INTRODUCTION
In many ecosystems, a large proportion of plants regularly produce both seeds and ramets (Eriksson Reference ERIKSSON, DE KROON and VAN GROENENDAEL1997, Klimeš et al. Reference KLIMEŠ, KLIMEŠOVA, HENDRIKS, VAN GROENENDAEL, DE KROON and VAN GROENENDAEL1997, Vallejo-Marin et al. Reference VALLEJO-MARIN, DORKEN and BARRETT2010). Ramets are plant parts with the the potential of physiological independence and can therefore be considered offspring. This is true within the understorey of neotropical forests where clonal species are found in many common families, including Marantaceae, Piperaceae and Bromeliaceae (Lasso et al. Reference LASSO, DALLING and BERMINGHAM2012, Matlaga & Horvitz Reference MATLAGA and HORVITZ2009, Svenning Reference SVENNING2000, Villegas Reference VILLEGAS2001). Within the understorey, beneath the intact canopy of moist neotropical forest, light levels can be less than 2% of full sun (Clark et al. Reference CLARK, CLARK, RICH, WEISS and OBERBAUER1996). However, the availability of light can be much greater under tree-fall gaps but these microsites are rare in space and time (Chazdon et al. Reference CHAZDON, PEARCY, LEE, FETCHER, MULKEY, CHAZDON and SMITH1996). A literature exists addressing the influence of canopy structure (e.g. gaps vs. closed canopy) on the dispersal of sexual propagules (seeds) (Augspurger & Franson Reference AUGSPURGER and FRANSON1988, Puerta-Piñero et al. Reference PUERTA-PIÑERO, MULLER-LANDAU and CALDERÓN2013) but a complementary literature investigating dispersal of clonal offspring has not yet developed. Specifically, it is not known if the dispersal of clonal offspring is influenced by canopy openness and plant size.
The spatial movement of clonal plants (e.g. placement of clonal offspring in space relative to the parent) has been approached from a limited perspective within the clonal plant literature. ‘Clonal mobility’ (or ‘vegetative mobility’), defined as the horizontal expansion of a clonal plant over time, has been quantified at the species-level and used in community-level analyses to understand patterns of diversity over time (Moora et al. Reference MOORA, OPIK, ZOBEL and ZOBEL2009, Tamm et al. Reference TAMM, KULL and SUMMUL2002). However, the causes and consequences of variation in clonal mobility between individuals within a species has received little attention. A specific knowledge gap that has been identified is the influence of environmental heterogeneity on clonal mobility (Moora et al. Reference MOORA, OPIK, ZOBEL and ZOBEL2009).
Previous research investigating the influence of light availability on the dispersal distance of clonal offspring has done so using species with growth-forms that utilize rhizomes and/or stolons. It has been found that in higher-light environments internode lengths are shorter (de Kroon & Hutchings Reference DE KROON and HUTCHINGS1995, de Kroon & Knops Reference DE KROON and KNOPS1990, Traveset et al. Reference TRAVESET, MORAGUES and VALLADARES2008); therefore, the clonal offspring are dispersed a shorter distance from the parent plant compared with dispersal in lower-light environments. This strategy is interpreted as allowing clonal plants to monopolize areas with high light availability by placing many ramets in the high-resource environment. It remains unclear how light availability influences clonal dispersal of species that do not utilize stolons or rhizomes. Our study species Goeppertia marantifolia has a growth-form found among several other members of the Zingiberales which has received little attention in the clonal-plant literature. Goeppertia marantifolia produces bulbils (clonal offspring) on above-ground shoots after fruiting has occurred. Clonal offspring can develop leaves and roots while atop the parent's shoot. On average, the parent plant's shoot contacts the soil surface after 6 mo, which allows bulbils to root directly (D. Matlaga unpubl. data). Dispersal of clonal offspring in species that produce above-ground bulbils may respond positively to light availability. In higher light environments parent plants may obtain a larger size, having longer shoots that disperse offspring further.
We used the neotropical understorey herb Goeppertia marantifolia as a model system to investigate the influence of parent plant size (leaf area) and canopy openness on clonal offspring dispersal. The goal of our study was to test the hypothesis that both size and canopy openness would have a positive effect on dispersal distance of clonal offspring.
METHODS
Study species and site
We studied the dispersal of Goeppertia marantifolia (Standl.) Borchs. & S.Suárez (Marantaceae; formerly known as Calathea marantifolia; Borchsenius et al. Reference BORCHSENIUS, SUÁREZ and PRICE2012) clonal offspring within the secondary forest surrounding Sirena Biological Station, Corcovado National Park (8°28′49″N, 83°35′22″W), on the Pacific coast of Costa Rica. This tropical wet forest receives more than 5 m of rain annually (Hartshorn Reference HARTSHORN and JANZEN1983) with distinct rainy (May–November) and dry seasons (December–April) (Sirena Biological Station unpubl. data). Goeppertia marantifolia (Marantaceae) is a neotropical understorey herb occurring in wet to semi-deciduous forest from central Ecuador to Honduras (Kennedy Reference KENNEDY1978). The sequence of events for sexual and clonal reproduction of G. marantifolia has been described in some detail by Matlaga & Horvitz (Reference MATLAGA and HORVITZ2009).
Data collection
We established eight plots in August 2004 and censused these plots biannually (August and March) until 2007. Plots were selected to capture the range of canopy openness in which G. marantifolia occurs within the study site. Plots were selected by first locating 93 patches of G. marantifolia along established trails, and the canopy openness of each patch was estimated using the canopy scope technique (Brown et al. Reference BROWN, JENNINGS, WHEELER and NABE-NIELSEN2000). Patches were randomly chosen from the ends of the canopy openness continuum at our study site with four plots having relatively high canopy openness and four plots with relatively low canopy openness. The dimensions and area of each plot differed, but each was located in a relatively uniform light environment and contained approximately 100 G. marantifolia individuals. Each plant was individually marked using an aluminium tag fixed to the ground at the plant's base using a flag with a metal stake. Clonal offspring were marked with a plastic cuff while atop their parent plant's shoot. If clonal offspring were rooted, the distance to their parent plant was recorded. Canopy openness was estimated directly above each plant using the canopy scope technique during the August census (Brown et al. Reference BROWN, JENNINGS, WHEELER and NABE-NIELSEN2000). Canopy scope score is significantly correlated with per cent canopy openness, measured using fish-eye photography, within the secondary forest of Corcovado (r2 = 0.695, P = 0.0001, D. Matlaga unpubl. data). The length of each leaf was recorded for all individuals during March and August censuses. The total leaf area of each plant was estimated using leaf lengths and the regression relationship between leaf length and leaf area specific to G. marantifolia at Corcovado (Horvitz & Le Corff Reference HORVITZ and LE CORFF1993).
Data analysis
We used a path analysis model (Mitchell Reference MITCHELL, Scheiner and Gurevich2001, Wright Reference WRIGHT1960) to determine the relationship among our independent variables, light availability (measured as canopy scope score, Brown et al. Reference BROWN, JENNINGS, WHEELER and NABE-NIELSEN2000) and parent plant size (measured as total leaf area), and the influence of the independent variables on our dependent variable, clonal-offspring dispersal distance. This analysis places correlations into an assumed cause-effect structure. We constructed a simple path model judged to be realistic for our study system based on previous research and our experience with G. marantifolia at Corcovado (Matlaga & Horvitz Reference MATLAGA and HORVITZ2009). In our model, light availability directly influences parent plant leaf area, which is consistent with studies showing faster growth and larger size among neotropical broad-leaved herbs in tree-fall gaps compared with the shaded understorey (Matlaga & Horvitz Reference MATLAGA and HORVITZ2009). In our model, parent plant size (leaf area) directly affects the dispersal of clonal offspring. This is based on our observation that plants with greater leaf area have longer shoots which have the potential to disperse clonal offspring farther. In our path model, light also acts as a latent variable influencing dispersal. This is based on our observation that light availability influences the density of vegetation surrounding G. marantifolia which has the potential to obstruct the shoot of a parent plant as they lower to the ground, thereby reducing dispersal distance. Therefore, light availability may be negatively associated with clonal offspring dispersal. The size of the path coefficients were estimated using standardized partial regression coefficients (betas) using the lmbeta() function within the ‘QuantPsyc’ package in the R program. The unexplained variation in the dependent variables (parent plant leaf area and clonal offspring dispersal distance), denoted as U in the path model, was calculated as U = (1−R2)1/2 (Mitchell Reference MITCHELL, Scheiner and Gurevich2001).
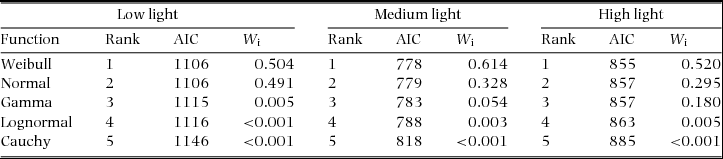
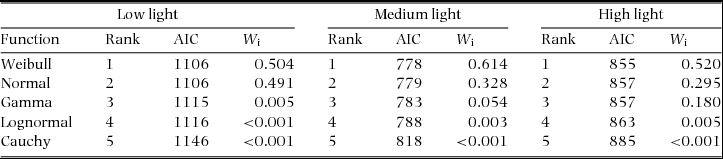
We investigated the properties of the clonal offspring dispersal kernel separately for three light-availability ranges due to the results of our path analysis demonstrating that light availability strongly influenced clonal offspring dispersal. We created our three light-availability categories with the goal of having approximately equal sample sizes. Our light-availability categories included low light (Canopy Scope Score (CSS) = 0.08–0.19, N = 119), medium (CSS = 0.19–0.4, N = 81) and high light (CSS = 0.44–0.8, N = 82; Appendix 1). Using the fitdist function within the ‘fitdistplus’ package in R, which uses maximum likelihood to fit distributions to data, we characterized the clonal-offspring dispersal kernel using five common distributions (Gamma, Weibull, Lognormal, Normal and Cauchy). We characterized dispersal kernels separately for plants in low, medium and high light. The fitdist function produces parameter estimates and Akaike's Information Criterion (AIC) values when fitting each distribution to data. The ‘raw’ AIC score is a relative measure of the quality of fit of the distribution to the data (Burnham & Anderson Reference BURNHAM and ANDERSON2002). To evaluate the goodness of fit for each distribution we calculated the Akaike weights (Wi ) which provide a continuous measure of the strength of support for a given model (distribution). Akaike weights can be interpreted as the probability that a given model is the ‘best’ model (meaning the least amount of explanatory power lost) given the data and the suite of candidate models (Wagenmakers & Farrell Reference WAGENMAKERS and FARRELL2004).
RESULTS
Overall, results from the path model indicate that a significant proportion of the variance in clonal offspring dispersal was explained (Table 1). Canopy openness had a strong, positive direct effect on both parent plant leaf area and the distance clonal offspring were dispersed (Figure 1, Table 1). Parent plant leaf area, however, had only a weakly positive effect on the distance clonal offspring were dispersed (Figure 1, Table 1).
Table 1. Results of path analysis estimating the relationship between Goeppertia marantifolia clonal offspring dispersal in Corcovado National Park, Costa Rica and causal factors. Analysis is demonstrating the direct, indirect and total effect of the independent variable on the dependent variables. ∗ P < 0.05, ∗∗∗P < 0.001. R2 represents the variation explained by all the standardized variables.


Figure 1. Path diagram for the dispersal distance of Goeppertia marantifolia clonal offspring (N = 282) in Corcovado National Park, Costa Rica. Key provides the approximate magnitude of the direct effect coefficients indicated by line widths. See text for the exact values and significance levels of the variables. ∗ P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Scatterplots are included that demonstrate the relationships summarized in path arrows.
The dispersal distributions for each of the individual canopy openness categories, low, medium and high canopy openness, were best described by Normal and Weibull distributions (Appendix 2,3). The average dispersal distances increases from low light (79 cm) to high light (124 cm) (Appendix 4).
DISCUSSION
Studies have demonstrated that within tropical forests understoreys, variability in canopy openness has the potential to influence both the demography (Schleuning et al. Reference SCHLEUNING, HUAMÁN and MATTHIES2008) and seed dispersal (Wenny & Levey Reference WENNY and LEVEY1997) of plants. Canopy openness, therefore, may influence both the population growth rate and the population spread rate of understorey plants. Because many tropical understorey plants utilize clonal reproduction an obstacle to our understanding of the spatial population dynamics of these species has been the gap in our knowledge of how canopy heterogeneity influences clonal dispersal. Results from our study demonstrate that canopy openness has the potential to positively influence the dispersal of clonal offspring in G. marantifolia, a species that produces above-ground clonal bulbils. In addition, we demonstrated that although light influences clonal dispersal, the distribution of clonal dispersal distances is best described by a Weibull distribution in all light environments.
Detailed experimental research has shown that clonal plants that grow laterally using stolons or rhizomes can actively change their morphology and physiology in response to resource heterogeneity. This ‘clonal foraging’ leads to internodes that are shorter in resource-rich patches, allowing for a greater amount of root and leaf mass per surface area for resource capture (de Kroon & Hutchings Reference DE KROON and HUTCHINGS1995). This is specifically true for clonal plants that respond to light heterogeneity. In high-light patches internodes are shorter, allowing for more shoots per area, and internode angles promote placement of ramets in high light (Henry & Aarssen Reference HENRY and AARSSEN2001, Wang et al. Reference WANG, XI, CHUN-HUA and DAN2016, Xiao et al. Reference XIAO, YU and WANG2006). Our results using a bulbil-producing growth-form demonstrate the opposite result. We found a positive relationship between light availability and clonal dispersal distance. However, due to the nature of this growth-form where clonal offspring are produced above-ground apically, it seems likely that resource conditions resulting in longer above-ground shoots will also result in greater clonal dispersal distances. This situation creates the possibility that clonal offspring will leave the high light patch where the parent plant is located; however, if the clonal offspring remain in the same light environment its chances experiencing shading from their parent plant may be lessened by increased dispersal. It is difficult to know how generalizable our results are within this growth-form since little research has been conducted (but see Ronsheim Reference RONSHEIM1994, Reference RONSHEIM1997).
On average, G. marantifolia clonal offspring dispersed 40% further under high canopy openness compared with low canopy openness. This is probably due to greater light availability resulting in larger plants with longer shoots that were able to disperse clonal offspring further. Because population spread is determined by dispersal and demography to understand the influence of variability in canopy openness on the spatial population dynamics of this clonal understorey herb we must also consider the influence of canopy openness on propagule survival. Matlaga & Horvitz (Reference MATLAGA and HORVITZ2009) conducted a transplant experiment where clonal offspring were transplanted into gap centres, gap edges and the shaded understorey. This study discovered that clonal offspring had approximately 20% lower survival in gap centres compared with gap edges and the understorey. Clonal propagules may experience higher stress in high-light environments, compared with the shaded understorey, during the transition to independence from their parent plant. Therefore, the potential exists for the contrasting effects of canopy openness on clonal offspring dispersal (positive effect) and survival (negative effect) to cancel each other out resulting in similar rates of population spread for G. marantifolia across the understorey light gradient. However, to fully investigate this issue a model of population spread for G. marantifolia must be developed that incorporates the light-dependent demography of all life cycle stages.
ACKNOWLEDGEMENTS
The authors thank M. Gei, J. Chastaine and T. Matlaga for assistance in the field. MINAE (Costa Rica) issued permit no. 01339. Logistical support was provided by L. Gilbert and E. Deinert. The manuscript was improved by comments from T. Matlaga, H. de Kroon, D. Janos, B. Whitlock, O. Gaoue and C. Garcia-Robledo. Financial support by the James W. MacLamore Fellowship at the University of Miami is gratefully acknowledged. The research was conducted in compliance with Costa Rican law.

Appendix 1. Histogram displaying the frequency of Goeppertia marantifolia clonal offspring in Corcovado National Park, Costa Rica observed dispersing across a canopy-openness gradient quantified using canopy scope scores. Bars indicate canopy openness categories of low (CSS 0.08–0.19, N = 119), medium (C; 0.19–0.4, N = 81) and high openness (D; 0.44–0.8, N = 82).

Appendix 2. Histograms displaying probability density function (PDF) for Goeppertia marantifolia clonal offspring dispersal in Corcovado National Park, Costa Rica at low canopy openness (Canopy Scope Score (CSS) 0.08–0.19, N = 119), medium openness (CSS 0.19–0.4, N = 81) and high openness (CSS0.44–0.8, N = 82) and with Normal and Weibull fitted distributions.
Appendix 3. Goodness-of-fit criteria (AIC and AIC weights (W i) used to evaluate the fit of five functions for the distribution of Goeppertia marantifolia clonal offspring dispersal in low light (Canopy Scope Score (CSS) 0.16–0.20; N = 119), medium light (CSS 0.24–0.4; N = 81) and high light (CSS 0.44–0.8; N = 82) in Corcovado National Park, Costa Rica.
Appendix 4. Descriptive statistics for the distribution of Goeppertia marantifolia clonal offspring dispersal in low light (Canopy Scope Score 0.16–0.20; N = 119), medium light (CSS 0.24–0.4; N = 81) and high light (CSS 0.44–0.8; N = 82) in Corcovado National Park, Costa Rica. SD = standard deviation.