INTRODUCTION
Parkinson’s disease (PD) is a progressive neurologic disease affecting over a million Americans and over ten million people worldwide (Parkinsons Disease Foundation, 2016). While the cardinal symptoms of PD (tremor, rigidity, bradykinesia, and postural instability) are motoric in nature, individuals with PD also suffer from non-motor symptoms, many of which adversely affect quality of life (Weerkamp et al., Reference Weerkamp, Tissingh, Poels, Zuidema, Munneke, Koopmans and Bloem2013), such as changes in cognition and mood (Barone et al., Reference Barone, Antonini, Colosimo, Marconi, Morgante, Avarello and Ceravolo2009; Martinez-Martin, Rodriguez-Blazquez, Kurtis, & Chaudhuri, Reference Martinez‐Martin, Rodriguez‐Blazquez, Kurtis and Chaudhuri2011).
As many as 80% of individuals with PD develop cognitive impairments (Aarsland, Andersen, Larsen, Lolk, & Kragh-Sorensen, Reference Aarsland, Andersen, Larsen, Lolk and Kragh-Sorensen2003; Cummings, Darkins, Mendez, & Hill, Reference Cummings, Darkins, Mendez and Hill1988; Pirozzolo, Hansch, Mortimer, Webster, & Kuskowski, Reference Pirozzolo, Hansch, Mortimer, Webster and Kuskowski1982), which do not reliably respond to available pharmacological and surgical treatments (Fournet, Moreaud, Roulin, Naegele, & Pellat, Reference Fournet, Moreaud, Roulin, Naegele and Pellat2000; Owen, Iddon, Hodges, & Summers, Reference Owen, Iddon, Hodges and Summers1997; Skeel et al., Reference Skeel, Crosson, Nadeau, Algina, Bauer and Fennell2001). Although many cognitive domains are impacted by PD, the current study focuses on a few domains that are frequently reported to be impaired: speed of processing, memory, and executive function abilities, such as planning, inhibition, and set shifting (Saint-Cyr, Trepanier, Kumar, Lozano, & Lang, Reference Saint-Cyr, Trepanier, Kumar, Lozano and Lang2000; Taylor, Saint-Cyr, & Lang, Reference Taylor, Saint-Cyr and Lang1986).
Furthermore, working memory and executive function abilities predict impairments in language production in PD (Bastiaanse & Leenders, Reference Bastiaanse and Leenders2009; Colman et al., Reference Colman, Koerts, van Beilen, Leenders, Post and Bastiaanse2009; Troche & Altmann, Reference Troche and Altmann2012). Language output in PD is often more sparse and less informative than that of healthy peers (Altmann & Troche, Reference Altmann and Troche2011; Bayles, Reference Bayles1990; Murray, Reference Murray2000). Furthermore, narratives produced by individuals with PD are less fluent (e.g., contain more false starts and word repetitions), less grammatically complex, and shorter than those of controls (Altmann & Troche, Reference Altmann and Troche2011; Murray, Reference Murray2008). Troche and Altmann (Reference Troche and Altmann2012) demonstrated significant relationships between cognitive abilities and many aspects of language, suggesting that if cognitive abilities improve, some aspects of language may also improve.
Notably, persons with PD who display impairments in cognition frequently also present neuropsychiatric and mood symptoms (Emre, Reference Emre2003). Depressive symptoms are experienced by as many as 90% of people with PD (Slaughter, Slaughter, Nichols, Holmes, & Martens, Reference Slaughter, Slaughter, Nichols, Holmes and Martens2001), and depression is the single strongest predictor of quality of life in PD, even after accounting for motor function (Jones, Malaty, Price, Okun, & Bowers, Reference Jones, Malaty, Price, Okun and Bowers2012). Additionally, apathy without depression is seen in 17–30% of individuals with PD and may contribute to changes in activity level and quality of life (Jones, Butterfield, et al., Reference Jones, Butterfield, Song, Lafo, Mangal, Okun and Bowers2015; Jones, Marsiske, et al., Reference Jones, Marsiske, Okun, Bowers, Jones, Butterfield and Bowers2015). Consequently, a non-pharmacological intervention for individuals with PD that improves or protects against mood impairments while improving cognition, language, and motor function would be extremely valuable.
The current study tests whether aerobic exercise may be an effective treatment for both cognitive and language impairments as well as mood disorders in people with PD. Many studies have documented that aerobic exercise can improve memory and executive dysfunction, and reduce severity of depression in otherwise healthy older adults (Blumenthal, Babyak, Moore, & et al., Reference Blumenthal, Babyak and Moore1999; Erickson et al., Reference Erickson, Voss, Prakash, Basak, Szabo, Chaddock and Kramer2011). Improvements are attributed to exercise-related increases in the release of several neuromodulators responsible for proliferation of synapses and dendritic branching (Cotman & Berchtold, Reference Cotman and Berchtold2002; Dishman et al., Reference Dishman, Berthoud, Booth, Cotman, Edgerton, Fleshner and Zigmond2006). These exercise-induced changes, in turn, have led to: increased volume of white and gray matter (Colcombe et al., Reference Colcombe, Erickson, Scalf, Kim, Prakash, McAuley and Kramer2006), increased hippocampal volumes (Erickson et al., Reference Erickson, Voss, Prakash, Basak, Szabo, Chaddock and Kramer2011), and improved functional connectivity in both the Default Mode Network and the Frontal Executive Network (Voss et al., Reference Voss, Prakash, Erickson, Basak, Chaddock, Kim and Kramer2010). Moreover, the impact of aerobic exercise is similar in impaired elderly populations, such as individuals with mild cognitive impairment (Suzuki et al., Reference Suzuki, Shimada, Makizako, Doi, Yoshida, Ito and Kato2013), Alzheimer’s disease (Coelho et al., Reference Coelho, Andrade, Pedroso, Santos-Galduroz, Gobbi, Costa and Gobbi2013), or Huntington’s disease (Harrison et al., Reference Harrison, Busse, Openshaw, Rosser, Dunnett and Brooks2013), suggesting that exercise-related changes in brain function may occur despite brain pathology.
The few exercise intervention studies in PD have also found positive results. Uc and colleagues (Reference Uc, Doerschug, Magnotta, Dawson, Thomsen, Kline and Grabowski2014) reported significant improvements in gait speed, depression, and one measure of executive function, as well as in disease severity in 43 individuals with PD who performed aerobic exercise three times a week for 6 months. Unfortunately, Uc et al. did not have a control group, which is an important consideration when participants have a degenerative disease. Cruise et al. (Reference Cruise, Bucks, Loftus, Newton, Pegoraro and Thomas2010), using a combined aerobic and strength-training regimen, also reported cognitive improvement following exercise training, but no changes in depression. While Cruise et al. included a no-contact control group, the scores of the two groups were not compared. Other studies in PD assessed combined types of exercise (Tanaka et al., Reference Tanaka, de Quadros, Santos, Stella, Gobbi and Gobbi2009) or strength-training (David et al., Reference David, Robichaud, Leurgans, Poon, Kohrt, Goldman and Corcos2015), and have found benefits in aspects of executive function. Thus, aerobic exercise has potential as an intervention for cognition and mood PD, although studies with appropriate control groups are needed.
One open question in the exercise literature concerns the resiliency of exercise-induced changes in cognition. Particularly in the PD exercise literature, it is typical for only one of many cognitive tests to improve (e.g., Cruise et al., Reference Cruise, Bucks, Loftus, Newton, Pegoraro and Thomas2010; Uc et al., Reference Uc, Doerschug, Magnotta, Dawson, Thomsen, Kline and Grabowski2014). The current study addresses this issue in two ways. First, because no single cognitive measure can adequately assess ability in an entire domain, the current study adopted a statistical approach called parcellation (Little, Rhemtulla, Gibson, & Schoemann, Reference Little, Rhemtulla, Gibson and Schoemann2013). Parcellation theory recommends using several measures of each construct of interest to triangulate on a participant’s true ability in that domain. Second, participants were assessed on cognition and language pre- and post-intervention in both single and dual task conditions. During dual tasks, cognitive resources are shared between the two tasks, so performance typically declines in one or both tasks due to insufficient resources to maintain performance in both tasks at the single task level (Kahneman, Reference Kahneman1970).
We hypothesized that any improvements in cognition would be shared between the cognitive and motor tasks during a dual task, but not a single task. Thus, small improvements in cognition would first be detectable in a single task; whereas, cognitive improvements would only manifest in dual tasks if the effect were very robust. However, declines in cognition might manifest first in dual task conditions where cognitive resources were already divided, and it is already difficult to maintain performance in both concurrent tasks. An alternative view would suggest that any improvements or declines in cognition would first manifest in dual task performance, because that is where the demand for cognitive resources is greatest.
As a corollary to these predictions, executive function tasks were expected to be most sensitive to cognitive improvement or decline, because they are highly demanding of cognitive resources. Similarly, language production was predicted to change in concert with cognitive abilities. Additionally, based on previous findings in the literature (e.g., Uc et al., Reference Uc, Doerschug, Magnotta, Dawson, Thomsen, Kline and Grabowski2014), it was expected that mood and symptoms of disease severity on the Unified Parkinson’s Disease Rating Scale (UPDRS; Goetz et al., Reference Goetz, Fahn, Martinez-Martin, Poewe, Sampaio, Stebbins and LaPelle2007) would also improve.
METHODS
Participants
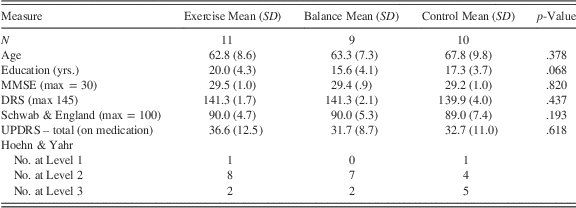
Forty individuals diagnosed with idiopathic PD enrolled in the study from the Center for Movement Disorders and Neurorestoration in Gainesville, Florida. Three participants quit before completing initial assessments, after which participants were randomized into three groups, aerobic exercise, stretch-balance (the contact-equivalent control group), or control (no-contact control group). Four participants did not complete post-intervention testing. Three additional participants completed the intervention and post testing but had incomplete data and were excluded from analysis. Thus, 30 participants were included in the final analyses. Demographic information on participants included in analyses appears in Table 1. Information on participants enrolled in the study but excluded from analysis is in the Supplementary Materials.
Table 1 Descriptive information for participants when they entered the study

MMSE=Mini Mental Status Examination; DRS=Dementia Rating Scale-2; UPDRS=Unified Parkinson’s Disease Rating Scale.
Participants were diagnosed with idiopathic PD according to UK Brain Bank criteria, which was confirmed by a movement disorders neurologist. Modified Hoehn and Yahr scale scores ranged between 1 and 3 in the “on” medication state (Hoehn & Yahr, Reference Hoehn and Yahr1967). Participants had a stable response to anti-parkinsonian and/or psychotropic medication. Participants with secondary or atypical Parkinsonism, or severe, unpredictable episodes of motor fluctuation were excluded. Potential participants with a history of falls as shown by a score greater than one in the fall item of the UPDRS Part II were excluded under advisement of the Institutional Review Board to minimize risk to participants. Individuals were excluded from the study if they used medications known to interfere with cognitive function (e.g., anticholinergics), or had symptoms of mild cognitive impairment or dementia as indicated by a score less than 25 on the Mini Mental Status Examination (MMSE; Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975), a history of psychiatric disturbance (e.g., major depressive disorder or generalized anxiety), or cardiovascular disease.
All participants signed an Informed Consent form approved by the University of Florida Health Sciences Institutional Review Board. Participants were evaluated using the UPDRS while on medication, which was administered by trained personnel and was video-recorded for later scoring by a movement disorders neurologist blinded to treatment condition.
Tasks
General assessments
Disease severity was assessed using the UPDRS. Participants completed the Dementia Rating Scale (DRS; Mattis, Reference Mattis1988) as a measure of general cognition. Mood assessments included the Beck Depression Inventory-II (BDI; Beck, Steer, & Brown, Reference Beck, Steer and Brown1996), the Apathy Scale (Marin, Biedrzycki, & Firinciogullari, Reference Marin, Biedrzycki and Firinciogullari1991), and the Beck Anxiety Scale (Beck & Steer, Reference Beck and Steer1993). Sleep was assessed using the Pittsburgh Sleep Index (Buysse, Reynolds, Monk, Berman, & Kupfer, Reference Buysse, Reynolds, Monk, Berman and Kupfer1989).
Experimental tasks
Stimuli for cognitive and language tasks were presented by a laptop running MediaLab (Jarvis, Reference Jarvis2006b) and DirectRT software (Jarvis, Reference Jarvis2006a). Responses for all experimental tasks were oral and recorded via a wireless microphone for later scoring by trained research assistants using Audacity software (Bland, Busam, Gunlogson, Mekkes, & Saunders, Reference Bland, Busam, Gunlogson, Mekkes and Saunders2006). Participants received a different list of stimuli at each test session. While the primary outcome measures reported here varied between accuracy and response times (RTs) across tasks, tasks were scored for both wherever possible. Scores on secondary outcome measures are found in the Supplementary Materials.
Cognitive tasks covered three domains: speed of processing, attention/memory, and executive function. Because no single task can adequately assess a particular cognitive construct like speed of processing or executive function, several tasks were used to assess performance in each domain (Little et al., Reference Little, Rhemtulla, Gibson and Schoemann2013).
Speed of processing tasks
Assessment of speed of processing initially included four tasks: simple attention, 0-back, Stroop Colors, and digit symbol. However, inspection of the data and correlations between variables led to the additional inclusion of the Stroop Color Word task (Lezak, Howieson, Loring, Hannay, & Fischer, Reference Lezak, Howieson, Loring, Hannay and Fischer2004) in this composite. The primary outcome measure for all speed of processing tasks was the RT measured from stimulus onset to voice onset. The Simple Attention task presented a large, blue star centered on the screen at intra-stimulus intervals of 750, 1250, 1750, or 2250 ms. Participants said “Go!” when they saw the star, followed by the next trial. In the 0-back task (Nystrom et al., Reference Nystrom, Braver, Sabb, Delgado, Noll and Cohen2000), participants saw a fixation asterisk followed by a tic-tac-toe figure with two black dots in adjacent or diagonally adjacent squares. Participants said “Yes” if the current figure matched a prespecified target figure (25% of 40 trials), or “No” otherwise.
In the Stroop Colors task (Lezak et al., Reference Lezak, Howieson, Loring, Hannay and Fischer2004), participants saw a centered fixation asterisk, and then said the color of font in which four upper-case Xs appeared. In the Stroop Color Words task, following a fixation asterisk, participants saw a color word (e.g., BLUE, RED, GREEN) printed in an incongruent color and said the color of the font in which the word appeared. In an oral version of the Digit Symbol Substitution task (Lezak et al., Reference Lezak, Howieson, Loring, Hannay and Fischer2004), participants saw an array of nine digits, each paired with a Korean letter, at the top of the screen, and 750 or 1250 ms later a larger version of one of the Korean letters appeared below it. Participants said the number of the digit associated with the large Korean letter. With the exception of the 0-back task which had 40 trials total, all speed of processing tasks had 20 trials. With the exception of the simple attention task, all tasks featured a one second blank screen between trials.
Attention/ memory tasks
Two verbal and two visual attention-memory tasks were included. Accuracy was the primary outcome measure. In the two verbal tasks, digit span forward and backward (Wechsler, Reference Wechsler1997), participants heard increasingly long lists of digits and repeated them in verbatim or reverse order, respectively. Testing continued until the participant erred on both trials of one list length. The score was the proportion of lists (of 14) repeated successfully. The non-verbal tasks comprised 1- and 2-back tasks (Nystrom et al., Reference Nystrom, Braver, Sabb, Delgado, Noll and Cohen2000) using the tic-tac-toe figures described above. As in the 0-back task described above, 1- and 2-back trials began with a fixation asterisk and ended with a 1000 ms blank screen. In the 1-back task, participants said “Yes” when the current figure matched the immediately preceding figure (25% of 40 trials), and “No” otherwise. In the 2-back task, participants said “Yes” if the current figure matched the figure two trials before (25% of 40 trials), and “No” otherwise. The primary outcome measure for these n-back tasks was the proportion correct on “yes” trials. Scores for the secondary outcome measures, RTs for correct trials, are presented in the Supplementary Materials.
Executive function tasks
The three measures were included in the executive function composite. Stroop interference was calculated by subtracting RTs on the Stroop Color-Words task from RTs on the Stroop colors task. Thus, higher magnitude negative scores signified greater slowing in Stroop Color-Words relative to Stroop Colors. In the visual memory updating task (Fougnie & Marois, Reference Fougnie and Marois2006), participants saw the words “New Trial,” followed by 1–4 tic-toe-toe figures individually presented for 2000 ms each, and then a horizontal array of the same number of figures. Participants verified whether the figures in the array matched the figures just presented. Stimuli were randomized so participants were unaware how many figures they would have to remember. The outcome measure was the proportion of correct responses in the 16 trials. In the operation span task (Conway et al., Reference Conway, Kane, Bunting, Hambrick, Wilhelm and Engle2005), participants were asked to remember a set of six non-rhyming consonants presented for 500 ms. They then verified whether a series of 1–4 one-step arithmetic problems were correct (e.g., 3+2=5 “Yes”; 4–1=5 “No”), and then recalled the previously presented letters when cued. The outcome measure was the proportion of letters recalled correctly in order across the 20 trials. In the latter two tasks, trials were separated by a 1000 ms blank screen.
Language task
Since people with PD have difficulty with language at the sentence and discourse level (Altmann & Troche, Reference Altmann and Troche2011), a sentence generation task previously used with people with PD (Troche & Altmann, Reference Troche and Altmann2012) assessed language production. Participants produced a sentence to describe each of 20 black-and-white line drawings from Bock, Loebell, & Morey (Reference Bock, Loebell and Morey1992) and Kempler (Reference Kempler2003) depicting a single event involving two or three characters, as shown in Figure 1. Participants were instructed to describe each picture using a grammatical sentence that mentioned each character in the picture without using pronouns. Outcome measures included fluency, grammaticality, and complete information. A fluent response included no “ums” or “uhs,” false starts, or long pauses between words. A grammatical sentence included appropriate articles and auxiliary verbs, correct subject-verb agreement, and no other grammatical errors. Complete information was defined as mentioning all characters pictured plus an appropriate action. Reliability of scoring using Cronbach’s alpha was excellent (fluency .950, grammaticality .936, completeness .905).

Fig. 1 Sample of stimuli with two or three entities in the picture for the language production task (Kempler, Reference Kempler2003).
Procedure
The 12 experimental tasks described above were administered twice, once in a quiet room and once while riding a stationary bicycle, the dual task condition, before and after the intervention. Participants pedaled at their chosen rates against minimal resistance. At least two experimenters were present during dual task sessions, one explaining and running the cognitive tasks and one running the motion capture system to track cycling speed. Only one experimenter administered the single task testing. Experimenters administering post-intervention assessments were blinded to group membership. To control for learning effects, the order of single and dual task assessment was counterbalanced across participants and was the same pre and post-intervention.
Physical training
After completing all intake testing, participants were randomly assigned to the aerobic exercise, stretch-balance, or no-contact control groups. Participants in the aerobic exercise and stretch-balance groups came to the UF Center for Exercise Science three times a week for 16 weeks for personal training with a cardio-pulmonary resuscitation (CPR) certified, fitness specialist familiar with PD. Following warm-up stretching, exercise duration progressed from an initial 20 min per session to the maximum 45 min by increasing exercise time by 5 min each week. Participants wore a heart rate monitor during all aerobic training. Aerobic exercise, performed on a treadmill, began at low intensity [50% maximal heart rate (HR) reserve] and increased by 5% each week to a maximum of 75% HR. The stretch-balance group did stretching exercises as outlined in the American Parkinson’s Disease Foundation publication “Be Active” (American Parkinson Disease Association, 2009), most of which are performed while sitting. Subsequently, they performed balance tasks on a force platform with visual feedback displayed on a large computer monitor. Attendance in both groups was high (>94% of scheduled visits). Participants in the no-contact group continued their regular daily activities and were discouraged from initiating any physical training during the study. Levels of physical activity within the control group were monitored monthly by phone interview and questionnaire (Godin & Shephard, Reference Godin and Shephard1997).
Analyses
Scoring
For all experimental cognitive tasks, the dependent variable for each domain was the mean of Z-scores across all tasks in that domain at each time point (e.g., Anderson-Hanley et al., Reference Anderson-Hanley, Arciero, Brickman, Nimon, Okuma, Westen and Zimmerman2012). This was calculated by pooling subjects’ scores for all conditions on a task and calculating the Z-scores. In this way, change relative to all conditions could be determined. Z-scores for tasks with RTs as the primary measure were sign reversed, so higher positive scores signified faster responses. Using Z-scores allowed the inclusion of tasks with different outcome measures (e.g., RTs and accuracy) or very different means (e.g., very fast and very slow RTs) in the same analysis without biasing results. Dependent variables for the language task comprised the proportion of responses meeting each of the three criteria described above. Similarly, raw scores from the UPDRS, general cognition, and mood assessments comprised the dependent variables in those analyses.
Changes in disease severity and mood were assessed with mixed repeated measures analyses of variance in which group (aerobic, balance, control) was a between-subjects variable, and time (pre, post) was a within-subjects, repeated measure. Change within UPDRS domains was assessed with a multivariate analysis of variance (MANOVA) in which subtest scores on mood, activities of daily living, motor, and medication were component variables. The general cognition, mood measures, and sleep were assessed with group × time analyses of variance (ANOVAs) within each domain. Changes in cognition and language production were analyzed using group × time × single/dual task analyses of covariance (ANCOVAs) in which order of single and dual tasks was covaried to control for learning effects. Significant interactions were explored with the appropriate post hoc tests, with Bonferroni correction for multiple comparisons. Alpha was set to .05 for all analyses. All statistics were computed using SPSS 22 (IBM, 2013).
RESULTS
Disease Severity and Mood
Scores for UPDRS, general cognition, mood and sleep are found in Table 2. The MANCOVA over UPDRS scores found no significant effects; neither did the analysis of DRS scores. In the analyses of mood and sleep scores, only BDI scores revealed a significant group by time interaction, F(2,27)=5.219, p=.003, η2 .344. Post hoc within-group t tests revealed significant increases in BDI scores of the control group at post, t(9)=3.161, p=.012, but no change in BDI scores among aerobic or stretch-balance groups (both p>.3).
Table 2 Scores on measures of severity and mood measures before and after the intervention

* Significant group by time interaction, p<.05.
‡ within group change, p<.05.
UPDRS=Unified Parkinson’s Disease Rating Scale; ADL=activities of daily living; DRS=Mattis Dementia Rating Scale-2; BDI=Beck Depression Inventory 2; Apathy=Apathy Scale; Anxiety=Beck Anxiety Index; Sleep=Pittsburgh Sleep Quality Index.
Cognition
Speed of processing
As shown in Table 3, the ANCOVA analyzing speed of processing revealed a significant main effect of dual task, F(1,26)=8.131, p=.008, η2=.238, with faster scores in the dual task (M=+.030, SD=.749) than the single task (M=−.038, SD=.700). Additionally, the group by time interaction was significant, F(2,26)=4.113, p=.028, η2=.240. RTs of the control group were faster at post (M=+.187, SD=.545) than pre (M=−.242, SD=.938), t(9)=2.631, p=.027, which was not significant with Bonferroni correction for three comparisons (i.e., p=.017). The stretch-balance group showed a similar pattern, with faster response times at post (M=−.060; SD=.703) than pre (M=−.153; SD=.665), but this was not significant, t(8)=2.267, p=.053. The aerobic group showed little change in RTs on these tasks, t(10)<1.
Table 3 Single and dual task scores for speed of processing tasks (RTs), pre and post training in the three groups

Note. Mean Z-scores are reversed in sign so that higher positive scores indicate improvement, faster responses.
* Significant group by time interaction, p<.05.
‡ Significant dual task benefit, p <. 05.
Attention-memory
The ANCOVA examining performance in attention/working memory accuracy (see Table 4) found no significant effects.
Table 4 Single and dual task scores (accuracy) for attention-memory tasks as a percent of possible accurate trails, pre and post training in the three groups

Note. Analyses found no significant effects.
Executive function
The ANCOVA examining executive function performance revealed a nonsignificant trend for higher scores in the dual task (M=+.077; SD=.757) than in the single task (M=−.077; SD=.671), F(1,26)=3.749, p=.064, η2=.128. Additionally, as shown in Table 5, there was a significant three-way interaction between group, time, and dual task, F(2,26)=3.440, p=.047, η2=.209. Paired-sample t tests comparing pre and post scores, separately in single and dual tasks, were completed for each group, leading to six comparisons (required p-level of .008 with Bonferroni correction). Executive function scores of the aerobic group improved significantly in the single task (mean difference=+.409; SD=.403), t(10)=3.358, p=.007, but remained steady in the dual task. In contrast, dual task performance in the control group showed a tendency to decline at post (mean difference=−.288; SD=.403), t(9)=2.260, p=.050, with no significant change (p=.244) in the single task. Performance of the stretch-balance group remained stable, both p>.6 (Figure 2).

Fig. 2 Composite scores for executive function tasks in the single and dual task conditions before and after the intervention by the three groups. (* signifies p ≤ .05).
Table 5 Single and dual task scores for executive function tasks, pre and post training in the three groups

Note. Analysis revealed a significant effect of dual task (p=.034) and a significant group by time by exercise interaction (p=.033).
* Significant dual task benefit, p <. 05.
‡ Significant group by time by dual task interaction, p<.05.
Language
Language task scores are presented in Table 6. The ANCOVA analyzing completeness of information in sentences revealed a significant interaction between group and time, F(2,26)=4.440, p=.022, η2=.255. To explore this interaction, a post hoc univariate ANCOVA with difference scores (post–pre) as the dependent variable, controlling for task order, with Bonferroni correction was computed. The aerobic group (M Change=+.092, SD=.187) improved significantly more in completeness of information than the stretch-balance group (M Change=−.083; SD=.140; p=.008), but did not differ from the control group (M Change=+.068; SD=.132; p=1.00), nor did the stretch-balance and control groups differ (p=.103). The analysis of fluent responses revealed a significant main effect of time, F(1,26)=5.412, p=.028, η2=.172. Participants were more fluent during post testing (M=.700; SD=.266) than during initial testing (M=.597; SD=.288). Additionally, in the analysis of fluency, the main effect of dual task was significant, F(1,26)=5.355, p=.028, η2=.161. Participants were more fluent in the dual task. The analysis of grammaticality scores found no significant effects.
Table 6 Single and dual task scores for the sentence production task, pre and post training in the three groups

Note. Analyses found only a significant group by time interaction in the completeness of response and a main effect of time in the analysis of fluent responses.
* Significant group by time interaction, p<.05.
¥ Significant main effect of time, p<.05.
DISCUSSION
The current study compared the effects of aerobic exercise in PD on disease severity, mood, cognitive and language outcomes relative to effects in both no-contact and contact-equivalent control groups. Furthermore, the study tested performance in both single and dual task conditions. The results demonstrated that the 16-week aerobic exercise intervention elicited significant improvement in executive function in the single task and potentially also improvements in language content in people with PD. In contrast, on timed tasks, control participants responded faster at post, while the aerobic group showed only minimal changes in response times. Finally, participants responded faster in speed of processing tasks, spoke more fluently in the language task, and were marginally more accurate in executive function tasks in dual task rather than in single task conditions.
Our primary finding was that, following training, the aerobic group demonstrated robust improvement in executive function (+.409 SD), but only in the single task condition. This pattern was evident in all three executive function measures, as shown in Table 5. In contrast, the balance group showed no change, and executive function performance in the control group worsened (−.26 SD), but only in the dual task condition. This pattern of response was predicted in the case of relatively small treatment effects. Specifically, it was argued that cognitive improvements due to exercise would first manifest in the single task, because any improvement in cognitive resources would be diluted by the additional attentional burden of the dual task (Kahneman, Reference Kahneman1970).
Similarly, because available cognitive resources are shared between the dual tasks, it was predicted that small declines in cognition might first be noticeable in the dual task. This might explain the drop in executive function performance in the control group while cycling. Notably, there was no change in performance for any group on the DRS, an assessment of general cognition. Thus, in very high functioning, highly educated individuals, such as those in the current study, very demanding tasks in which scores are far from ceiling, such as the executive function tasks used in the current study, may be best for detecting cognitive change due to exercise.
Surprisingly, there was no change in attention-memory performance, even in the two most difficult tasks, digit span backward and 2-back. A potential explanation is that these two tasks relied heavily on immediate, verbatim recall and sustained attention, rather than executive function. Consistent with this argument, in a meta-analysis, digit span tasks dissociated from working memory tasks requiring more manipulation or distraction, such as operation span (Bopp & Verhaeghen, Reference Bopp and Verhaeghen2005). In the 2-back, only two stimuli were in the focus of attention at a time, minimizing executive function demands.
In contrast, the operation span and visual memory updating tasks required both executive function and working memory. The operation span task required six letters to be recalled after attention had been shifted to a different task, and, thus, was actually a dual task. In the visual memory updating task, incorrect comparison arrays differed from targets only by reversing the order of consecutive figures or off-setting the dots in one figure by one square. Thus, this task was very difficult. Additionally, stimuli were presented randomly, so participants did not know how many figures they would have to recall, requiring memory representations to be flexible enough that participants could add to them if required, as opposed to the verbatim recall needed in digit span and 2-back. Therefore, digit span backward and 2-back may not have improved, because they were not as dependent on executive function as operation span and visual memory updating.
The findings of the current study are consistent with the three previous studies examining effects of aerobic exercise on cognition in PD. Uc and colleagues (Reference Uc, Doerschug, Magnotta, Dawson, Thomsen, Kline and Grabowski2014) documented significant improvements in depression and resistance to interference on the Eriksen flanker task (Eriksen & Eriksen, Reference Eriksen and Eriksen1974), but no significant changes on the Stroop task or other executive function tasks. Uc and colleagues also reported improvement in UPDRS scores, which were not found in the current study. The difference may be attributable to their larger sample size (i.e., 43 participants) or the longer training time (6 vs. 4 months). Unlike the current study, Uc et al. did not include a contact-equivalent control group. Thus, it is unknown how much improvement in that study was due to social stimulation.
In another study, Nocera, Altmann, Sapienza, Okun, and Hass (Reference Nocera, Altmann, Sapienza, Okun and Hass2010) reported the effects of a 12-week treadmill exercise intervention in one individual with PD, which yielded improvements in verbal fluency and the Stroop task. Finally, it is difficult to compare the current study to the exercise study by Cruise et al. (Reference Cruise, Bucks, Loftus, Newton, Pegoraro and Thomas2010), which reported some improvement in verbal fluency and visual working memory, because it also lacked a contact-equivalent control group and did not directly compare performance of the exercise and no-contact control groups. Therefore, despite several differences, the previous studies and the current one have observed improvements in executive function tasks in individuals with PD following aerobic exercise interventions.
Consistent with Uc et al. (Reference Uc, Doerschug, Magnotta, Dawson, Thomsen, Kline and Grabowski2014), the current study adds to the accumulating evidence that aerobic exercise training can be protective against the increasing incidence of depressive symptoms in people with PD. Mood disorders are common in PD and can be traced to both progressive neurophysiological changes in the limbic system and to the everyday burden of the disease (Cooper, Sagar, Jordan, Harvey, & Sullivan, Reference Cooper, Sagar, Jordan, Harvey and Sullivan1991; Leentjens, Reference Leentjens2004). Considering that both the aerobic exercise and stretch-balance groups here showed similarly stable BDI scores, this positive effect may stem from thrice weekly interactions with people outside the home, rather than a particular intervention. Therefore, future research should test whether social interventions involving multiple events per week may be similarly protective against depressive declines for people with PD.
The aerobic group also improved in completeness of responses in the language task significantly more than the stretch-balance group. Unexpectedly, the control group also improved somewhat in completeness, paralleling their nonsignificant improvements in single task executive function. A possible explanation was that these changes might be related. In fact, changes in completeness of response correlated significantly with changes in single task executive function, r(30)=.390, p=.033. This suggests that executive function plays an important role at the conceptual level of language production. This does not, however, address why the control group improved at all in executive function at post. Their pretest performance was very poor; thus, improvements may simply represent regression to the mean.
An additional, unanticipated effect in the current study was that the control group responded faster in speed of processing tasks at post than they had initially. This pattern is remarkably consistent across single and dual conditions in five tasks. Similar changes in the stretch-balance group were not significant, and the aerobic group showed no change in RTs on these tasks. There are two possible explanations for this. One is that this is also a case of regression to the mean, as overall response times were longer in the control group than in the other two groups at initial testing. A second possibility is that faster speeds on cognitive tasks in PD represent a type of speed-accuracy trade off. Accuracy on two speed of processing tasks (simple attention, Stroop colors) was at ceiling for all groups, and accuracy on two other speed tasks dropped at post in the control group (0-back and Stroop Color Word; see Supplementary Information). Thus, faster responses on speed of processing tasks may not necessarily indicate improved performance, but instead signify a type of cognition festination during which participants respond very quickly before they have fully processed the stimuli. Tracking speed of processing over time in PD may yield new insights into cognitive effects of the disease.
Surprisingly, there were also faster RTs in speed of processing tasks during dual tasks relative to single tasks. Similarly, Altmann et al. (Reference Altmann, Stegemöller, Hazamy, Wilson, Okun, McFarland and Hass2015) reported faster cycling during these identical tasks in this group at pretest; thus, both motor and cognitive tasks benefitted during the dual task. Altmann et al. attributed increases in cycling speed to a combination of exercise-related arousal and cognitive arousal stemming from the fast pace of the speeded tasks. The current findings support the suggestion in Altmann et al. that the right combination of tasks, an easy motor task with easy, fast-paced cognitive tasks, can improve performance in both.
One limitation of this study was that cognitive testing was only performed while on medication. Future exercise studies in PD should also assess performance off medication. Additionally, due to randomization, groups differed somewhat in age, although not significantly; even so, initially groups were remarkably similar in disease severity, general cognition, and mood level. Indeed, the groups may have been so homogeneous that the findings of the current study may not generalize to more diverse groups of individuals with PD. Another limitation was the small sample size, which limited power to detect small changes.
In summary, the current study adds to the literature on aerobic exercise in PD in several ways. First, the study compared the effects of aerobic exercise on cognition to both contact-equivalent and no-contact control groups, helping to control for effects of both social interaction and passage of time. The results also are consistent with the suggestion that small improvements in cognition following aerobic training will first be observable in single task settings, but declines in cognition may first be evident in dual task performance. Moreover, results demonstrate that a dual task can selectively improve performance in a variety of tasks. The study also documents that changes in language content may be related to changes in executive function. Most importantly, these findings add to the growing evidence that aerobic exercise can positively impact performance on mood and executive function in people with PD.
ACKNOWLEDGMENTS
We acknowledge the support of the National Institute of Aging (R21AG0033284-01A2) at the National Institutes of Health, as well as the National Parkinson Foundation Center of Excellence, and the UF-INFORM database. We thank J. Katherine Bock and Daniel Kempler for sharing their picture materials with us. We also thank the UF Language over the Lifespan lab and UF Neuromechanics lab for their help and support with this project. The authors have no conflicts of interest with respect to this study.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S135561771600076X