial use. The first-order condition for the second stage (antimicrobial use) decision is
The first-order condition gives a standard result of private marginal benefit of antimicrobial use equal to marginal cost of antimicrobial use and, assuming the Implicit Function theorem holds, antimicrobial demand is
![]() $a^{\ast} =a(g,\rho, \tilde{a}, \tilde{g},c) $
. The marginal rate of substitution between a and g is
$a^{\ast} =a(g,\rho, \tilde{a}, \tilde{g},c) $
. The marginal rate of substitution between a and g is
From this relationship we have our first hypothesis:
Hypothesis 1. More antimicrobials are used by households that graze more on communal grazing lands.
Optimal antimicrobial use in relation to the baseline disease risk, conditional on the grazing rate is
implying a second hypothesis:
Hypothesis 2. A higher background disease prevalence is associated with higher antimicrobial use.
The first stage first-order condition for grazing is (after applying the envelope theorem based on the first-order condition for antimicrobial demand) is
which indicates that the marginal value of grazing is equal to the marginal cost of disease exposure due to grazing, accounting for optimal response to antimicrobial use. Communal grazing demand is
![]() $g^{\ast} =g(\rho, \tilde{a}, \tilde{g},c)$
, which includes the same arguments as a* (except g itself).
$g^{\ast} =g(\rho, \tilde{a}, \tilde{g},c)$
, which includes the same arguments as a* (except g itself).
Losses from livestock illness are represented by
![]() $l^{\ast} = v({g^{\ast}})(\gamma (g^{\ast} ;\tilde{g}, \tilde{a},\rho)\break\alpha(a^{\ast};\tilde{a}))$
, and dependent on (endogenous) grazing and antimicrobial use. Losses increase with increase at the margin from communal grazing by
$l^{\ast} = v({g^{\ast}})(\gamma (g^{\ast} ;\tilde{g}, \tilde{a},\rho)\break\alpha(a^{\ast};\tilde{a}))$
, and dependent on (endogenous) grazing and antimicrobial use. Losses increase with increase at the margin from communal grazing by
![]() $\partial l^{\ast} /\partial g^{\ast} =v_{g} \gamma \alpha +v\gamma _{g} \alpha $
and decrease at the margin from antimicrobial use by
$\partial l^{\ast} /\partial g^{\ast} =v_{g} \gamma \alpha +v\gamma _{g} \alpha $
and decrease at the margin from antimicrobial use by
![]() $\partial l^{\ast} /\partial a^{\ast} =v\gamma \alpha _{a} $
(evaluated g* and a* in both cases).
$\partial l^{\ast} /\partial a^{\ast} =v\gamma \alpha _{a} $
(evaluated g* and a* in both cases).
From a social welfare perspective, private decisions about communal grazing and antimicrobial use have impacts beyond the household through
![]() $\tilde{g}$
and ã. To examine the implications of these inter-household impacts, assume there are N+1 identical households as described above, and define
$\tilde{g}$
and ã. To examine the implications of these inter-household impacts, assume there are N+1 identical households as described above, and define
![]() $\tilde{g}= \sum\nolimits^{N}_{j=1} g= Ng$
and
$\tilde{g}= \sum\nolimits^{N}_{j=1} g= Ng$
and
![]() $\tilde{a}= \sum\nolimits^{N}_{j=1} a = Na$
. In other words, the sum of other households’ communal grazing and antimicrobial use increase or decrease the morbidity and mortality losses to a household. Given that
$\tilde{a}= \sum\nolimits^{N}_{j=1} a = Na$
. In other words, the sum of other households’ communal grazing and antimicrobial use increase or decrease the morbidity and mortality losses to a household. Given that
![]() $\tilde{a}= \sum\nolimits^{N}_{i=1} a$
, a one unit increase in one household's use of antibiotics adds one unit to ã, so the net externality of a household's antibiotic use on all N other households at the margin is
$\tilde{a}= \sum\nolimits^{N}_{i=1} a$
, a one unit increase in one household's use of antibiotics adds one unit to ã, so the net externality of a household's antibiotic use on all N other households at the margin is
where
![]() $\pi ^{\ast} =\pi (\rho ,c,\tilde{g}, \tilde{a})$
is the indirect profit function.Footnote
3
The net marginal external cost of antibiotic use across N identical users is
$\pi ^{\ast} =\pi (\rho ,c,\tilde{g}, \tilde{a})$
is the indirect profit function.Footnote
3
The net marginal external cost of antibiotic use across N identical users is
![]() $E_{\tilde{a}} = NE_{a}$
.
$E_{\tilde{a}} = NE_{a}$
.
The marginal externality of one household grazing on communal land due to contributions to disease incidence is similarly
![]() $E_{g} =N\partial \pi ^{\ast} /\partial \tilde{g}= N(v_{\tilde{g}}(1-\gamma \alpha)- v(\alpha\gamma_{\tilde{g}} + \gamma\alpha_{a}a^{\ast}_{\tilde{g}})),$
and the communitywide externality is
$E_{g} =N\partial \pi ^{\ast} /\partial \tilde{g}= N(v_{\tilde{g}}(1-\gamma \alpha)- v(\alpha\gamma_{\tilde{g}} + \gamma\alpha_{a}a^{\ast}_{\tilde{g}})),$
and the communitywide externality is
![]() $E_{\tilde{g}} =NE_{g}$
. Note that the externality in this case has three parts: (a) the negative effect on grazing productivity, (b) increased transmission risk due to grazing itself, and (c) increased antimicrobial resistance from the induced increase in antimicrobial use in response to higher transmission and disease risk from grazing.
$E_{\tilde{g}} =NE_{g}$
. Note that the externality in this case has three parts: (a) the negative effect on grazing productivity, (b) increased transmission risk due to grazing itself, and (c) increased antimicrobial resistance from the induced increase in antimicrobial use in response to higher transmission and disease risk from grazing.
3. Data and econometric methods
To test hypotheses 1 and 2 above, we run regressions to represent communal grazing and demand for antimicrobials, and a third regression to estimate the relationship between grazing, antimicrobial use, and livestock illness:
 $$\eqalign{ g^{\ast} &=g\lpar c,\rho ,\tilde{a}, \tilde{g} \rpar = g\lpar {\bi X}\rpar \cr a^{\ast} &=a\lpar g, c,\rho ,\tilde{a}, \tilde{g} \rpar = a\lpar g^{\ast}, {\bi X}\rpar \cr l^{\ast} & =l\lpar {g,c,\rho, \tilde{a}, \tilde{g}}\rpar = l\lpar g^{\ast}, a^{\ast}, {\bi X}\rpar .}$$
$$\eqalign{ g^{\ast} &=g\lpar c,\rho ,\tilde{a}, \tilde{g} \rpar = g\lpar {\bi X}\rpar \cr a^{\ast} &=a\lpar g, c,\rho ,\tilde{a}, \tilde{g} \rpar = a\lpar g^{\ast}, {\bi X}\rpar \cr l^{\ast} & =l\lpar {g,c,\rho, \tilde{a}, \tilde{g}}\rpar = l\lpar g^{\ast}, a^{\ast}, {\bi X}\rpar .}$$
Because the characteristics of our data define the specific estimation strategies we use, we first describe our data, and then describe our regression estimation procedures.
Data collection was performed by Washington State University, with a protocol approved by the Tanzania National Institute for Medical Research. Data were collected from 416 households in 13 villages, and the dataset is made up of one record (observation) per household (Caudell et al., Reference Caudell, Quinlan, Subbiah, Call, Roulette, Roulette, Roth, Matthews and Quinlan2017b). There are three main ethnic groups that inhabit the area of study, which ranges between Mount Meru and Mount Kilimanjaro in North Central Tanzania, and West to the Ngorogoro area (figure 1). The Arusha and Chagga populate the slopes of Mt. Meru and Mt. Kilimanjaro, respectively, while the Maasai mostly live in the surrounding steppe. Some Arusha live in lower lying areas interspersed among Maasai to the West of Arusha Town in Monduli District. The Chagga generally live in the higher rain fed, green regions around Mount Kilimanjaro, and their herds are generally small (mean herd size = 8.9) and more confined. The Maasai mostly live in the more arid lowland steppe plains. Their herds are relatively large (mean herd size = 345.5), and they mainly rely on communal grazing to feed their animals, given the predominant land tenure system in Tanzania. The green regions around Mount Meru are mostly inhabited by the Arusha. Again, with greater forage around the farms, they typically rely less on communal grazing than the Maasai.

Figure 1. Northern Tanzania from Mt Meru to the southern slopes of Kilimanjaro, West to Ngorogoro showing major highways and location of ethnic groups sampled.
Table 1 describes each of the variables used in the analysis, and table 2 provides summary statistics. Antimicrobial use (represented by a in our theoretical model) provides information about antimicrobial inventories on-hand in each household, which are used to develop an antimicrobial use index. The index indicates the presence of syringes/needles for antimicrobial injection and number of types of antimicrobials on-hand in a household at the time of survey enumeration.Footnote 4 The largest number for any household was 7, and the lowest was 0, therefore, our index ranges from 0 to 7. As such, our antimicrobial use data are treated as count data in our analysis (refer to figure 2). The average index value of antimicrobials on hand in a household is 1.69 (standard deviation 1.63) (table 2). One hundred fifty-seven out of the 382 households did not have any antimicrobials/syringes in their inventory. Virtually none of the Chagga households held antimicrobials or syringes, and virtually all Maasai households held some, while Arusha households varied more in their antimicrobial holdings.
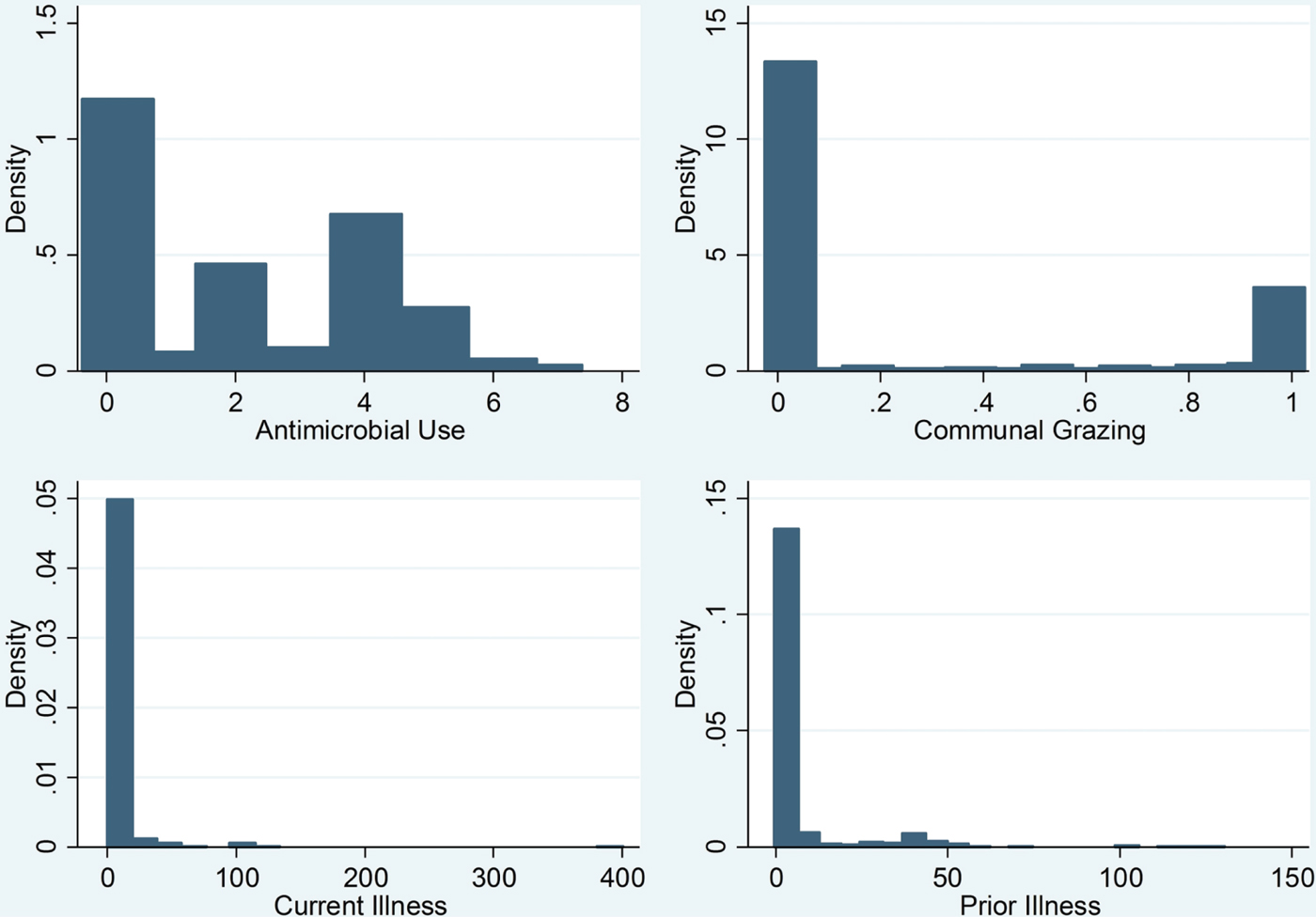
Figure 2. Histograms of communal grazing rates, antibiotic use, prior illness and current illness variables.
Table 1. Data description

Table 2. Summary statistics (N = 382)

Communal grazing (represented by g in our theoretical model) is the fraction of animals in a household's herd that are regularly grazed outside the household or compound, therefore g ranges in the unit interval.
We hypothesize that background disease prevalence, measured imperfectly in our data through recent history of local livestock illness, affects antimicrobial use through its impact on herd owner risk perceptions (Pingali and Carlson, Reference Pingali and Carlson1985; Dickie and Gerking, Reference Dickie and Gerking1996; McNamara et al., Reference McNamara, Green and Olsson2006; Clark, Reference Clark2013). Current illness is the number of animals reported sick during the time of the survey, and is a proxy for current and expected illness outcomes (represented by
![]() $v\gamma \alpha$
in the model). Prior illness is the number of animals reported sick in the past year prior to current illnesses. Figure 2 contains histograms of current illness, prior illness, antimicrobial use and communal grazing.
$v\gamma \alpha$
in the model). Prior illness is the number of animals reported sick in the past year prior to current illnesses. Figure 2 contains histograms of current illness, prior illness, antimicrobial use and communal grazing.
Sheep/goats, and cattle herd size are important control variables in the analysis. They relate to total herd livestock value (v) and, in conjunction with grazing rates, determine the number of animals grazed on communal land. The Chagga and Arusha who inhabit Mt. Meru and Mt. Kilimanjaro often practice zero-grazing because the rainfall in these regions is plentiful and land is limited, while the Maasai generally use communal grazing, and live in more arid conditions. About 50 per cent of the sample consists of Maasai households and the other 48 per cent of the sample is made up of Chagga and Arusha. About 2 per cent of the sample households belong to other ethnicities.
Other variables that we hypothesize may explain the differences in the antimicrobial use include total household income, household size, and method of consultation regarding livestock health. The household income variable is measured in Tanzanian Shillings (10,000s). Crop inventory, and sale were converted into monetized value, and added to the cash income to indicate total household wealth. Cash income is a good indicator of wealth for Chagga and Arusha, but Maasai may have less cash income, and more crop income. Therefore, recent sale prices of the produce were multiplied by crop stored and sold quantities to estimate cash value of the crops.
The variable Govt. Vet. is an indicator variable equal to 1 if a household uses a professional veterinarian for livestock health consultation, and zero if a household uses traditional methods or over-the-counter antimicrobials. Distance to urban is the natural logarithm of distance between the household and an urban center, and Distance to market is the logarithm of distance between the household and a market. We include these variables as proxies to control for access to antimicrobials, livestock health services, and both livestock and human population density.
4. Empirical model
We developed two econometric models to test our hypotheses: (a) a model of communal grazing rates conditional on household characteristics, and (b) an instrumental variables model of antimicrobial use conditional on grazing patterns, prior livestock disease, and household characteristics. A third regression model is used to estimate the relationship between livestock illness rates, communal grazing and antimicrobial use.
A fractional Probit regression is used to model the grazing practices of the households because the dependent variable, communal grazing (g), is the proportion of a herd grazed bounded by zero and one (see figure 2).Footnote 5 Following Papke and Woolridge, (Reference Papke and Woolridge1996), the conditional expectation of the grazing rate is
where
![]() ${\Phi}({\bi X})$
is a standard normal cumulative distribution function and
X
includes rainfall, ethnicity dummy variables and other available exogenous controls that may explain grazing patterns, and η
i
is a random disturbance. The fractional Probit model is estimated via quasi-maximum likelihood using the Stata® 14 fracreg routine.
${\Phi}({\bi X})$
is a standard normal cumulative distribution function and
X
includes rainfall, ethnicity dummy variables and other available exogenous controls that may explain grazing patterns, and η
i
is a random disturbance. The fractional Probit model is estimated via quasi-maximum likelihood using the Stata® 14 fracreg routine.
Antimicrobial use is count data, with a high proportion of zeros. A zero-inflated Poisson model is used for estimation using Stata® 14 zip routine. This specification implicitly assumes that the decision to use antibiotics is not systematically different from the decision about how much or how many antimicrobials to use. The first stage in the econometric model characterizes the probability of a household having no antimicrobials on hand (a = 0). Following Greene (Reference Greene2011), Prob
![]() $[a=0{\vert}g,{\bi X}]=F(a{\vert }g,{\bi X}).$
The probability of nonzero antimicrobials on hand is
$[a=0{\vert}g,{\bi X}]=F(a{\vert }g,{\bi X}).$
The probability of nonzero antimicrobials on hand is
![]() $\hbox{Prob}\lsqb {a=j{\vert }{\bi X},a>0} \rsqb =(1-F(a\vert g,{\bi X}))=\exp ({-\lambda } )\lambda ^{j}/j!$
; where
$\hbox{Prob}\lsqb {a=j{\vert }{\bi X},a>0} \rsqb =(1-F(a\vert g,{\bi X}))=\exp ({-\lambda } )\lambda ^{j}/j!$
; where
![]() $\lambda =\lambda (g,{\bi X})$
and is the conditional mean of the Poisson process. Hence,
$\lambda =\lambda (g,{\bi X})$
and is the conditional mean of the Poisson process. Hence,
![]() $E\lsqb {a{\vert }g,{\bi X}} \rsqb =F^{\ast}0+({1-F({a{\vert }g,X} )} )^{\ast}E\lsqb {a{\vert }g,{\bi X},a \gt 0} \rsqb =\break ({1-F({a{\vert }g,{\bi X}})})\lambda.$
$E\lsqb {a{\vert }g,{\bi X}} \rsqb =F^{\ast}0+({1-F({a{\vert }g,X} )} )^{\ast}E\lsqb {a{\vert }g,{\bi X},a \gt 0} \rsqb =\break ({1-F({a{\vert }g,{\bi X}})})\lambda.$
F is estimated as a logit such that
![]() $\lsqb q=1{\vert }g,{\bi X} \rsqb =\exp ({\bi X}^{\prime}{\bf \delta})/1+\hbox{exp}({\bi X}^{\prime} {\bf \delta})$
, which results in the following probability regression that can be estimated using maximum likelihood, motivated in Greene, (Reference Greene2011):
$\lsqb q=1{\vert }g,{\bi X} \rsqb =\exp ({\bi X}^{\prime}{\bf \delta})/1+\hbox{exp}({\bi X}^{\prime} {\bf \delta})$
, which results in the following probability regression that can be estimated using maximum likelihood, motivated in Greene, (Reference Greene2011):
where μ l captures producer heterogeneity. X constitutes all the factors described in the theoretical model, and g is the grazing rate. The second stage (the Poisson process) can be estimated using the canonical formulation motivated by Cameron and Trivedi, (Reference Cameron and Trivedi1986),
where
![]() $\varepsilon _{i} $
captures unobserved heterogeneity among households in the data sample.
$\varepsilon _{i} $
captures unobserved heterogeneity among households in the data sample.
Our theoretical model treats communal grazing (g) as a quasi-fixed choice variable and is endogenous in the intermediate run, depending on several factors including land grazing characteristics and land tenure. We therefore apply a two-stage approach to estimation of the antimicrobial use regression by replacing the actual value of g with the predicted values of g from a regression of grazing on a set of explanatory variables. In two-stage instrumental variable estimation, an adjustment must be made to attain consistent covariance estimates (Greene, Reference Greene2011), which we perform.Footnote 6 Evidence of over-dispersion was found in the data, which could be due to heterogeneity in household preferences or the nature of the process generating the excess zeros (Mullahey, Reference Mullahey1986). The Vuong test (Vuong, Reference Vuong1989) suggests that the excess zeros are generated by a separate process, justifying a zero-inflated Poisson regression.
A final regression estimates the relationship between current livestock illness, antimicrobial use, and grazing rates. Current illness is also a count variable (see figure 2) and a Vuong test suggests that a zero-inflated Poisson regression is justified. The standard errors are again adjusted for instrumental variable use as they were in the antimicrobial regression.
5. Results
We present results for the grazing regression, the antimicrobial use regression, and the illness regression in turn. In the grazing regression (table 3), the coefficient for rainfall is negative, large and statistically significant (p<0.001), consistent with grazing intensity being higher in arid environments whereas feed and fodder is brought to livestock in the high rain fed areas and grazing is only used as an extensive margin. The coefficients for ethnicity indicators, Arusha and Chagga, are also negative relative to the Maasai; this is consistent with the grazing behaviors of these ethnicities.
Table 3. Communal grazing regression. Fractional probit results

Notes: Number of observations = 382. ***indicates statistical significance at 1% level.
These ethnic dummy variables are excluded instruments for the second stage antimicrobial regression, where ethnic grazing practices are hypothesized to be more long-term, historical phenomena shaped by environment, culture and land tenure issues, while antimicrobial use is a modern risk management phenomenon that is more fluid and not subject to these factors. Therefore, controlling for ecological factors and livestock and human densities, ethnic background is taken to affect antimicrobial use through livestock management practices like communal grazing.
Distance to Urban and Distance to Market are proxies for human and livestock densities and market access. Urban areas tend to be located at higher rainfall regions within our sample, and distances to markets tend to be short. Their associated coefficients are consistent with higher communal grazing rates in steppe environments that receive lower rainfall and support lower human and livestock densities.
For the antimicrobial use regression, the results show a positive relationship between communal grazing and antimicrobial use, corroborating hypothesis 1 (table 4). The null hypothesis that communal grazing does not affect antimicrobial use is rejected at 1 per cent level of significance (p<0.001) in the logit regression, while we fail to reject the null hypothesis in the case of Poisson regression. These results suggest that, conditional on controls, communal grazing is associated with higher antimicrobial use, but does not (statistically speaking) influence how many types of antimicrobials a household will use. This pattern might result if households tend to use a broad-spectrum antimicrobial (like oxytetracycline in our sample) for all disease challenges, or if they face just one or a few diseases for which one antimicrobial will suffice. Overall, a 10 per cent increase in the communal grazing rate is associated with about 7 per cent increase in antimicrobial use evaluated at sample means (i.e.
![]() $(\partial a/\partial g)/({\tilde{g}/\bar{a}}))$
. It is worth noting that communal grazing captures herd-contact imperfectly, and there could be other mechanisms of herd contact like watering holes and livestock transactions.
$(\partial a/\partial g)/({\tilde{g}/\bar{a}}))$
. It is worth noting that communal grazing captures herd-contact imperfectly, and there could be other mechanisms of herd contact like watering holes and livestock transactions.
Table 4. Antimicrobial use regression. Zero-inflated poisson

Notes: *, **, *** indicate statistical significance at 10, 5 and 1% respectively. N = 382.
a The model predicts the outcomes of zero observations and therefore reported signs for the estimates here are for the probability of not choosing antibiotics.
b The predicted values from the regression summarized in table 4 are used as the instrument for communal grazing.
The analysis also shows a positive effect of prior illness on antimicrobial use, which is consistent with hypothesis 2. A unit increase in the prior illness number is associated with an increase in antimicrobial usage of 0.87 and the coefficient is significant at 5 per cent level for both the Logit and Poisson regression components. This result is consistent with how reference levels and loss aversion play a role in behavior (Kahneman et al., Reference Kahneman, Knetsch and Thaler1991; Tversky and Kahneman, Reference Tversky and Kahneman1991). ‘Availability bias’ can be another reason why farmers may use more antimicrobials after experiencing salient illness events in the recent past (Tversky and Kahneman, Reference Tversky and Kahneman1973). To the extent that herd owners understand the disease risks associated with communal grazing and use prior illness as an indicator of underlying disease risk, they can affect subjective risk assessment and therefore the extent of antimicrobial use.
Higher household income is associated with higher antimicrobial adoption rates, but not the number of antimicrobial types used. If household production and consumption are separable activities, if livestock husbandry is a purely financial enterprise, and if antimicrobial use is solely providing benefits in terms of reduced livestock morbidity and mortality, we might expect household income to have no effect on antimicrobial use (Marsh et al., Reference Marsh, Yoder, Deboche, McElwain and Palmer2016). There are several reasons why income may have an effect. First, if liquidity constraints affect the ability of households to purchase antimicrobials, then income may affect antimicrobial purchases (Carter and Yao, Reference Carter and Yao2002). Second, antimicrobial use for livestock may provide human health benefits to the extent that antimicrobial use in household livestock mitigates zoonotic disease incidence. Third, while livestock may be an important economic asset, household herds and their wellbeing may hold cultural significance beyond their market, income and consumption value (Quinlan et al., Reference Quinlan, Rumas, Naisikye, Quinlan and Yoder2016).
Rainfall is also positively related to antimicrobial use, given grazing practices and other controls. Higher rainfall can support taller grass, which can lead to high tick intensity in herds and may lead to higher disease transmission risk and more frequent use of antibiotics, antiprotozoans and acaricides.
The method of consultation that households use can also influence antimicrobial use. Our results show that use of govt. vet is associated with an intercept shift of −0.3; a lower rate of antimicrobial holding and use. Although we cannot identify underlying drivers of this result and variation in use of veterinary services is closely tied to the three primary ethnic groups identified in this study, it is consistent with professional advice acting to reduce antimicrobial use (all else constant) relative to private use by herd owners. Note, however, that the use of veterinary services is an endogenous decision, likely affected by the cost of and access to professional services. Large herd owners may choose to make antimicrobial use decisions on their own depending on the fee structure of professional veterinary health care providers, and fees may be larger in rural areas relative to urban/peri-urban settings due to travel cost differences.
Distance to urban does not appear to be correlated with antimicrobial use. Nevertheless, distance to market is associated with a decrease in antimicrobial use, perhaps due to higher acquisition costs or, perhaps, less disease challenge through inter-herd contact.
Table 5 provides illness regression results with one regression that excludes antimicrobial use as a regressor (Regression 1) and one that includes an instrument for antimicrobial use.Footnote 7 Regression 1 shows that an increase in communal grazing is associated with a higher incidence of sick animals. This is consistent with hypothesis 1, suggesting that communal grazing may lead to higher rates of illness through higher transmission.
Table 5. Current illness, zero-inflated poisson regression

Notes: **, *** indicate statistical significance at 5 and 1% respectively.
a The predicted values from the regression summarized in table 5 is used as the instrument for antimicrobial use. N = 382.
This first regression can be interpreted as a reduced form regression in which household demand for antimicrobials is implicit, and it is included primarily as a robustness check to compare with Regression 2.
Regression 2 (columns 3 and 4 of table 5) includes an instrumental variable for antimicrobial use (the predicted values from the regression in table 4). The associated negative parameter under ‘No Sick Animals’ indicates that the probability of having no illness is negatively associated with antimicrobial use. The positive coefficient under ‘Number of Sick Animals’ indicates that there is a positive association between antimicrobial use and the number of sick animals. To relate this result back to our theoretical model, recall that the marginal effect of antimicrobial use on illness is
![]() $\partial l^{\ast} /\partial a^{\ast} =v\gamma \alpha _{a} <0$
, suggesting we would expect to see a negative relationship between antimicrobial use and illness. Note, however, that our current illness measure is the number of current reported illnesses, and therefore better characterized as vγ rather than
$\partial l^{\ast} /\partial a^{\ast} =v\gamma \alpha _{a} <0$
, suggesting we would expect to see a negative relationship between antimicrobial use and illness. Note, however, that our current illness measure is the number of current reported illnesses, and therefore better characterized as vγ rather than
![]() $v\gamma \alpha .$
As such, our available metric is an incomplete measure of illness because it does not measure the degree or duration of illness – the characteristics of illness that therapeutic antimicrobial use would most likely affect. Thus, the positive relationship between antimicrobial use and current illness in this regression is consistent with a scenario in which antimicrobial use is primarily therapeutic instead of preventive, which, based on out-of-sample anecdotal field evidence, appears to be the case in most households. Conditional on grazing practices, illness frequency increases when background risk increases
$v\gamma \alpha .$
As such, our available metric is an incomplete measure of illness because it does not measure the degree or duration of illness – the characteristics of illness that therapeutic antimicrobial use would most likely affect. Thus, the positive relationship between antimicrobial use and current illness in this regression is consistent with a scenario in which antimicrobial use is primarily therapeutic instead of preventive, which, based on out-of-sample anecdotal field evidence, appears to be the case in most households. Conditional on grazing practices, illness frequency increases when background risk increases
![]() $(\gamma _{\rho} >0)$
, and hypothesis 2 is that antimicrobial demand increases with background illness risk, and so the estimated positive relationship between antimicrobial use and current illness is likely picking up this signal and its influence on therapeutic antimicrobial use.
$(\gamma _{\rho} >0)$
, and hypothesis 2 is that antimicrobial demand increases with background illness risk, and so the estimated positive relationship between antimicrobial use and current illness is likely picking up this signal and its influence on therapeutic antimicrobial use.
6. Conclusion
Infectious disease management and grazing decisions are important elements of agropastoral livestock husbandry. Our results show strong relationships between communal grazing, livestock illness, and antimicrobial use. We estimate the impact of grazing patterns and prior livestock illnesses on antimicrobial demand using a zero-inflated Poisson regression model. Identification within this framework is achieved by making use of the variation in ethnicity of households in our sample. We also examine the relationship between current illness rates, grazing and antimicrobial use. Our results show that disease risk perceptions and communal grazing play important roles in determining disease outcomes and the demand for antimicrobials.
The paper relies on communal grazing and prior illness as indicators of underlying risk over which herd owners make antimicrobial use decisions. Communal grazing is directly linked with exposure and disease transmission, while prior illness is informative about current risk and can also have a psychological framing effect on pastoralists’ beliefs about disease outcomes. Both communal grazing and prior illness are positively related to an increased probability of having antimicrobials on hand for livestock use. Prior livestock illness in the last month also is positively related to having more antimicrobials on hand. In turn, we find that communal grazing is positively related to the current number of sick animals, as is antimicrobial use. While we control for endogeneity using an instrumental variable approach, the positive relationship between antimicrobial use and current illness could reflect therapeutic antimicrobial use rather than a practice of using antimicrobials for prophylaxis (disease prevention).
There are externalities associated with both communal grazing and antimicrobial use. Although communal land tenure has important strengths as a property rights regime, especially in terms of risk management in volatile climate zones (Agrawal, Reference Agrawal2001; Ostrom, Reference Ostrom2015), it can incentivize overgrazing (Ciriacy-Wantrup and Bishop, Reference Ciriacy-Wantrup and Bishop1975; Runge, Reference Runge1981), and potentially be associated with disease transmission externalities to the extent that herds pass on disease to other herds sharing the communal grazing land. Antimicrobial use has two potentially offsetting effects that can be magnified by communal grazing. First, antimicrobial use that reduces the intensity and duration of pathogen shedding can reduce pathogen transmission to other herds, but it might also lead to a larger fraction of pathogen populations being antimicrobial resistant, leading to reduced effectiveness of future antimicrobial use. We show that through its potential to increase disease transmission rates, communal grazing may also exacerbate overuse of antimicrobials from an economic efficiency perspective.
Therefore, for optimal communal grazing and antimicrobial use in terms of economic efficiency, it is important to align the private benefits of communal grazing and antimicrobial use with the social benefits related to the two activities. Although our data do not allow examination of how antimicrobial use in this context influences development of antimicrobial resistance, understanding incentives for antimicrobial use in agropastoralist systems may help devise strategies to limit the emergence and persistence of antimicrobial resistance in these populations.









