Introduction
Polyphenism is a general phenomenon in aphids. Different morphs appear during the life-cycle of aphids (Dixon, Reference Dixon1998), and such polyphenism has been reported for many species (Kawada, Reference Kawada, Minks and Harrewijn1987; Dixon, Reference Dixon1998). Because the different morphs are genetically identical during the parthenogenetic phase, aphid polyphenism is a case of phenotypic plasticity whereby environmental conditions, often perceived by the mother, influence the phenotype (Dixon, Reference Dixon1998). The environmental factors of crowding, temperature and host plant quality were found to be particularly important for induction of winged forms (Kawada, Reference Kawada, Minks and Harrewijn1987; Dixon, Reference Dixon1998). In addition, natural enemies have been found to induce production of winged offspring in pea aphid, Acyrthosiphon pisum (Dixon and Agarwala, Reference Dixon and Agarwala1999; Weisser et al., Reference Weisser, Braendle and Minoretti1999; Sloggett and Weisser, Reference Sloggett and Weisser2002; Kunert and Weisser, Reference Kunert and Weisser2003), and the cotton aphid, Aphis gossypii (Mondor et al., Reference Mondor, Rosenheim and Addicott2005). At least for pea aphids, wing induction caused by natural enemies seems to be a general phenomenon since they react to important enemies such as ladybird adults and larvae, parasitoids, lacewing larvae and hoverfly larvae (Weisser et al., Reference Weisser, Braendle and Minoretti1999; Sloggett and Weisser, Reference Sloggett and Weisser2002; Kunert and Weisser, Reference Kunert and Weisser2003). For the cotton aphid, so far only the influence of the convergent ladybird, Hippodamia convergens Guérin-Méneville, has been tested (Mondor et al., Reference Mondor, Rosenheim and Addicott2005).
For the black bean aphid, Aphis fabae, and the vetch aphid, Megoura viciae, wing induction caused by natural enemies was tested by exposing aphids to the odour of the predator-aphid complex or the tracks left by predators, but no wing induction was observed (Dixon and Agarwala, Reference Dixon and Agarwala1999). The authors attributed this to the fact that these aphids are toxic or of poor quality to the predator (Adalia bipunctata Linnaeus) used in the experiment (Hodek and Honěk, Reference Hodek and Honěk1996). However, since both aphid species were attacked and killed by the predator (Dixon and Agarwala, Reference Dixon and Agarwala1999), this explanation seems to be not very convincing. If the presence of a predator indicates that there is also some future risk of being attacked on the same plant, by the same predator individual or by other predators or parasitoids, wing induction can be an adaptive strategy to reduce the future risk of predation (Weisser, Reference Weisser, Woiwod, Reynolds and Thomas2001).
As Dixon and Agarwala (Reference Dixon and Agarwala1999) stated for A. fabae, it might be important that this species is frequently ant-attended and, therefore, better protected against natural enemies. Thus, it may not need to escape from predators by inducing the winged morph. Dixon and Agarwala (Reference Dixon and Agarwala1999) exposed target aphids only to traces left by natural enemies. However, if there are other cues involved in wing induction, as is known for the pea aphid (Kunert et al., Reference Kunert, Otto, Röse, Gershenzon and Weisser2005), wing induction may not be found with the experimental design used by Dixon and Agarwala (Reference Dixon and Agarwala1999).
Here, we examine whether M. viciae and A. fabae react with wing induction to natural enemies if they are exposed to all possible cues involved with predation in a situation where aphids are confronted directly with a natural enemy. Chrysoperla carnea is a highly polyphagous species, which has a normal larval development if fed with M. viciae (New, Reference New, Minks and Harrewijn1988). In the experiments, colonies of M. viciae and A. fabae were, therefore, confronted with C. carnea to test for natural enemy-induced wing production.
Material and methods
Experimental animals and plants
For the experiments, two aphid species, A. fabae and M. viciae, were used. Both aphid species were originally collected near Jena (Thuringia, Germany) on Vicia faba Linnaeus and V. sepium Linnaeus (Fabaceae), respectively. One aphid per species was used to start the culture. Both aphid species were then reared and studied on three-week-old plants of V. faba (variety ‘The Sutton’, Nickerson-Zwaan, UK). To prevent aphids and predators from escaping, each plant was caged in an air-permeable cellophane bag.
Second-instar larvae of C. carnea were used as predators. They were obtained from a commercial supplier (Katz Biotech Services, Welzheim, Germany) and reared on aphid-infested bean plants until they reached the second larval stage. C. carnea larvae used for the experiment with M. viciae were fed with M. viciae, larvae used for the A. fabae experiment were fed with A. fabae.
Plants, aphids and lacewing larvae were raised under long-day conditions (16:8, L:D) in climate chambers at 20°C and 75% RH. Because even air- and water-permeable cellophane bags reduce air movement, it is likely that plants and animals caged in these bags were exposed to higher values of relative humidity.
Experimental design
Three experiments were conducted, one with A. fabae and C. carnea and two with M. viciae and C. carnea. All three experiments were carried out in the same way and under the same conditions as the rearing of plants and insects. To minimize maternal effects, a ‘split-brood-design’ was used. Therefore, aphid lines were established which emanated from one aphid each. Each line was then used as one replication consisting of one plant with the predator (treatment) and one control plant. For the A. fabae experiment and the first M. viciae experiment, 15 lines of aphids were established. For the second M. viciae experiment, 22 lines were established. To initiate each line, one single adult aphid was placed on a bean plant and allowed to reproduce for three days. After this time, the adult aphid was removed and the offspring were reared until they reached the last nymphal (fourth) instar, or early adult, stage. Then, they were transferred to new plants (three per plant: A. fabae experiment, three plants; M. viciae experiments, two plants), where they could reproduce for another three days and were then taken off the plant. The offspring were reared until the fourth instar before they were used for the experiment.
In the first M. viciae experiment, each line consisted of 20 fourth instar aphids, ten on each control plant and ten on each predator treatment plant. In the second M. viciae experiment, 15 aphids were used for each control plant and predator treatment plant, respectively. In the A. fabae experiment, 35 aphids were used on each control and plant predator treatment plant. One lacewing larva was added to each predator treatment plant. After three days, which represents the first part of the experiment, the remaining adult aphids were counted and transferred to new plants (surviving adult aphids from one plant were transferred together to a new plant). The offspring remained on the plant until they reached the adult stage. In the predator treatment, the lacewing larvae were removed at the point of adult aphid transfer and new second instar lacewing larvae placed onto the new predator treatment plants (one per plant). The adult aphids and predators stayed on the new plants for another three days, the second part of the experiment. After the three days, adult aphids were taken off the plant and counted. Predators were also removed. The offspring were again reared until adulthood. The offspring of both experimental parts were taken off the plant when they reached the adult stage and were frozen for later counting and morph determination.
Statistics
The results are presented as mean±standard error. For the comparison of the number of aphids a paired t-test was used after testing for normality. If data were not normally distributed they were square root transformed. After that the data fulfilled the requirements of a parametric test, and the paired t-test was performed using the transformed data. When data transformations were necessary, these are mentioned in the relevant result section. A linear regression was utilized to analyze the relationship between the number of aphids and the percentage of winged offspring. For both tests, the software package SigmaStat for Windows, version 2.03 was used.
The effect of aphid number and presence of the predator on the proportion of winged offspring were analysed using a generalized linear model (glm). In order to deal with overdispersion, a quasibinomial distribution was used in the models instead of a binomial distribution. Whenever possible, models were simplified by removing non-significant terms (Crawley, Reference Crawley2002). The analyses were done using the statistical program R, version 2.0.1 (Venables et al., Reference Venables and Smith2002). The tests used are indicated with the results.
Results
Megoura viciae
Number of surviving aphids
Experiment with ten initial aphids per plant
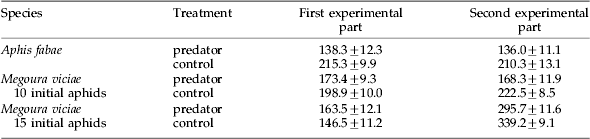
In both experimental parts, the total number of surviving aphids was lower in the predator treatment than in the control (table 1). In the second experimental part, the difference was significant (paired t-test: sqrt transformed data, t=−4.332, P⩽0.001), while the difference in the first experimental part just failed the significance level of 5% (paired t-test, t=−2.116).
Table 1. Mean (±SE) number of surviving aphids (adults and offspring) after the first and second parts of all experiments.
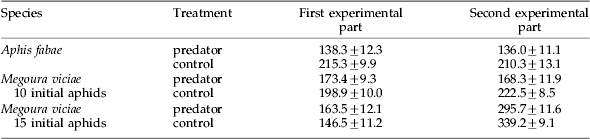
Experiment with 15 initial aphids per plant
The number of all surviving aphids was not significantly different in the first experimental part (paired t-test, t=1.418). However, in the second experimental part, the number of all surviving aphids was significantly lower in the predator treatment than in the control (paired t-test, t=−4.357, P⩽0.001; table 1).
Wing induction
Experiment with ten initial aphids per plant
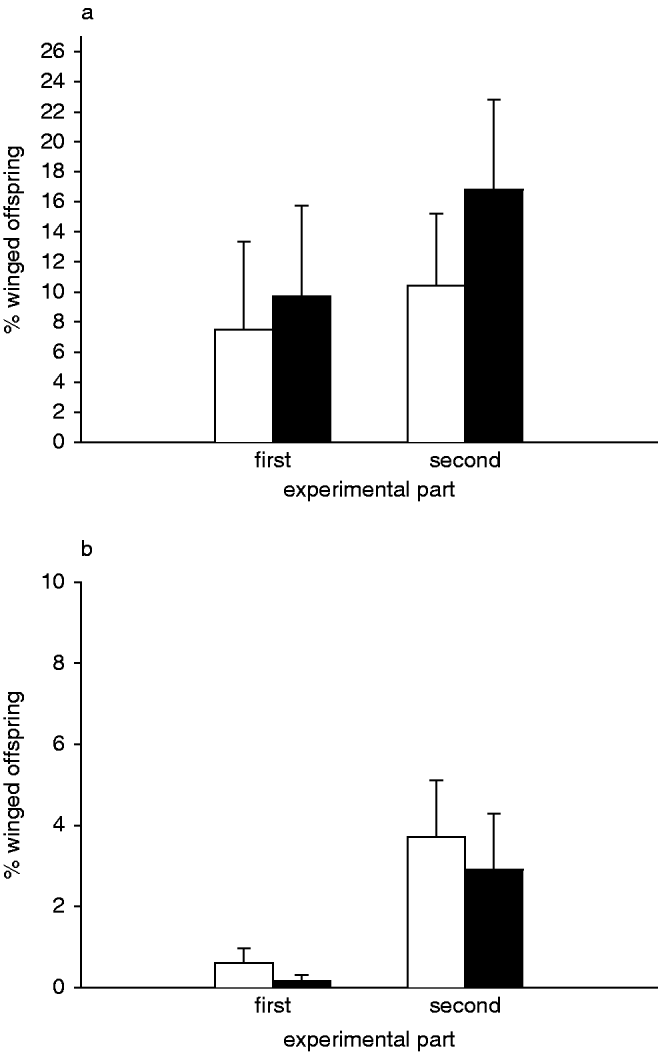
In both experimental parts, there was no significant effect of the presence of the predator or the number of aphids at the end of an experimental part on the percentage of winged offspring (glm: predator presence: first experimental part, t=0.959; second experimental part, t=0.041; aphid number: first experimental part, t=1.105; second experimental part, t=−1.689; fig. 1a).
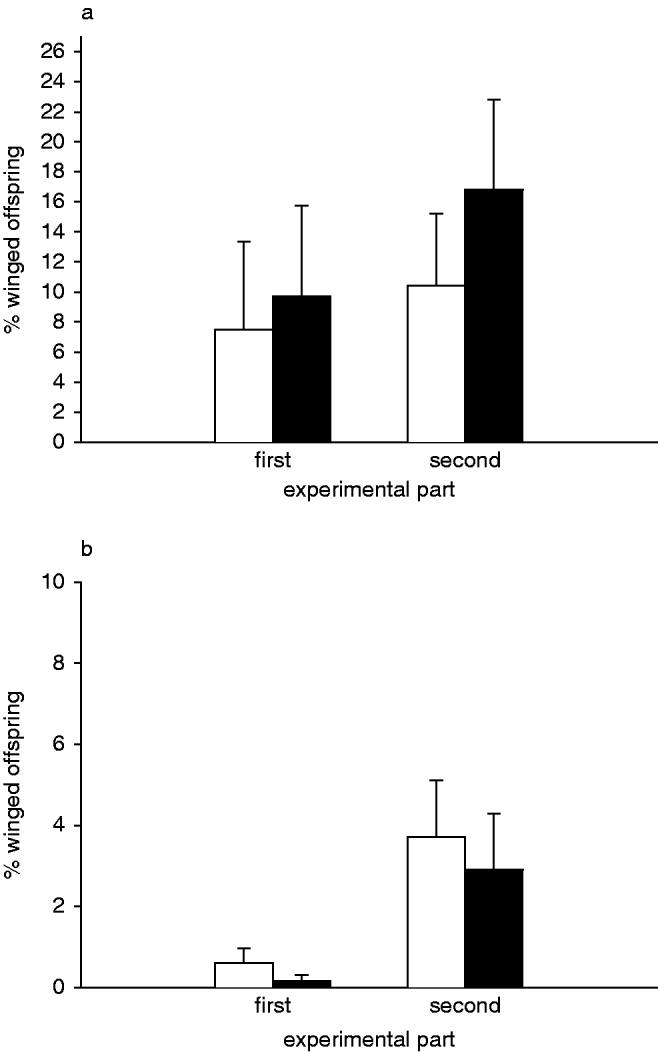
Fig. 1. Percentage of winged morphs of Megoura viciae among the offspring produced during the experiment with (a) ten initial aphids; and (b) 15 initial aphids (first experimental part, days 1–3; second experimental part, days 4–6) (□, control; ■, predator treatment).
Experiment with 15 initial aphids per plant
As in the first M. viciae experiment, the percentage of winged offspring was neither dependent on the presence of the predator (glm: first experimental part, t=−1.237; second experimental part, t=−0.550) nor on the number of aphids at the end of each experimental part (glm: first experimental part, t=−1.030; second experimental part, t=−0.163; fig. 1b).
Aphis fabae
Number of surviving aphids
In both experimental parts, the number of surviving aphids (adults and offspring) was significantly higher on the control plants than on the predator treatment plants (paired t-test: first experimental part, sqrt transformed data, t=−7.979, P⩽0.001; second experimental part, t=−11.061, P⩽0.001; table 1).
Wing induction
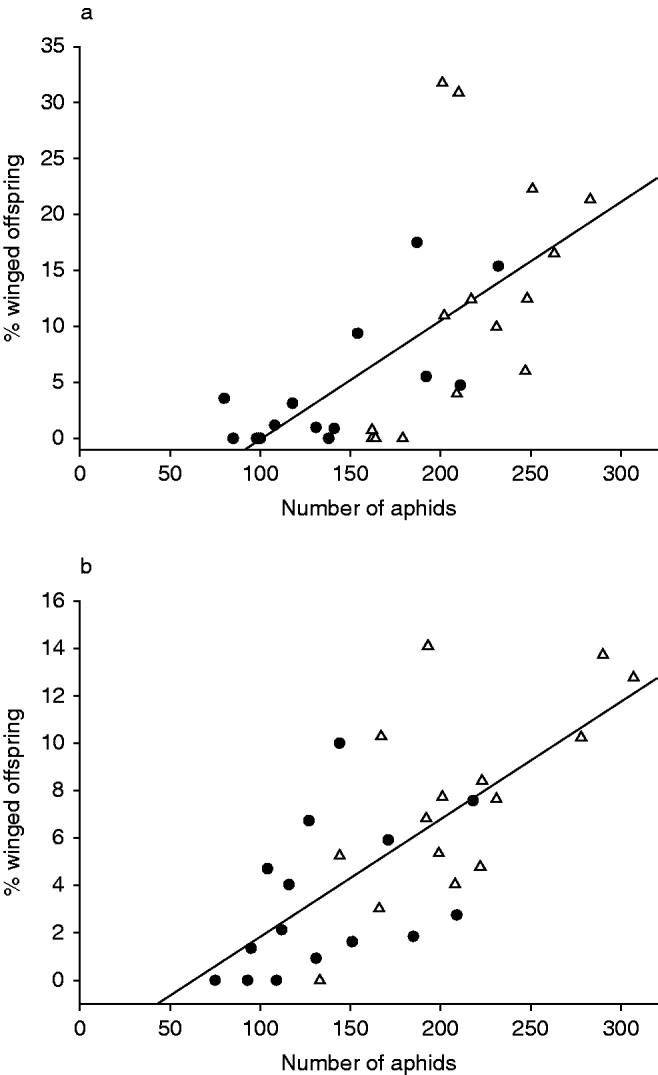
During both experimental parts, the percentage of winged offspring was lower in the predator treatment than in the control. However, only the number of aphids had a significant influence on the proportion of winged offspring (glm: first experimental part, number of aphids, t=3.644, P=0.001; presence of predator, t=−0.136, n.s.; second experimental part, number of aphids, t=4.799, P<0.001; presence of predator, t=−1.153, n.s.). A linear regression showed that in both experimental parts the percentage of winged offspring is dependent on the number of aphids (first experimental part, r²=0.429, P⩽0.001, F=21.067; second experimental part, r²=0.505, P<0.001, F=28.509; fig. 2).
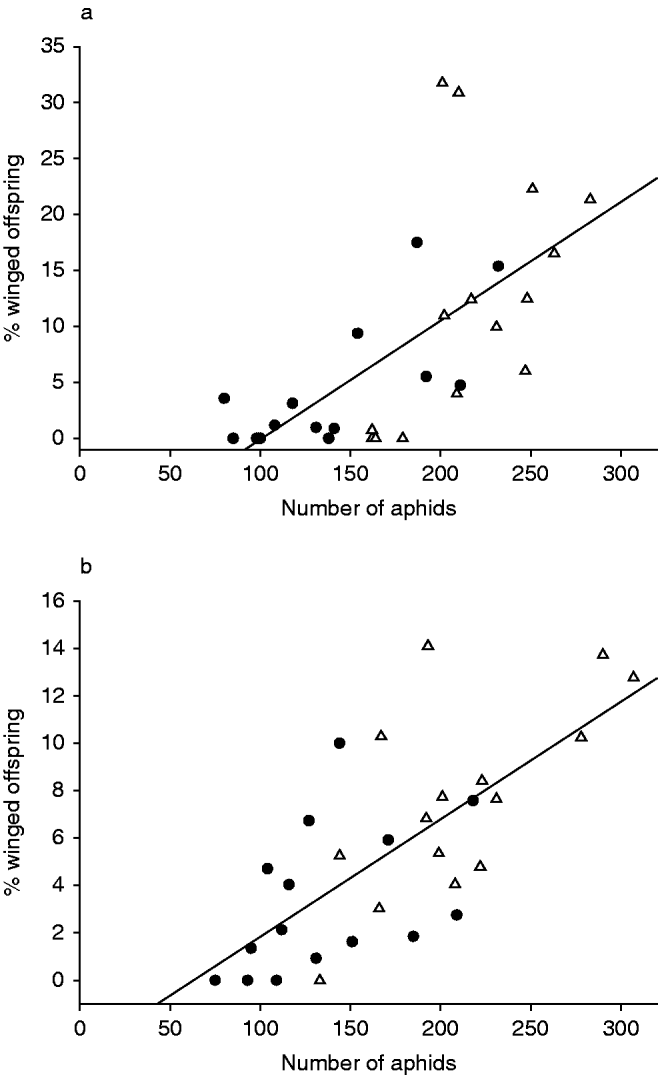
Fig. 2. The relationship between the numbers of surviving black bean aphids (Aphis fabae) after an experimental part and the percentage of winged offspring produced during this period. (a) First experimental part, % winged offspring=(0.106×number of aphids)−10.678; (b) second experimental part, % winged offspring=(0.050×number of aphids)−3.120 (●, predator treatment; △, control).
Discussion
The results clearly show that, in M. viciae and A. fabae, the presence of natural enemies does not lead to a higher percentage of winged morphs among the offspring, in agreement with the findings of Dixon and Agarwala (Reference Dixon and Agarwala1999). Thus, even when a true predator of the two species is used and when all cues associated with predation are available in the aphid colony, the aphids do not increase the proportion of winged morphs among the offspring. An alternative explanation – that winged morphs are induced in the presence of the lacewing predator but that there is selective predation by lacewing larvae on the induced alatiform nymphs, resulting in no apparent increase in winged morphs at the adult stage – is unlikely. We have carried out extensive tests with pea aphids and have found no selectivity in C. carnea larvae for either alatiform or apteriform nymphs (Kunert et al., unpublished data). In any case, the preference of lacewing larvae would have to be very strict to completely override such a wing induction effect.
In the following, the results of the experiments will be discussed species by species. In the M. viciae experiments, neither the presence of the predator nor the number of aphids on a plant, i.e. the aphid density, had a significant influence on wing induction. The predator was active as indicated by the on-average reduced aphid number in the predator treatments. Our result appears to be contrary to experiments carried out by Lees (Reference Lees1967), who found that M. viciae reacted to crowding with wing induction, which formed the basis of his hypothesis that a tactile stimulus is the important cue for wing induction. In our experiments, aphid densities were lower than in Lees' (Reference Lees1967) experiment and the contact rate was likely to be higher in Lees' work, since he caged aphids in small specimen tubes. But even with the higher density of 15 aphids, which corresponds to the pea aphid densities in previous wing induction experiments (Kunert and Weisser, Reference Kunert and Weisser2003), we found no influence of aphid density on wing induction. Together, it appears that different aphid species, or even clones within a species, differ in their sensitivity to natural enemies, as well as in their sensitivity to contact.
For A. fabae, the situation is different. The proportion of winged offspring of A. fabae was positively correlated with aphid density, confirming findings by Shaw (Reference Shaw1970). Contrary to M. viciae, A. fabae is very often ant-attended. Wing induction in the presence of natural enemies may not be very plausible in ant-attended species, as winged morphs that leave the natal plant would lose the advantages provided by the ants present on the current plant, such as protection against predators and parasitoids. Furthermore, it was found that ants react to the aphid alarm pheromone, trying to protect the aphid colony from natural enemies (Nault et al., Reference Nault, Montgomery and Bowers1976), making leaving after predator attack even less likely. However, wing induction caused by natural enemies could be found in A. gossypii, an aphid species which is also ant-attended (Mondor et al., Reference Mondor, Rosenheim and Addicott2005). Thus, a simple relationship between ant-attendance and enemy-induced wing induction cannot be assumed.
The number of aphid species tested for wing induction due to natural enemies is now four, and it appears that particular aphid-predator interactions may play a role in aphid polyphenism. It might be possible that conditions such as aphid density, predator consumption rates and aphid species-specific sensitivity to contact or disturbance play important roles in the induction of winged morphs by natural enemies. Further investigations of aphid wing induction by natural enemies are, therefore, required.
Acknowledgements
We thank Ingrid Jakobi for help with the rearing of plants and animals and Katz Biotech Services for providing lacewing larvae. This study was funded by grant Nr. WE 2618/2-2 of the Deutsche Forschungsgemeinschaft (DFG).