Introduction
Nearly all the tropical and subtropical countries of the world maintain mosquito vector research, surveillance and control programmes, principally in relation to dengue, malaria, some arboviral encephalitis and mosquito-borne filariasis. Some of these diseases have resurged or have shown a significant increase in the number of cases over the last two decades, to such an extent that the World Health Organization (WHO) has called for improvements in research, as well as vector surveillance and control methods (Tun-Lin et al., Reference Tun-Lin, Kay and Barnes1995).
Ecological research on mosquitoes varies according to the different interests, objectives and opportunities of the researcher. Some researchers prefer to sample adult mosquito populations, but collection methods cannot be universally applied to all species due to differences in activity patterns (e.g. light traps do not collect diurnal species), or feeding preferences (e.g. human-bait only attracts anthropophilic species), and locating adult individuals in natural refuges is nearly impossible. Many researchers prefer to sample the aquatic larval stages because it is relatively easy to locate the appropriate water sources. Nevertheless, the standardization of samples has been a problem in relation to the number of dips taken according to the size of the aquatic habitat, the delimitation of the sample area and the number of larval development sites that should be sampled in relation to the area (Service, Reference Service1993).
Mosquito surveillance programmes are frequently based on sampling of mosquito larvae or pupae (Service, Reference Service1993). For dengue, the house, recipient, Breteau and other indexes are usually calculated despite the fact that no correlation has been detected between high index values and the incidence of cases of dengue in some studies (Bang et al., Reference Bang, Brown and Onwubiko1981; Tidwell et al., Reference Tidwell, Williams, Tidwell, Peña, Gwinn, Focks, Zaglul and Mercedes1990). The principal problem of these indexes is that they do not consider the density of the species at each aquatic site relative to the volume or area of water (Ibáñez-Bernal & Gómez-Dantés, Reference Ibáñez-Bernal and Gómez-Dantés1995). For malaria control programmes, the WHO Expert Committee (OPS, 1977) suggested taking ten or more dipper-samples at each breeding place; but, again, they did not consider the area of water present.
In this study, we present a method to obtain the mosquito species richness and abundance (diversity parameters) of use in ecological research and vector surveillance programmes. These parameters can only be compared between areas, seasons, or years, or before and after a specific control intervention, if the procedure is standardized. If a standardized procedure is used, it is possible to correlate the number of human cases of vector-borne disease with the presence and relative abundance of the vector mosquito species and, moreover, to correlate climate and other environmental parameters with mosquito population changes and disease outbreaks in order to apply preventative or control measures. We present an example of a case study, with the recommended methodology for richness, abundance and diversity analysis, and discuss its usefulness in basic research and vector surveillance.
Standardized sampling method
Design of study and areas to be sampled
Combinations of historical and ecological factors determine the specific faunistic composition in an area. It is possible to expect differences in mosquito diversity between sites with different altitudes, vegetation type, urban and natural areas, or types of breeding places. It is important to design the study considering the scale and objectives. For example, to determine the mosquito fauna in a geographical basin, representative zones can be selected within an altitude gradient; whereas, if the relationship between mosquito diversity and vegetation type or between urban and wild areas is of interest, it will be important to select corresponding representative areas. Moreover, from an epidemiological point of view, it is important to select human settlements that can be compared, to provide an estimate of the risk of transmission, e.g. localities with known cases of a mosquito-born disease vs. localities with little or no history of such disease. It is also useful to compare the same sample area at different times considering annual or seasonal fluctuations in climatic conditions, to obtain information on the richness, composition and abundance of vector species.
Delimitation of the sampling area
Diversity studies performed in different regions with distinct animal and plant assemblages have demonstrated one hectare (2.47 acres) to be a reliable sample area for quantitative ecological studies (Colwell & Coddington, Reference Colwell and Coddington1994; Vreugdenhil et al., Reference Vreugdenhil, Meyrat, House, Mateus, Stapf and Linarte2003). Based on preliminary studies performed by us in a costal-tropical area of Mexico with a great variety of mosquito aquatic habitats, we demonstrated that it is possible to sample meticulously all the aquatic habitats in a one hectare area with the effort of two people working for one day. Species accumulation curves, determinant coefficients and percentage of collected fauna were prepared or calculated from each day's sampling data. The hectare area was randomly selected to represent a specific homogeneous area to be evaluated over a period of time (Adler & Lauenroth, Reference Adler and Lauenroth2003). Nevertheless, the number of representative hectare sites to be sampled will depend on the total area of interest and the objectives of the study.
For urban areas, a map of the locality can be used to divide it into a grid of one hectare quadrants. Each quadrant is assigned a number, and a statistical randomization procedure is applied to select one or more quadrants for sampling. In the case that quadrants cannot be easily defined on a map, a single point may be selected randomly and used to define a one hectare area around it. In the case of natural, non-urban areas, the sampling area could be chosen taking into account hectare units that include all the typical and representative landscape elements, applying the same procedure as used to select the quadrants. Clearly, it is important to exclude a priori areas that are poor in aquatic habitats, like pasture fields, dunes, desert zones, etc. that are unsuitable for mosquito development.
Sampling effort
Inside each selected hectare area, all the existing aquatic habitats must be sampled. A commercial mosquito-dipper (330 ml) is taken to represent the unit effort. Additionally, a siphon collector for mosquito larvae is used for those habitats that are difficult to reach or in which the dipper could not be used, such as rock or tree holes. The volume of water extracted by siphoning needs to be the same as that of the dipper to guarantee that the collection effort will be the same. The results are expressed as the number of larvae per dipping-unit effort. Mosquito larvae are not homogeneously distributed among breeding sites, and this well-recognized problem has been discussed by Service (Reference Service1993). Consequently, in large bodies of water, the dipping procedure must be targeted at suitable mosquito microhabitats, such as the pool borders or protected zones with standing water, and should avoid sampling of open-waters.
Water collections must be sampled with a proportional number of dips in accordance with their respective sizes, as the number of species or individuals increases proportionally with the size of the water body (Service, Reference Service1993). To calculate the water body size, we recommend surface area as a measurement unit, instead of volume, because it is easier to calculate and permits a more reliable estimation of mosquito density, considering the need for the immature stages to breathe air. Standardization of the number of dips in accordance with the surface area of the water body is as follows: number of dips, water surface area (m2): 1, <0.25; 2, 0.26–1.0; 3, 1.1–3.0; 4, 3.1–5.0; 5, 5.1–7.0; 6, 7.1–9.0, and so on. If the number of dips in accordance with the size of the water body is then applied, the results of the sampling may be used for population comparisons using appropriate statistical tests. This sampling regime is based on the Arrhenius theory that describes the relationship between species number and area and the nested plot technique (Vreugdenhil et al., Reference Vreugdenhil, Meyrat, House, Mateus, Stapf and Linarte2003).
Field data
General information on the area under study, including biotic and abiotic parameters, should be recorded. Based on the sampling format proposed by Belkin et al. (Reference Belkin, Schick, Galindo and Aitken1967), we developed a format suitable for the collection of some useful information. The format includes four general aspects: (i) data on the locality or sampled area, (ii) water body quantitative parameters, (iii) water body qualitative parameters and (iv) the mosquito species and number of individuals obtained by sampling. All these data are integrated in a multivariate database.
This field schedule provides information on species richness, the relative abundance of each species and other environmental parameters. Species richness data record the species that are present in the area at one time, which is useful for species inventory studies and important to identify the presence of medically-important species. The relative abundance of each species is recorded, so it is possible to detect changes in population numbers between areas or seasons and correlate these changes with climate and epidemiological data of vector-borne diseases or compare the abundance of a vector before and after a control intervention. Data on species richness and abundance are required to estimate mosquito diversity. The environmental parameters can be evaluated independently to identify factors affecting the presence/absence or abundance of each species.
Suggested method for data analyses
The sample effort efficiency (SEE) can be evaluated by species accumulation curves (Colwell & Coddington, Reference Colwell and Coddington1994; Magurran, Reference Magurran1998; Moreno, Reference Moreno2001), or by using non-parametric estimators of species richness such as the Abundance-based coverage estimator (ACE) and Chao2 (Chao, Reference Chao1987; Colwell & Coddington, Reference Colwell and Coddington1994; Chazdon et al., Reference Chazdon, Colwell, Denslow, Guariguata, Dallmeir and Comiskey1998). To eliminate the effect of the sequence in which samples are added, the samples must be randomized using a larger number than the sample size, e.g. if there are 7000 specimens, the randomization number could be set to 10,000. This procedure can be performed simply using the EstimateS web-program (Colwell, Reference Colwell2005).
There are a number of criteria already employed in the literature (Soberón & Llorente, Reference Soberón and Llorente1993; Colwell & Coddington, Reference Colwell and Coddington1994), but species-richness abundance, as well as sample-species-richness abundance are more reliably calculated. Data fit to the model can be evaluated based on the coefficient of determination (R 2). After checking several types of curve fittings, data may be fitted to the Clench model (Fagan & Kareiva, Reference Fagan and Kareiva1997; Moreno & Halffter, Reference Moreno and Halffter2000). According to this model, the probability of finding more species increases over time and with increasing collection effort. This procedure has demonstrated to perform robustly under a variety of conditions and situations for a number of taxa (Jiménez-Valverde & Hortal, Reference Jiménez-Valverde and Hortal2003). The Clench model is expressed as Sn=an/1+bn, where a is the increase rate of species sampled from the beginning of the collection, b is the slope at the end of the curve, and n is the cumulative number of samples. Using the parameters a and b, the inventory quality (IQ=a/(1+bn)2) can be evaluated by examining the slope at the end of the curve, whereas the proportion of the recorded fauna (%RF) is calculated using the formula: %RF=S obs/(a/b), where S ob=observed richness. Finally, the theoretical number of species (TN) is extrapolated using the formula TN=(a/b) (Jiménez-Valverde & Hortal, Reference Jiménez-Valverde and Hortal2003).
Diversity analysis
The spatial unit is taken to be each of the single hectares sampled, and the alpha, beta and gamma diversity estimates (Whittaker, Reference Whittaker1972) are obtained according to the study objectives, for example, urban vs. natural areas, comparisons between areas with different altitudes or vegetation, etc. The temporal unit is considered to be each of the samples taken at different seasons in each hectare plot. Of course, data are more reliable if the study is conducted over a long period of time, but it is possible to make comparisons between seasons, years or before and after an insecticide application.
Alpha diversity
This community atribute can be usefully expressed using species richness (S), the Simpson index (D) and the alpha diversity Fisher's index (F) (Fisher et al., Reference Fisher, Corbet and Williams1943; Magurran, Reference Magurran1998; Moreno, Reference Moreno2001). Statistical differences of diversity between sample units are determined by the Solow test (Solow, Reference Solow1993), using the values of the Simpson index (D) obtained for each kind of habitat or season. The Simpson and Fisher indices, as well as the Solow test of the Simpson's index values, may be calculated by using the Species Diversity and Richness III 3.02 program (Pisces Conservation Ltd., 2006).
Beta diversity
This parameter may be calculated using the complementarity index (C AB) proposed by Colwell & Coddington (Reference Colwell and Coddington1994), which describes the rate of dissimilarity in the species composition between biota pairs. This is obtained in two different ways: (i) total richness for both unit samples combined or (ii) the number of species present in both unit samples. Results can be expressed as a simple percentage (Moreno, Reference Moreno2001).
Gamma diversity
It is possible to express gamma diversity in different forms (Moreno, Reference Moreno2001); but, like species richness, this parameter is useful in providing a general measurement of the landscape (Hulbert, Reference Hulbert1971). It is expressed as the overall number of species in the landscape. Whittaker curves of abundance richness are plotted to help in the description of gamma diversity (Hulbert, Reference Hulbert1971; Magurran, Reference Magurran1998).
An example of a study case
To illustrate the application of this methodological process, we have described an example using data obtained from a mosquito fauna study performed on the Gulf-coast of Mexico. La Mancha (LM) is a coastal locality in Veracruz State, Mexico (96°36′N, 96°22′W), where dengue outbreaks have been recorded regularly. No previous studies of the mosquito fauna of this area had been performed; however, we expected to observe a high number of species and high abundance because of the warm and humid climatic conditions, with summer rainfall, an annual precipitation of 1200–1500 mm and an annual mean temperature of 22–26°C. Dominant winds come from the north (Moreno-Casasola, Reference Moreno-Casasola1982). Between November and February the climatic conditions change, with a decrease in temperature, scarcity of rain and often high wind speeds (Castillo & Carabias, Reference Castillo and Carabias1982). The vegetation is semi-evergreen forest, tropical deciduous forest, mangrove, dune vegetation and swamp forest, ranging in altitude from 20 to 1150 m a.s.l. (SECOMVER, Reference Esparza2000).
Selection of the sampling area
Two landscape units were determined in LM: an urban zone (UZ), inside the town, and a wild zone (WZ), consisting of a natural patch of semi-evergreen forest. Mosquito sampling was conducted on six occasions during two years; two samples were taken in each season: rainy season (RS, in September 2004 and 2005), cold season (CS, in January 2005 and 2006) and dry season (DS, in May 2005 and 2006). Diversity analyses were performed considering the following landscapes: (i) as a whole, (ii) the urban zone (UZ) and (iii) the wild zone (WZ), independently; and considering the season: (i) as a whole, (ii) rainy season (RS), (iii) cold season (CS) and (iv) dry season (DS). One hectare sampling areas were chosen to be representative of the UZ and the WZ. In both zones, all the water bodies were located and sampled, except for a few tree holes that were impossible to sample due to their height in trees. Each sampling unit consisted of a 330 ml dipper. Each one of the water bodies was sampled with a number of dips in proportion to the water surface area. The field data and water body general characteristics were recorded. All collected larvae were preserved for taxonomical identification and quantification in the laboratory (Ibáñez-Bernal & Martínez-Campos, Reference Ibáñez-Bernal and Martínez-Campos1994).
Sample effort efficiency (SEE)
To explore the potential of this method, the SEE was checked two different ways. (i) Using the data from one month of sampling (September, RS) that included the evaluation of one hectare areas for each of the urban and natural zones, as well as the complete area. In this case, evaluation of the dipping unit effort was achieved by fitting data to the Clench model. (ii) All data from both sampling years were included and the non-parametric ACE and Chao2 estimators were calculated in addition to the Clench model. In both cases, the SEE was evaluated for LM as a complete area, as well as for the UZ and WZ, following all the methods recommended above, except where otherwise specified.
Results
A total of 2985 culicid larvae were collected, belonging to 24 species and eight genera. The dominant species were Culex coronator Dyar and Knab, 1906 (23.58%), Cx. nigripalpus Theobald, 1901 (19.33%), Aedes scapularis (Rondani, 1848) (15.48%), Cx. quinquefasciatus Say, 1823 (14.44%), Deinocerites cancer Theobald, 1901 (5.63%), Ae. aegypti (Linnaeus, 1762) (4.72%) and Cx. iolambdis Dyar, 1918 (4.72%) (table 1).
Table 1. Mosquito species abundance from La Mancha, Veracruz State, Mexico. Data from two years of sampling (September 2004 to May 2006).

Ab=total abundance; %=percentage of abundance.
Key, abbreviated name of species.
In September (RS), 14 species were collected; nine species from UZ and six from WZ. According to the Clench model, the total number of species that might have been collected was 17.3 for the complete area (LM), 10.21 from UZ and 8.02 from WZ. Thus, the percentage of fauna collected consistently exceeded 70% of the total fauna estimated to be present (i.e. LM=80.92, UZ=88.0 and WZ=74.77). The species accumulation curves, based in the Clench model, show an asymptotic tail for LM, UZ and WZ (fig. 1). The coefficient of determination (R 2) was close to 1.0 in each of the three cases (LM=0.99, UZ=0.98 and WZ=0.99), indicating a close fit of empirical data to the Clench model. The IQ for the three cases (LM, UZ and WZ) was 0.06, suggesting an accurate and reliable species inventory.

Fig. 1. Species accumulation curves with Clench model. September data (rainy season). (a) La Mancha as a whole, (b) urban zone and (c) wild zone.
LM (UZ+WZ)
In total, 24 species were captured. Following the Clench model estimates, a total of 28.5 species might have been captured. The capture, therefore, represents 84.0% of the estimated total number of species present. In the case of ACE and Chao2 estimates, 100% of species were obtained (24 species). For the whole area (UZ+WZ), the three species accumulation curves, based on Clench, ACE and Chao2, showed a clear asymptotic tendency (fig. 2). The coefficient of determination (R 2=0.99) indicated an excellent fit of data to the Clench model. The IQ=0.04 value supports the concept of a reliable inventory.

Fig. 2. Species accumulation curves of La Mancha as a whole by (a) Clench model and (b) ACE and Chao2 estimators.
Urban zone (UZ)
A total of 16 species were collected in the urban zone. According to the Clench model, 18.8 species might have been collected, so we obtained 84.7% of the estimated total number of species. Using ACE and Chao2, we obtained 100% of the estimated number of species (16 species). The three species accumulation curves, based on Clench, ACE and Chao2, all showed an asymptotic tendency (fig. 3). The coefficient of determination (R 2=0.98) indicated close agreement between our data and the model, and the inventory quality value (IQ=0.05) was considered reliable.

Fig. 3. Species accumulation curves of the urban zone of La Mancha by (a) Clench model and (b) ACE and Chao2 estimators.
Wild zone (WZ)
A total of 16 species were collected in the WZ. According to the Clench model, 19.5 species might have been collected, so 82.2% of the estimated total number of species was collected. The ACE estimate indicated that 100% of the species were collected (16.4), whereas the Chao2 estimate (N=15) was lower than the actual number of species collected. The three species accumulation curves, based on the Clench, ACE and Chao2 models, all tended to an asymptote (fig. 4). The coefficient of determination (R 2=0.99) indicated close agreement between data and model values and the inventory quality value (IQ=0.08) indicated that the species inventory was accurate and reliable.

Fig. 4. Species accumulation curves of the wild zone of La Mancha by (a) Clench model (b) ACE and Chao2 estimators.
Spatial diversity
The regional richness (S) of mosquitoes in LM was 24 species (gamma diversity). The UZ and the WZ each had a species richness of 16 species. The Simpson index (D) diversity value for LM was 6.76 and the Fisher index (F) value was 3.56. The diversity of the WZ was slightly greater in both indexes (D=4.30; F=2.59) compared to the UZ diversity values (D=4.04; F=2.43). This difference was found to be not significant when a Solow test was applied to compare the two zones (P=0.28). Eight species were shared between zones (C AB=0.67), namely, Culex nigripalpus, Aedes scapularis, Deinocerites cancer, Cx. iolambdis, Haemagogus regalis Dyar and Knab, 1906, Ae. taeniorhynchus Wiedemann, 1821, Ae. aegypti and Anopheles albimanus Wiedemann, 1820 (fig. 5).

Fig. 5. Abundance-richness of urban and wild zones of La Mancha.
Temporal scale
Sampling seasons
The highest species richness (S) was recorded in the RS with 18 species; whereas a lower richness (11 species) was recorded in the CS and DS (table 2). The Solow test detected highly significant differences between the sampling seasons comparisons (P=0.0001). Beta diversity between the sampling seasons varied more than 50%, with the RS sharing the highest number of species (8 spp.) with CS and DS (table 2). Each of the sampling seasons had a number of exclusive species, although some species, like Cx. quinquefasciatus and Cx. coronator, were present and abundant in all seasons. Other species, like Ae. aegypti, appeared in the DS and their abundance increased in the RS (fig. 6).

Fig. 6. Abundance-richness of La Mancha according to season.
Table 2. Temporal diversity. Data from two years of sampling (September 2004 to May 2006).
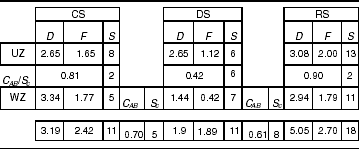
D=Simpson index; F=alfa diversity Fisher's index; S=species richness.
C AB/S c=beta diversity; S c=shared richness.
UZ=urban zone; WZ=wild zone.
The highest diversity was found at the UZ in the RS and DS, whereas diversity was highest in the WZ during the CS. In all cases, the Solow test detected highly significant differences between comparisons of UZ and WZ in each season (P=0.0001). The DS was the season with the greatest number of shared species (six species). On the other hand, the RS, with the highest species richness and abundance, shared only two species between zones (fig. 7).

Fig. 7. Abundance-richness of urban and wild zones of La Mancha according to season.
Abundance
Based on the data presented in table 1, it is possible to identify those species of medical importance as anthropophilic species and vectors of human pathogens. The number of individuals of every species was recorded, and it is possible to analyze changes in species abundance over seasons (figs 6 and 7). In this way, the site and the moment in which significant population increases occur (fig. 5) can be identified. Moreover, it is possible to correlate abundance with epidemic outbreaks. If this kind of study is made prior and posterior to a mosquito intervention programme, it would be possible to evaluate accurately the efficacy of the control measures. Taking advantage of epidemiological data recorded by a dengue monitoring programme in Veracruz State, it is possible to correlate the abundance of the mosquito vector with increases in the number of cases of dengue and to correlate these phenomena with other climatic factors that may be used as indicators of epidemiological risk. As an example, a dengue outbreak was recorded in September 2005 in LM, with a total of six cases (L. Hidalgo, 2006, personal communication). Comparing this event with the abundance of Ae. aegypti between seasons (see Aeae; fig. 7), we can see that the vector population increased during this period (UZ in RS), concurrent with the appearance of the disease in humans.
Discussion
The design of an easy and effective method to assess the diversity of mosquitoes that could help determine not only key components of the community but also the abundance of each species, particularly those involved in the transmission of pathogens, is a well recognized problem (see Service, Reference Service1993). Researchers commonly design sampling methods based on a set of special circumstances that meet their particular objectives (García & Micieli, Reference García and Micieli2000; Berti et al., Reference Berti, Gutiérrez and Zimmerman2004; Pérez-Pacheco et al., Reference Pérez-Pacheco, Rodríguez-Hernández, Lara-Reyna, Montes-Belmont, Ramírez-Valverde and Martínez-Martínez2004; Rubio-Palis et al., Reference Rubio-Palis, Menare, Quinto, Magris and Amarista2005), but results can be extremely difficult to compare between different regions or over time. Nearly all vector programmes are focused on one or a small number of species, and data are often expressed using indexes that do not consider vector abundance, resulting in a loss of reliability and generality (OPS, 1995).
The difficulties of standardization in mosquito sampling begin with some operational aspects and conclude with some theoretical problems. The different types of dippers used affect the samples because the sample volume usually varies according to the model (Service, Reference Service1993). It is very common that, in developing countries, the dippers vary greatly depending on the specialist and the research project, resulting in data that are difficult or impossible to compare.
The accurate numeric representation of a mosquito larval population depends on the appropriate attributes of the dipper and the standardization of the number of dips taken, according to the size of the water body. The number of unit samples, taken from each water body, is another aspect that must be considered, because this affects abundance data. There are many examples in which the rationale for the number of dips per water body appears to lack any foundation (Pérez-Pacheco et al., Reference Pérez-Pacheco, Rodríguez-Hernández, Lara-Reyna, Montes-Belmont, Ramírez-Valverde and Martínez-Martínez2004; Rubio-Palis et al., Reference Rubio-Palis, Menare, Quinto, Magris and Amarista2005). Some works are based on the sampling of each water container by means of only one dip, no matter their size (Calderon-Arguedas et al., Reference Calderon-Arguedas, Troyo and Solano2004), whereas others present variation based on ten dips (SSA/OPS, 1989; Oria et al., Reference Oria, Stein and Gorodner2000), 30 (Rubio-Palis et al., Reference Rubio-Palis, Menare, Quinto, Magris and Amarista2005), 50 (Berti et al., Reference Berti, Gutiérrez and Zimmerman2004), or 100 dips (García & Micieli, Reference García and Micieli2000). Other researchers may suggest taking dips during different periods of time (Walter et al., Reference Walter, Valle, Naupay, Tineo, Rosas and Palomino2003). In the case of surveillance programmes, no systematized collection method has been recommended. The intention of the present study was not to obtain the absolute density of species but a standardized relative abundance that could be compared among areas, or in the same area, at different times of the year.
The systematization of the number of dips in accordance with the water collection surface area results in an accurate assessment of larval density. The variability of the micro-environmental condition and their influence on larval distribution in the water body has been discussed by Service (Reference Service1993). It is often impossible to determine the water volume in a natural water body, so it is better to estimate its surface area, which is a parameter of biological relevance given the air-breathing habits of mosquito larvae.
Another problem is the number of water bodies that should be sampled in a faunistic study. For anopheline species that usually breed in river pools or lakes, the water bodies are usually sampled without determining the area in which they are located (Tineo et al., Reference Tineo, Medina and Fallaques2003; Berti et al., Reference Berti, Gutiérrez and Zimmerman2004). In contrast, in dengue vector surveillance programmes, it is common to consider a predetermined proportion of houses in search of water containers (Rojas et al., Reference Rojas, Soca, Mazzarri, Sojo and Poleo2003), but these houses are not delimited within a sampling area and are normally selected based on the subjective criteria of the collector.
In other studies dealing with diversity indexes for mosquitoes, lack of adequate sample sizes resulted in data that were unsuitable for analysis (Marquetti et al., Reference Marquetti, González, Aguilera and Navarro1999). There are few studies in which the sample area has been delimited for Aedes surveillance. One good example is the work of Stein et al. (Reference Stein, Oria and Almirón2002), in which all the water bodies within a one hectare area were examined and all the larvae in each and every container were quantified. We consider this procedure to be excessive because it is impossible to know the absolute density of a cohort and, moreover, unnecessary. A comparable estimation of the density of one or several species at different times or areas is desirable, as an estimation of the state or condition of the population of a particular species. Delimiting a hectare area as a sampling unit has some benefits. (i) It is possible to examine all the water bodies present using limited manpower (people-hours). (ii) It is possible to count the total number of breeding sites that indicate the likely epidemiological situation in the zone. (iii) All or most of the species present in an area are likely to be included in the sampling procedure and, combined with standardization of dipping. (iv) It allows estimation of the relative abundance of each species in relation to other species or to the total numbers of larvae captured. (v) It provides comparable data on changes in species abundance over time. (vi) Comparisons can be made among the faunistic data of different localities. (vii) The efficacy of mosquito control measures can be evaluated and compared by sampling before and after a specific intervention.
Aside from the usefulness of the method for mosquito inventories and ecological research, this method is likely to be of value for periodic surveillance in mosquito vector control programmes, principally because it includes an estimation of the mosquito density, currently absent in the indexes used worldwide. All the environmental and habitat parameters obtained can be analyzed for each species to identify the type of breeding container, the physico-chemical demands of the different species and the principal factors that determine the abundance and composition of mosquito populations.
Acknowledgements
F.S. Mendoza Palmero received a doctorate scholarship (171 223) from CONACyT for studies performed in the Instituto de Ecología A.C. (INECOL, Mexico). This work was partially financed by the Servicios de Salud de Veracruz (SESVER) and by project 902-07-816 (S. Ibáñez-Bernal) in the Departamento de Biodiversidad y Ecología Animal, INECOL. We thank Rogelio Macías (INECOL), Victor Parra (UADY, Mexico), Lawrence J. Hribar (Florida Keys Mosquito Control District, USA), and Trevor Williams (INECOL) for useful comments on an earlier version of the manuscript. We also thank Ruth H. Xoliot, Leopoldo Hidalgo, Ricardo Bello, Francisco Del Angel, Francisco Luna and Israel Villa (SESVER, Mexico), and Imelda Martínez, Alberto Rísquez and Darío Navarrete (INECOL) for additional support.











