INTRODUCTION
Infection with the blood fluke Schistosoma mansoni is acquired in tropical regions when skin is in contact with infested fresh water (Lademann et al. Reference Lademann, Burchard and Reisinger2000). Following the penetration of the host skin, infectious larvae (cercariae) immediately transform into schistosomula. After a lung passage (pre-adults), the parasites finally move to the portal venous system, where they mature and form couples.
The oral uptake of large amounts of erythrocytes by adult schistosomes has been described previously. Adult male schistosomes ingest about 4×104 erythrocytes per hour; female worms, which produce about 300 eggs per day, ingest approximately 10 times more red blood cells than the males (Lawrence, Reference Lawrence1973; Bogitsh, Reference Bogitsh1989). The earlier developmental stages, schistosomula and pre-adults, are usually not able to ingest erythrocytes because of their undersized mouth (Crabtree and Wilson, Reference Crabtree and Wilson1980). So, only 4% of the pre-adults were found to have decomposed erythrocytes within the digestive system that appeared as a black mass of haem-pigment (Clegg, Reference Clegg1965). Previous studies showed that the parasites ingest macromolecules like albumin, dextrans, immunoglobulins and phospholipids orally (Bennett and Caulfield, Reference Bennett and Caulfield1991; Furlong et al. Reference Furlong, Thibault and Rogers1992). Bovine serum albumin (BSA), dextrans of different molecular weights or IgG, linked to fluorescein isothiocyanate (FITC), could be detected in the caecum and the oesophagus of in vitro-transformed schistosomula (Bennett and Caulfield, Reference Bennett and Caulfield1991). Furthermore, rhodamine-labelled bovine serum albumin was detected within the entire digestive system of adult worms in vitro. Both, BSA and haemoglobin are digested within the gut by several helminthal proteases and peptidases (Delcroix et al. Reference Delcroix, Sajid, Caffrey, Lim, Dvorak, Hsieh, Bahgat, Dissous and McKerrow2006). However, information regarding the albumin uptake of the developmental stages, especially in the definite host, is still lacking.
The aim of our study was to test whether schistosomula, pre-adults and adult worms of S. mansoni are able to ingest human serum albumin (HSA) in vitro as well as in an animal model.
MATERIALS AND METHODS
Animals
An African strain of S. mansoni, maintained in Biomphalaria glabrata snails (Brasilian strain) and female NMRI outbred mice (Harlan & Winkelmann, Germany), was used throughout this work. The experimental protocols were according to the German animal protection law and approved by the regional animal care and use committee.
Reagents for cell culture and parasite preparation
RPMI-1640 culture medium containing 13·3 μm phenol red and 2·05 mm L-glutamine and foetal calf serum (FCS) were obtained from GIBCO (Germany). FCS was heat inactivated at 56°C for 30 min before usage. Penicillin G (benzylpenicillin), streptomycin sulfate, heparin sodium salt, HEPES buffer (1 m) and Hanks balanced salt solution without phenol red and sodium bicarbonate (HBSS) were obtained from Sigma (Germany).
Albumin conjugate
Aminofluorescein bound to human serum albumin (Afl-HSA) was used to visualize the uptake of albumin within the digestive system. The free amount of aminofluorescein within the Afl-HSA solution was measured to be below 1%. Dichlorotriazinyl-aminofluorescein was covalently coupled in a 1:1 molar ratio to human serum albumin as previously described (Wunder et al. Reference Wunder, Müller-Ladner, Stelzer, Funk, Neumann, Stehle, Pap, Sinn, Gay and Fiehn2003).
In vitro experiments
Schistosomula
Cercariae were harvested from the snails and transformed into schistosomula by vortexing for 90 sec in order to separate tails and heads. Cercarial bodies were sedimented by incubation in HBSS (Ramalho-Pinto et al. Reference Ramalho-Pinto, Gazzinelli, Howells, Mota-Santos, Figueiredo and Pelliogrino1974). Then 50–70 of the organisms were cultured in 2 ml tubes with a 0·2 μm membrane lid (Eppendorf, Germany) containing 1 ml of schistosomula culture medium (SCM; RPMI-1640, 100 U/ml penicillin, 100 μg/ml streptomycin, 20 mm HEPES buffer and 5% FCS) and incubated at room temperature for 24 h in ambient air. The viability of the schistosomula was determined microscopically at 200-fold magnification. After 24 h the culture medium was changed and the schistosomula were then incubated at room temperature for 5 min, 10 min, 15 min or 5 h with 1 ml of SCM and 7·5 nmol/ml Afl-HSA. The schistosomula were then washed 3 times with schistosomula wash solution (RPMI-1640, 100 U/ml penicillin, 100 μg/ml streptomycin, 20 mm HEPES buffer) (Bennett and Caulfield, Reference Bennett and Caulfield1991). Between the washing steps schistosomula were centrifuged at 1000 g for 1 min.
Pre-adults
Two 6-week-old NMRI mice were infected with S. mansoni while sitting in a 50 ml water-bath containing 500 cercariae for 90 min. Six days after the infection the mice were sacrificed. The lungs were rinsed in situ with flush solution (FS; RPMI-1640, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% heparin) by using a sterile 20 ml syringe with a winged perfusion needle. This needle was inserted into the right cardiac ventricle and the left ventricle was punctured with another sterile needle. The FS was pressed into the blood circulation through the right ventricle and was recovered from the left ventricle to remove the blood from the lungs. The flushed lungs were dissected into small pieces with a scalpel and incubated in adult culture medium (ACM; RPMI-1640, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% FCS; El-Ridi et al. Reference El-Ridi, Ozaki, Inaba, Ito and Kamiya1997) at 37°C and 5% CO2 for 3 h. The pre-adults were separated from the lung tissue by a filter with a 200 μm mesh (Miller and Wilson, Reference Miller and Wilson1978). Then 30–50 pre-adults were cultivated in a 2 ml tube with a 0·2 μm membrane lid containing 1 ml of ACM at 37°C and 5% CO2 for 24 h. The viability of the pre-adults was determined microscopically at 200-fold magnification. The culture medium was changed and the pre-adults were incubated with 1 ml of ACM and 7·5 nmol/ml Afl-HSA at 37°C and 5% CO2 for 5 min, 10 min, 15 min or 5 h to examine the uptake of the conjugate over time. The pre-adults were then washed 3 times with adult wash solution (AWS; RPMI-1640, 100 U/ml penicillin, 100 μg/ml streptomycin). Between the washing steps the life stages were centrifuged at 1000 g for 1 min.
Adults
Two 6-week-old NMRI mice were infected with 300 cercariae of S. mansoni as described above and sacrificed 70 days after infection. To recover the adult worms the portal vein was dissected near the hilus region. Then a winged perfusion needle was inserted into the right cardiac ventricle and the FS was pressed into the blood circulation with a 20 ml syringe. The flushed worms were immediately transferred into AWS and washed until the culture medium was cleared from blood. Ten adult worms were cultured per cavity of 6-well plates (NUNC, Germany) containing 5 ml of ACM at 37°C and 5% CO2 for 24 h. The viability of the cultured worms was examined under the dissecting microscope at 5-fold magnification. The culture medium was changed and adults were incubated with 7·5 nmol/ml Afl-HSA per well at 37°C and 5% CO2 for 5 min, 10 min, 15 min or 5 h to examine the uptake of the conjugate. Another group was incubated with an equivalent dose of Afl-HSA for a prolonged period of 30 h to observe the degradation of the conjugate. After incubation the adult worms were washed 3 times with AWS.
In vivo experiments
Schistosomula
For recovering schistosomula 3 six-week-old mice were anaesthesized with 6 mg/kg bodyweight xylazin hydrochloride (Rompun® 2%, Bayer, Germany) and 90 mg/kg bodyweight ketamine hydrochloride (Ketamin 10%, Essex Healthcare, Germany). One centimetre of a mouse tail was then fixed in a tube, filled with water containing 500 cercariae, and incubated for 30 min. After 24 h, 0·4 ml of Afl-HSA (60·16 μmol/kg bodyweight) were injected into the tail vein of the infected mice. One day later, the mice were sacrificed and the tail skin, where cercariae had been allowed to invade, was removed. By using a scalpel the skin was chopped into small pieces and incubated in SCM for 3 h to assure the emigration of the schistosomula into the medium.
Pre-adults and adults
For the recovery of pre-adults and adults 5 six-week-old mice per stage were infected as described for the in vitro experiments. To examine the uptake of albumin by pre-adults and adults 0·4 ml of Afl-HSA (pre-adults: 60·16 μmol/kg bodyweight, adults: 42·97 μmol/kg bodyweight) were injected into the tail veins of the mice 6 days and 70 days after infection with cercariae, respectively. One day after the injection of Afl-HSA, all mice were sacrificed and the pre-adults and adults were isolated from the lungs or the portal vein as described above.
Fluorescence microscopy
Immediately after the final washing steps of the in vitro experiments or the recovery from the mice, the schistosomula and pre-adults were examined microscopically at 200-fold magnification and the adult worms at 100-fold magnification to detect green or blue fluorescence in the digestive, the excretory and the reproductive systems or the outer surface, respectively. All helminthic stages were examined alive under a Nikon Eclipse E 600 (NIKON GmbH, Düsseldorf, Germany) fluorescence microscope with FITC filters to detect green fluorescence (excitation filter (EX): 465–495 nm, barrier filter (BA): 515–555 nm) and a COHU high performance CCD camera. Because of the blue autofluorescence of the body surface, additional images were taken with DAPI filters (EX: 340–380, BA: 435–485) to visualize the body contours of the different helminthic stages. The images from FITC and DAPI filters were put on top of each other with the image processing software (4-Mac-Probe 4.0; Perceptive Scientific Instruments International Ltd, Chester, UK). However, because of worm movement in some cases the images were taken only with the FITC filters.
Control of autofluorescence
Ten schistosomula, 10 pre-adults and 5 male as well as 5 female adults of S. mansoni, served as controls without Afl-HSA treatment and were examined for green autofluorescence by fluorescence microscopy at 100- and 200-fold magnification with the FITC filters.
RESULTS
Ingestion of Afl-HSA in vitro
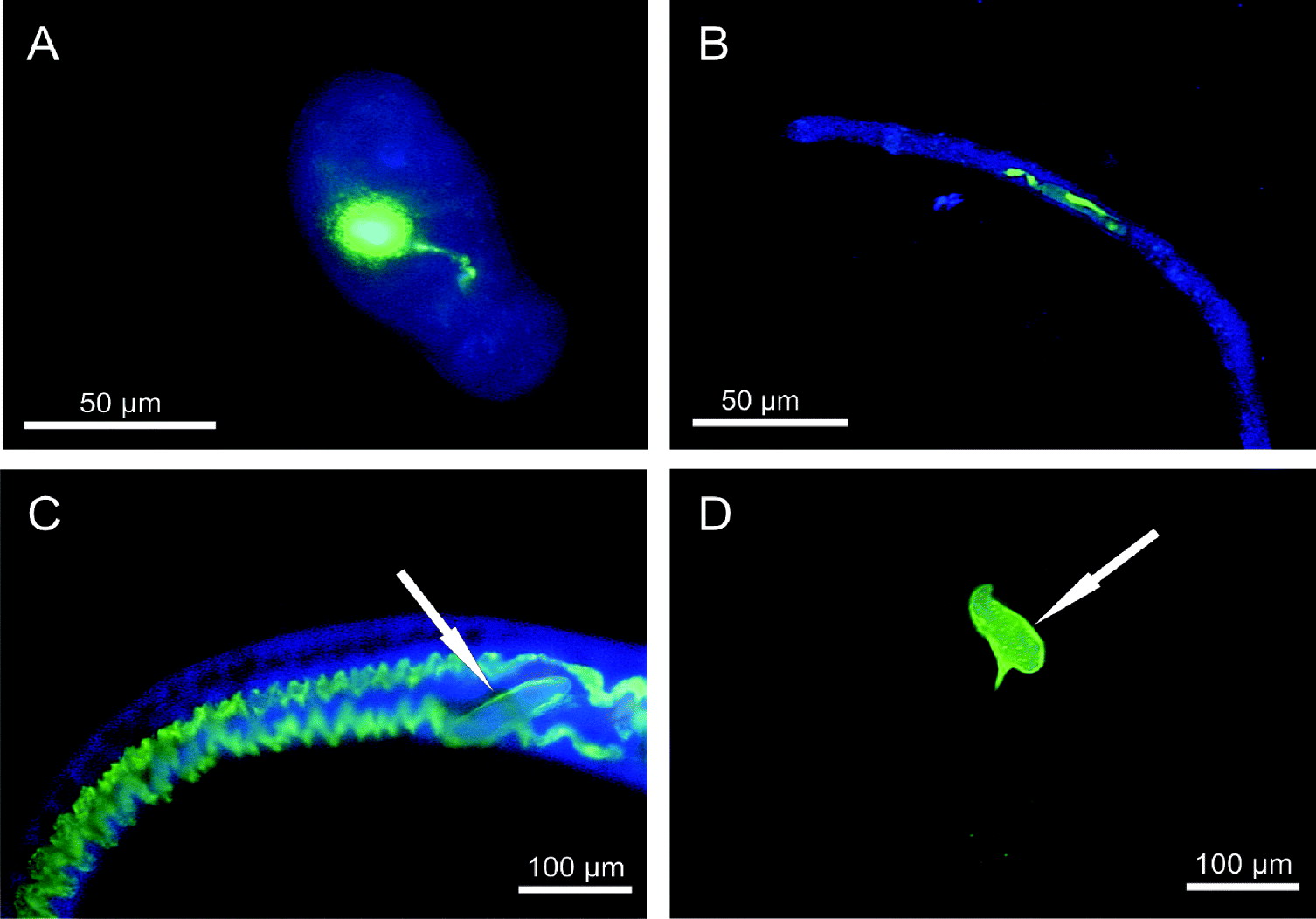
When schistosomula were incubated with 7·5 nmol/ml Afl-HSA for 5 min, an intense green fluorescence could be detected within the intestinal tract of all examined schistosomula. One of 4 (25%) showed the fluorescence only in the oesophagus, whereas 3 of 4 (75%) exhibited fluorescence in the oesophagus and the caecum. After 10 min, 15 min, 30 min and 5 h an intense green fluorescence within both oesophagus and caecum could be observed in all examined schistosomula (Fig. 1A, Table 1).

Fig. 1. In vitro ingestion of Afl-HSA by schistosomula (A), pre-adults (B) and adults (C) of Schistosoma mansoni, showing an intense green fluorescence in the oesophagus and the caecum after 5 h. In vitro accumulation (intense green fluorescence) of Afl-HSA in the flame cells of the excretory system of adults (D) of S. mansoni after 30 h. (Fluorescence microscopy; FITC filters for pictures A, B, C, D and DAPI filters for pictures A, B, C).
Table 1. Patterns of fluorescence of schistosomula, pre-adults and adults at different time-points after incubation with Afl-HSA in vitro

When pre-adults were incubated with the albumin conjugate for 5 min, an intense green fluorescence could only be observed in 1 of 11 (9%) examined stages. A slight green fluorescence within both oesophagus and caecum was observed in 7 of 11 (64%) whereas 3 of 11 (27%) exhibited the fluorescence only within the caecum. After 10 min, 7 of 8 (88%) showed an intense green fluorescence within the whole digestive system, 1 of 8 (12%) showed a slight green fluorescence within the caecum. After 15 min, 8 of 10 (80%) pre-adults exhibited an intense green fluorescence within the oesophagus and caecum. One of 8 (10%) exhibited the green fluorescence only within the caecum and another 10% did not show fluorescence within the digestive system. At the longer incubation periods of 30 min and 5 h, 10 and 86 pre-adults, respectively, showed an intense green fluorescence in the oesophagus and the caecum (Fig. 1B, Table 1).
When incubating adult worms with 7·5 nmol/ml Afl-HSA for 5 min, 3 of 4 (75%; 1♂, 2♀) adult worms did not show green fluorescence within the digestive system, whereas a slight green fluorescence within the oesophagus and the caecum could be observed in 1 of 4 (25%; 1♂). After 10 min a slight green fluorescence within the oesophagus and caecum could be observed in 40% (2/5; 2♂) of the examined adults. Another 40% (2/5; 1♂, 1♀) of adults showed no green fluorescence within the entire digestive system and only 20% (1/5; 1♀) showed an intense green fluorescence within the oesophagus and caecum. After 15 min, 43% (3/7; 2♂, 1♀) of the examined parasites showed a slight green fluorescence within the oesophagus and caecum, while 57% (4/7; 2♂, 2♀) exhibited no signs of green fluorescence. After 30 min, 5 h and 30 h all 9 (7♂, 2 ♀), 30 (17♂, 13♀) and 16 (11♂, 5♀) examined adults, respectively, showed an intense green fluorescence within the oesophagus and the caecum (Fig. 1C, Table 1). After the prolonged incubation period of 30 h, 7 of 16 (44%; 7♂) of the adults showed a green fluorescence of the flame cells of the excretory system (Fig. 1D). Afl-HSA did not bind to the outer surface of any of the examined stages in vitro.
Ingestion of Afl-HSA in vivo
All 60 schistosomula, recovered from the tail skin, and all 203 pre-adults, recovered from the lungs, showed an intense green fluorescence within the oesophagus and the caecum after treating infected mice with 60·16 μmol/kg bodyweight Afl-HSA for 24 h (Fig. 2A–B). All 105 adult worms (61♂, 44♀), recovered from the portal venous system of mice showed an intense green fluorescence within the entire digestive system after treating mice with 42·97 μmol/kg bodyweight Afl-HSA for 24 h (Fig. 2C). As in the in vitro experiments Afl-HSA did not attach to the outer surface of any examined life stage in vivo.
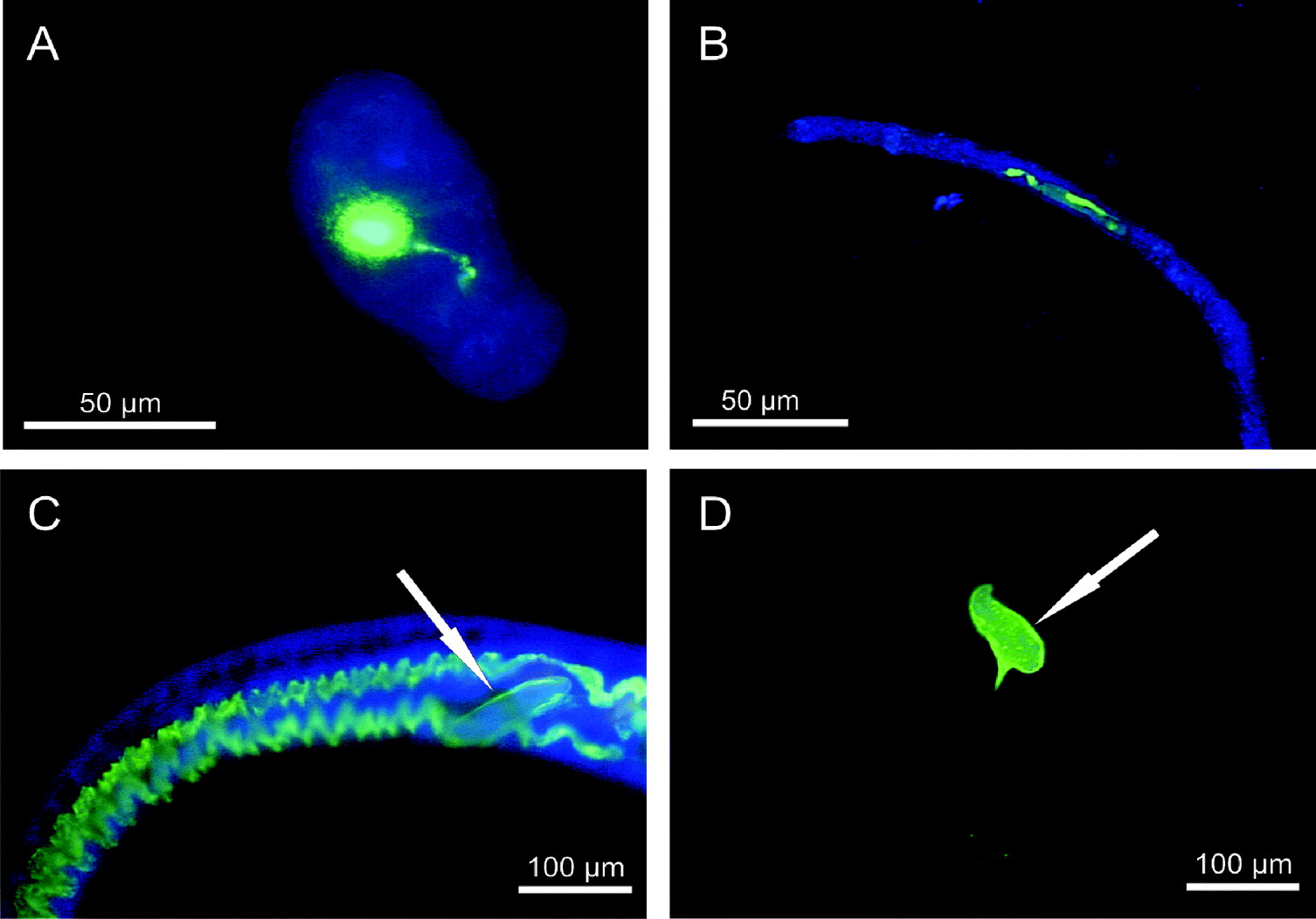
Fig. 2. In vivo ingestion of Afl-HSA by schistosomula (A), pre-adults (B, pre-adult is in the stretched phase of movement) and adults (C) of Schistosoma mansoni showing an intense green fluorescence of the entire digestive system after 24 h. Green autofluorescence of the eggs (white arrows) is detectable in treated (C) and untreated adult females (D). (Fluorescence microscopy; FITC filters for pictures A, B, C, D and DAPI filters for pictures A, B, C).
Control group
None of the 20 schistosomula, 8 pre-adults and 5 male adults, exhibited a green autofluorescence of the digestive system, the outer tegument, the excretory and the reproductive system. In none of the 5 female adults was green fluorescence of the digestive system detectable. In all females, an intense autofluorescence of the eggs, but not of the reproductive system or the excretory system was detectable (Fig. 2D). Three females showed a slight green autofluorescence of the tegument of the caudal part of the body.
DISCUSSION
In our in vitro experiments schistosomula and pre-adults ingested Afl-HSA rapidly within the first 10 min of incubation, as shown by intense green fluorescence within the entire digestive system of almost all examined life stages at this time-point. The same pattern was observed after 15 min, 30 min and 5 h of incubation. There was a time-dependent uptake of the Afl-HSA, suggesting that early developmental stages rapidly ingest proteins from the culture medium or serum. This hypothesis is supported by an earlier study that showed the ingestion of albumin by schistosomula of S. mansoni. Mechanically transformed schistosomula were found to ingest FITC-BSA within 30 min of incubation, exhibiting green fluorescence either in the oesophagus or the caecum. The authors proposed that ingested macromolecules enter the oesophagus by osmotic pressure and are transported to the caecum by peristalsis. The digestion of proteins by proteases in the caecum, and the uptake of the resulting peptides by cells of the gut epithelium reduce the osmotic pressure, so that the ingestion continues. The authors verified their theory by demonstrating, by thin-layer chromatography, the proteolytic degradation of FITC-BSA into peptides with a length of 10–15 amino acids within the gut (Bennett and Caulfield, Reference Bennett and Caulfield1991).
In contrast to the early developmental stages, adult worms exhibited an intense green fluorescence of the entire digestive system at the earliest after 30 min incubation with Afl-HSA. While ingestion of molecules seems to be based on the osmotic pressure in schistosomula and pre-adults, adult worms ingest molecules actively by suction created by the muscles of the oral sucker and the oesophagus (Halton, Reference Halton1997). Therefore, adult worms might need longer to ingest albumin and fill the entire digestive system.
Nutritional uptake via the tegument has been discussed for schistosomes previously. In the case of glucose, uptake occurs via specific schistosomal glucose transporter proteins (SGTP). SGTP1 and SGTP4, both localized in the tegument but not in the gut, are involved in the uptake of glucose (Fripp, Reference Fripp1967; Uglem and Read, Reference Uglem and Read1976; Skelly et al. Reference Skelly, Tielens and Shoemaker1998). Since we did not observe any green fluorescence of the outer worm surface in our experiments, there seems to be no albumin uptake via the tegument. The bright fluorescence within the lumen of the gut indicated an exclusively oral uptake of albumin by schistosomula, pre-adults and adult worms.
After a prolonged incubation period of 30 h we additionally observed an intense green fluorescence of the flame cells of the excretory system of adult worms in vitro. The microscopic morphology of the flame cells was similar to that described in previous studies (Bogers et al. Reference Bogers, Nibbeling, Van Marck and Deeler1994; Sato et al. Reference Sato, Kusel and Thornhill2003). Earlier studies reported that fluorescent dyes or proteins bound to fluorescent dyes are able to enter the excretory system directly. The fluorescent dyes resorufin and Texas Red – bovine serum albumin (TxR-BSA) entered the excretory system of adult schistosomes within 60 min of incubation (Sato et al. 2002; Wippersteg et al. Reference Wippersteg, Ribeiro, Liedtke, Kusel and Grevelding2003; Kusel et al. Reference Kusel, Oliveira, Todd, Ronketti, Lima, Mattos, Reis, Coelho, Thornhill and Ribeiro2006). The detailed uptake mechanisms still remain unclear, but it is discussed that e.g. resorufin enters the schistosomal body and is then excreted via the excretory system (Sato et al. 2004). Furthermore, tegumental damage resulted in the rapid labelling of the excretory system with the fluorescent markers resorufin and TxR-BSA in schistosomula and adults within several minutes (Wippersteg et al. Reference Wippersteg, Ribeiro, Liedtke, Kusel and Grevelding2003; Tan et al. Reference Tan, Thornhill, Al-Adhami, Akhkha and Kusel2003). However, in our experiments the fluorescence labelling of the excretory system did not occur rapidly, but only after 30 h of incubation and the albumin conjugate solution contained less than 1% free aminofluorescein, which did not accumulate on the body surface. Therefore we conclude that there was no direct uptake of the dye via diffusion from the medium or via strongly damaged tegumental areas. Since earlier studies, using interference RNA (RNAi) and specific protease inhibitors, reported on the digestion of bovine and mouse albumin by a schistosomal multi-enzyme network composed of cathepsin proteases and an asparaginyl endopeptidase, we assume, that albumin might be degraded within the gut lumen. Hence resulting peptide fragments bound to aminofluorescein might be taken up via gut cells and finally excreted via the excretory system (Delcroix et al. Reference Delcroix, Sajid, Caffrey, Lim, Dvorak, Hsieh, Bahgat, Dissous and McKerrow2006).
Since albumin is the most common protein and the biggest amino acid reservoir within human blood and, with respect to the previous studies regarding ingestion and digestion by schistosomes, albumin might be a major source of energy supply for schistosomula and pre-adults. Moreover, albumin seems to be an additional source of energy supply for adult schistosomes, as recent studies reported on the underdevelopment of both sexes of adult worms in undernourished mice, which were fed on a protein-deficient diet (Neves et al. Reference Neves, Machado-Silva, Pelajo-Machado, Oliviera, Coutino, Lenzi and Gomes2001; Barros et al. Reference Barros, Neves, De Moura and Machado-Silva2009).
Drug therapy of bilharziosis is currently not successful until the schistosomes have matured and reached the portal venous system, which is not earlier than 30 to 40 days after infection (Silva et al. Reference Silva, Menezes, Andrade de Oliveira and Andrade2003). As albumin is rapidly ingested and also digested by all schistosomal stages, it seems suitable as a carrier molecule for drugs. A carrier substance can enable enrichment of linked drugs in the target structures, such as in tumors or, as in our case, helminths. Albumin carrying the cytostatic drug methotrexate has successfully prevented the onset of rheumatoid arthritis (Wunder et al. Reference Wunder, Müller-Ladner, Stelzer, Funk, Neumann, Stehle, Pap, Sinn, Gay and Fiehn2003). It was used for prophylaxis of graft-versus-host disease in animals (Wolff et al. Reference Wolff, Frei, Hofmeister, Steiner, Kleine, Junghanss, Sievert, Terpe, Schrenk, Freund and Hartung2006) and for cancer treatment in clinical Phase I and II trials (Hartung et al. Reference Hartung, Stehle, Sinn, Wunder, Schrenk, Heegers, Kranzle, Frei, Fiebig, Heene, Maier-Borst and Queisser1999; Vis et al. Reference Vis, van der Gaast, van Rhijn, Catsburg, Schmidt and Mickisch2002; Bolling et al. Reference Bolling, Graefe, Lübbing, Jankevicius, Uktveris, Cesas, Meyer-Moldenhauer, Starkmann, Weigel, Burk and Hanauske2006). Systemic side-effects of methotrexate were greatly reduced when linked to albumin as a carrier.
Our findings of Afl-HSA ingestion, especially in early schistosomal stages, may be a step towards a potential drug-targeting treatment of early schistosomiasis with albumin bound to praziquantel or other anthelminthic drugs. Further studies have to show whether a drug bound to albumin has enhanced activity against juvenile and adult parasites.
ACKNOWLEDGEMENTS
The authors thank Dr Agnes Knopp (Division of Hematology and Oncology, University of Rostock) and Kathrin Sievert (Division of Experimental Surgery, University of Rostock) for their expert technical assistance.