Introduction
Treatment modalities for supraglottic squamous cell carcinoma include open supraglottic laryngectomy, transoral laser surgery, transoral robotic surgery and primary chemoradiotherapy.Reference Tomeh and Holsinger1
Radiotherapy (RT) and chemoradiotherapy have increasingly been used to treat laryngopharyngeal cancers located in the supraglottic region in the last few decades; however, these treatment modalities have several negative effects. Even long after treatment cessation, the persistence of severe mucositis, pain and dysphagia may be debilitating.Reference Iqbal, Chaw, Kovarik, Aslam, Jackson and Kelly2, Reference Rivelli, Mak, Martins, da Costa e Silva and de Castro3 The absence of pathological verification of nodal status in the neck may result in overtreatment with full curative radiation to both sides of the neck, in light of the significant possibility of regional metastasis. If radiation is used early as a primary treatment for resectable tumours, RT will not be an option when second tumours arise, which occurs in a significant portion of these patients.
Transoral laser surgery for supraglottic laryngeal tumours has been described by Weinstein and colleagues.Reference Weinstein, O'Malley, Snyder and Hockstein4 Despite its advantages, transoral laser surgery has some limitations, such as piecemeal resection of the tumour, manipulation of tissue only by one hand, the risk of disorientation of an unexperienced surgeon during surgery, and limited linear resection ability associated with the use of a microscope and laser light.
In addition to laser surgery, transoral robotic surgery has also been investigated for the treatment of supraglottic laryngeal carcinoma. Feasibility of the surgery has been verified, and early oncological and functional outcomes are encouraging.Reference Weinstein, O'Malley, Snyder and Hockstein4–Reference Durmus, Gokozan and Ozer7 Advantages of transoral robotic surgery include: high-quality three-dimensional imagination, wide exposure, en bloc resection of even big tumours, tremor filtration and free movement of angulated robotic arms.
The current study aimed to compare the functional and oncological outcomes of transoral robotic supraglottic laryngectomy and open supraglottic laryngectomy in patients with supraglottic carcinoma.
Materials and methods
Study design
A retrospective chart review was conducted of all patients with supraglottic carcinoma treated at our institute. All patients were discussed at the Tumor Board of the University of Health Sciences Head and Neck Cancer Center to work out a multidisciplinary treatment plan.
Surgical treatment was recommended to patients, having taken into account the following factors: patients’ preference, morbidity related to chemoradiotherapy, general performance and so on. All transoral robotic supraglottic laryngectomies were performed by one head and neck surgeon (CO). Open supraglottic laryngectomies were performed by two head and neck surgeons (BK and İD). Informed patients’ overall preferences, patients’ insurance status (robotic surgery is not covered by the national social insurance system) and access to the robotic surgery system (which is not available for all operation days) were considered when making decisions regarding the surgical method.
Patient population
The charts of patients with supraglottic carcinoma operated on between May 2010 and June 2014 were reviewed. During this period, 17 patients underwent transoral robotic supraglottic laryngectomy and 20 patients underwent open supraglottic laryngectomy.
Inclusion and exclusion criteria
All patients who underwent transoral robotic supraglottic laryngectomy or open supraglottic laryngectomy with curative intent for supraglottic squamous cell carcinoma were included in the study. There were no exclusion criteria.
Transoral robotic surgery
In the robotic surgery group, the da Vinci Robotic System (Intuitive Surgical, Sunnyvale, California, USA) was set as reported by Park et al.Reference Park, Lee, Lee, Lee, Choi and Chung8 Transoral robotic supraglottic laryngectomy was performed as previously described.Reference Weinstein, O'Malley, Snyder and Hockstein4, Reference Park, Kim, Byeon, Lee and Kim5
Transnasal intubation was preferred to keep the intubation tube away from the surgical area. A transoral Feyh-Kastanbauer retractor (Gyrus ACMI, Southborough, Massachusetts, USA) was used for wide exposure. A 30-degree, up-facing, binocular robotic camera, Maryland dissector and monopolar cautery spatula arm were used.
The first incisions were made superior to the ventral base of the epiglottis in a horizontal direction. The incision was continued through the pre-epiglottic space, up to hyoid bone and along the aryepiglottic folds. Dissection was continued inferiorly to the petiole of the epiglottis. Contralateral incisions were made above the glottis, into the ventricle and around the lesion. The specimen was resected and removed from the surgical field. The Maryland dissector and monopolar cautery spatula arm were used for all transoral robotic supraglottic laryngectomies.
Negative intra-operative margins were confirmed by histopathological analysis in all patients.
Open surgery
Open supraglottic laryngectomy was performed as reported by Alonso.Reference Alonso9 The procedure involved resection of supraglottic laryngeal tissues, including the false vocal folds, epiglottis and aryepiglottic folds, with the upper one-third of thyroid cartilage, by a transcervical approach. After resection, the remaining laryngeal tissues were approximated to the tongue base with sutures passing beyond the hyoid bone.
Neck treatment
Bilateral neck dissections were performed on all patients in light of the occult metastatic nature of the disease. The open supraglottic laryngectomy group underwent the neck dissections concurrently, but the transoral robotic supraglottic laryngectomy group underwent the neck dissections two to three weeks after the operation.
Post-operative adjuvant therapy
Indications for adjuvant RT, with or without chemotherapy, were based on histopathological evaluation of paraffin-embedded sections. It was recommended for patients with any of the following conditions: stage III or IV disease, extracapsular nodal spread, multiple lymph node involvement, or peri-neural or lymphovascular invasion.
Statistical methods
Age, sex, primary tumour subsite, tracheostomy requirement, decannulation time, oral feeding time, hospitalisation duration, additional therapies, follow-up period, early and late complications, and survival rates were recorded for both groups.
The Kolmogorov–Smirnov test was used to determine whether data were normally distributed. The Mann–Whitney U test was used to compare the continuous variables between the two groups. A p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS software package (version 19.0; SPSS, Chicago, Illinois, USA).
Results
Patients’ characteristics
Patients’ clinical characteristics are shown in Table 1. The mean age of patients was 62.2 years (range, 53–86 years) for the transoral robotic supraglottic laryngectomy group and 57.5 years (range, 39–78 years) for the open supraglottic laryngectomy group. The transoral robotic surgery and open surgery groups consisted of 2 (11 per cent) and 2 (10 per cent) females, respectively. The groups were similar in terms of age and gender distribution (p = 0.08 and p = 0.86 respectively).
Table 1. Clinical and pathological data for robotic and open surgery groups
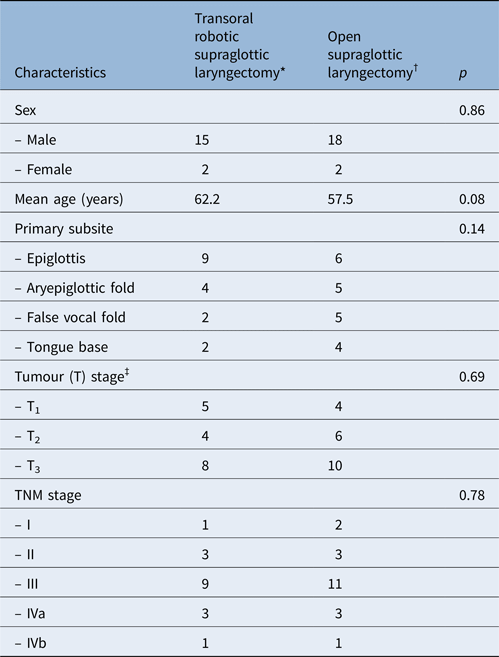
Data represent numbers of patients, except where indicated otherwise. *n = 17; †n = 20. ‡Pathologically confirmed. TNM = tumour–node–metastasis
In the transoral robotic surgery group, the primary tumour involved: the epiglottis (n = 9, 52 per cent), the epiglottis and aryepiglottic fold (n = 4, 25 per cent), the epiglottis and false vocal fold (n = 2, 11 per cent), and the tongue base and vallecula (n = 2, 11 per cent). In the open surgery group, it involved: the epiglottis (n = 6, 30 per cent), the epiglottis and aryepiglottic fold (n = 5, 25 per cent), the epiglottis and false vocal fold (n = 5, 25 per cent), and the vallecula and tongue base (n = 4, 20 per cent).
All patients underwent selective neck dissection, including levels II, III and IV, or modified radical neck dissections, bilaterally.
Tumours were staged according to the guidelines of the American Joint Committee on Cancer. In the transoral robotic supraglottic laryngectomy group, five patients were tumour stage T1, four were T2 and eight were T3. In the open supraglottic laryngectomy group, 4 patients were T1, 6 were T2 and 10 were T3. Regarding tumour–node–metastasis (TNM) stage, in the transoral robotic surgery group one patient was stage I, three were stage II, nine were stage III, three were stage IVa and one was stage IVb. In the open surgery group, 2 patients were stage I, 3 were stage II, 11 were stage III, 3 were stage IVa and 1 was stage IVb. There was no significant difference in tumour stage or tumour involvement subsites between groups (p = 0.78 and p = 0.14 respectively, Table 1).
Thirteen patients in the transoral robotic surgery group and 15 patients in the open surgery group received adjuvant RT, with or without chemotherapy. This was because one or more of the following conditions were present: stage III or IV disease, multiple lymph node involvement, and/or extracapsular spread.
Tumours were successfully removed in all patients. Multiple frozen biopsy specimens were studied. Negative margins were achieved intra-operatively in all patients.
Functional outcomes
We did not perform tracheostomy in the transoral robotic supraglottic laryngectomy group. However, one patient in this group required tracheostomy six months after the surgery because of bilateral vocal fold paralysis induced by RT. Patients in the open supraglottic laryngectomy group routinely had intra-operative tracheostomy. Mean decannulation time for the open surgery group was 34.7 days (range, 12–75 days).
Mean removal of the feeding tube occurred at 7 days (range, 0–12 days) in the transoral robotic supraglottic laryngectomy group and at 12.2 days (range, 7–53 days) in the open supraglottic laryngectomy group. There was a significant difference in oral feeding duration between groups (p < 0.01; Figure 1).
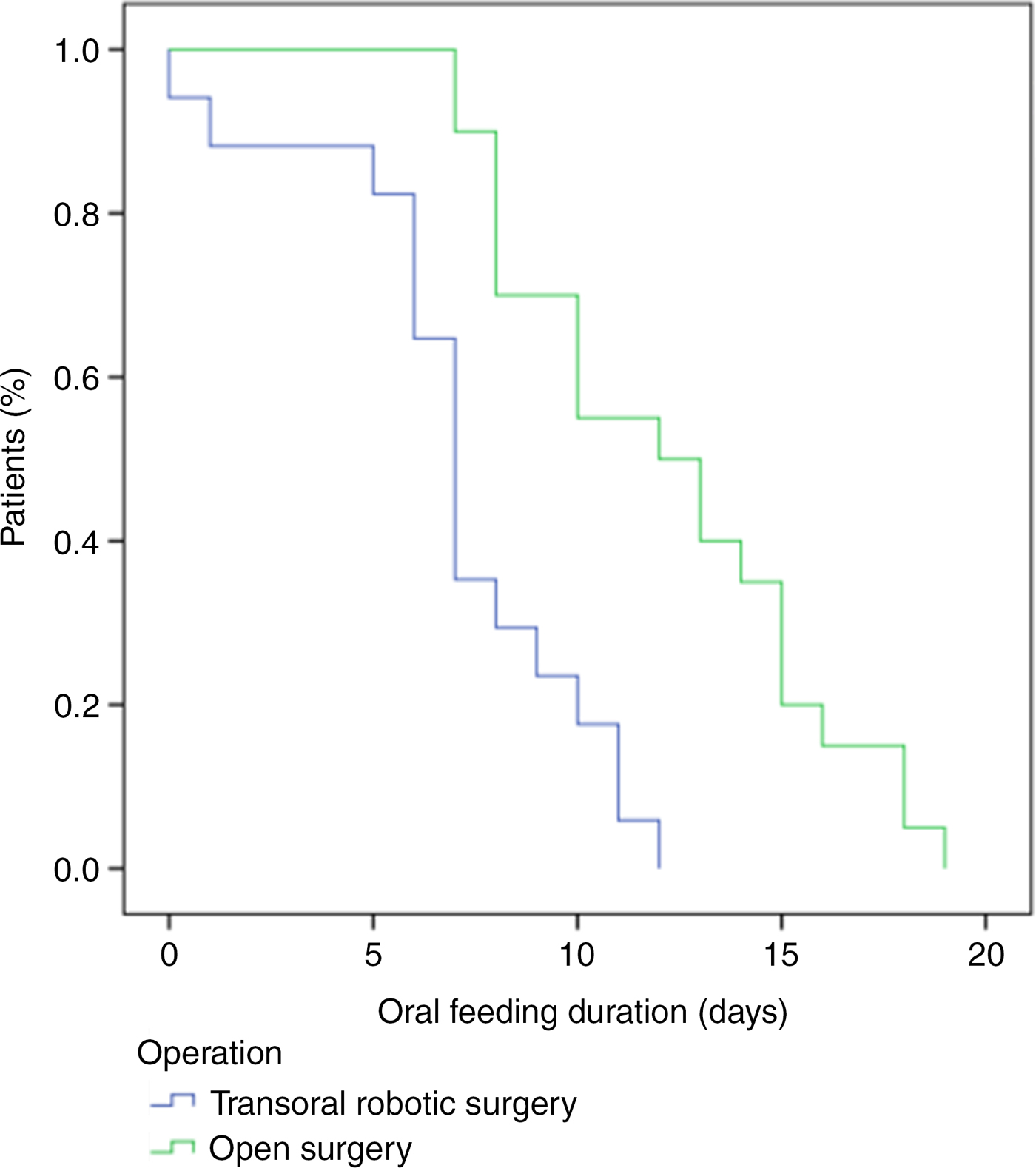
Fig. 1. Comparison of oral feeding duration for the transoral robotic supraglottic laryngectomy group and the open supraglottic laryngectomy group revealed a significant difference between the groups (p < 0.01).
Mean hospitalisation duration was 8.8 days (range, 0–14 days) and 14.7 days (range, 7–35 days) for the transoral robotic supraglottic laryngectomy group and the open supraglottic laryngectomy group, respectively; this difference was statistically significant (p = 0.001).
Treatment outcomes and overall survival
Mean follow-up duration was 25.8 months (range, 0–84 months) for the transoral robotic supraglottic laryngectomy group and 41 months (range, 21–82 months) for the open supraglottic laryngectomy group. One patient in the transoral robotic surgery group died on post-operative day 2 because of acute renal insufficiency. One patient from each group died following regional recurrence in the neck. Pathological assessment showed N3 neck involvement in both of these patients. All other patients were alive and disease-free when these data were collected.
The overall survival rate was 88 per cent for the transoral robotic supraglottic laryngectomy group and 95 per cent for the open supraglottic laryngectomy group. The disease-specific survival rate was 94 per cent for the robotic surgery group and 95 per cent for the open surgery group. There was no difference between groups in terms of overall survival and disease-specific survival rates (p = 0.22 and p = 0.49, respectively; Figures 2 and 3).

Fig. 2. Disease-specific survival for the transoral robotic supraglottic laryngectomy group and the open supraglottic laryngectomy group, analysed using the Kaplan–Meier method and compared using the log rank test (p = 0.49).
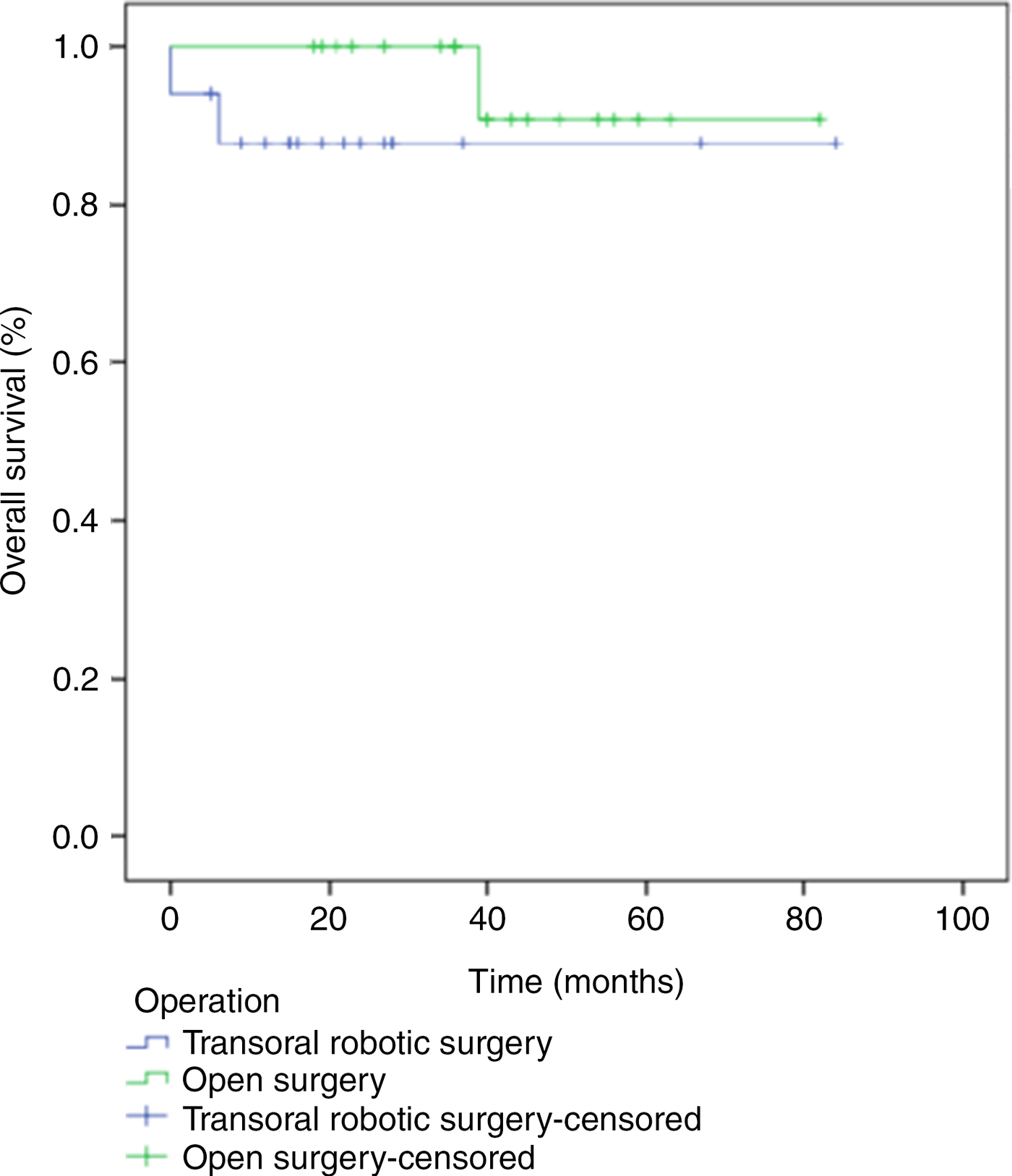
Fig. 3. Overall survival for the transoral robotic supraglottic laryngectomy group and the open supraglottic laryngectomy group, analysed using the Kaplan–Meier method and compared using the log rank test (p = 0.22).
There were no peri-operative complications in either group. No conversion from robotic surgery to an open approach was required in any patient. Two patients from the open surgery group and one patient from the robotic surgery group had pneumonia. One patient in the transoral robotic supraglottic laryngectomy group had vocal fold paralysis due to RT at six months post-operatively.
Discussion
Organ preservation, in addition to curing the cancer, is one of the main considerations in the treatment of supraglottic laryngeal carcinoma. Modern surgical treatments include transoral laser surgery, transoral robotic supraglottic laryngectomy and conventional open supraglottic laryngectomy. The outcome of these surgical modalities should be comparable to results obtained with total laryngectomy. In addition, respiration, phonation and swallowing function should be retained in order to improve the patients’ quality of life.
• Transoral robotic supraglottic laryngectomy provides a shorter recovery time for supraglottic laryngeal cancer patients than conventional surgery
• The main limitation of the procedure is inadequate exposure
• Robotic surgery for T4 supraglottic cancer has been described in the literature
• However, we maintain robotic supraglottic laryngectomy is more appropriate for tumours without extralaryngeal involvement
Traditionally, open surgery was the only surgical option for supraglottic laryngeal cancers. Open supraglottic laryngectomy is a well-defined surgery, and is still an option in some circumstances. However, the introduction of transoral surgical procedures for supraglottic lesions has shifted the surgical approach.
Transoral robotic supraglottic laryngectomy was introduced for supraglottic laryngeal cancer over a decade ago. It was originally described by Weinstein et al. for the treatment of T1, T2 and selected T3 tumours of the supraglottic larynx.Reference Weinstein, O'Malley, Snyder and Hockstein4
In our patient series, a temporary peri-operative tracheostomy was not needed in patients who underwent transoral robotic surgery. In contrast, the average decannulation time for open supraglottic laryngectomy was 34.7 days. Park et al. performed temporary tracheostomy on all patients who underwent transoral robotic supraglottic laryngectomy, with tube removal occurring at an average of 9 days post-operatively.Reference Park, Byeon, Chung, Choi and Kim10 Decannulation times were shorter in the transoral robotic surgery group than in open conventional surgery group.Reference Park, Byeon, Chung, Choi and Kim10 Some authors have preferred long intubation times after surgery, with an average extubation time of 24–48 hours instead of tracheostomy for transoral robotic supraglottic laryngectomy.Reference Mendelsohn and Remacle11, Reference Kayhan, Kaya, Altintas and Sayin12 A recent multicentre study of transoral robotic supraglottic laryngectomy reported that 24 per cent of the patients had temporary tracheostomy for an average of 8 days.Reference Razafindranaly, Lallemant, Aubry, Moriniere, Vergez and Mones13 However, the indications for tracheostomy were not clear in that study. This may be because of the nature of multicentre data collection and surgeon preference. Although we did not perform any peri-operative tracheostomies in the robotic surgery group, we believe that decisions about performing it should be made according to the patient.
Our mean follow-up period was 25.8 months for the transoral robotic supraglottic laryngectomy group and 41 months for the open supraglottic laryngectomy group. One patient in the robotic surgery group died at day 2 post-operatively because of acute renal insufficiency. There was one death in each group as a result of neck recurrence of N3 neck disease. There was no difference between the groups in overall survival or disease-specific survival rates.
Park et al. reported a mean follow-up period of 27.3 months for transoral robotic supraglottic laryngectomy patients; in addition, they did not find any significant difference in overall survival or disease-free survival rates between transoral robotic surgery and open surgery groups.Reference Park, Byeon, Chung, Choi and Kim10 The largest case series of patients who underwent transoral robotic supraglottic laryngectomy was reported by a French research group, but their mean follow-up period was short (14 months) and they did not report any survival rates, possibly because of the relatively short follow-up periods.Reference Razafindranaly, Lallemant, Aubry, Moriniere, Vergez and Mones13 Based on these findings, and the lack of any difference in survival rates between the transoral robotic surgery group and the open surgery group in our study, we suggest that transoral robotic supraglottic laryngectomy provides acceptable oncological results in comparison with open supraglottic laryngectomy.
Nasogastric tube dependence is one of the factors determining hospitalisation duration. In our study, nasogastric tube dependence was significantly shorter in the robotic surgery group because of the faster recovery of swallowing ability. Our patients in the transoral robotic surgery group were able to tolerate oral feeding at an average of 7 days (range, 0–12 days). None of our patients required gastrostomy. The aforementioned French research group reported that 24 per cent of patients who underwent transoral robotic supraglottic laryngectomy were able to start an oral diet within 24 hours of surgery, without a feeding tube requirement.Reference Razafindranaly, Lallemant, Aubry, Moriniere, Vergez and Mones13 The remaining patients had a nasogastric tube, with a median use of 8 days, and 9.5 per cent had definitive percutaneous endoscopic gastrostomy feeding.Reference Razafindranaly, Lallemant, Aubry, Moriniere, Vergez and Mones13 Park et al. also reported shorter swallowing recovery times for the transoral robotic supraglottic laryngectomy group than the open supraglottic laryngectomy group.Reference Park, Byeon, Chung, Choi and Kim10 Kayhan et al. were able to start oral feeding at a mean of 10.8 days in 13 patients who underwent transoral robotic supraglottic laryngectomy.Reference Kayhan, Kaya, Altintas and Sayin12 Ozer et al. reported that they were able to start oral feeding in all patients (n = 13) at post-operative day 1; however, two of the patients did not tolerate oral feeding and one of them needed temporary gastrostomy tube placement.Reference Ozer, Alvarez, Kakarala, Durmus, Teknos and Carrau6 We were not able to start an oral diet as soon as stated in these reports. Although the results of robotic surgery are better than those of open surgery, the robotic surgery patients still required intense swallowing therapy after the surgery.
One of the most precise reports regarding swallowing in transoral robotic supraglottic laryngectomy patients came from Mendelsohn et al.Reference Mendelsohn, Remacle, van der Vorst, Bachy and Lawson14 They reported that patients required 2–29 days (median, 4.5 days) for safe swallowing of solids, and 2–45 days (median, 5.5 days) for safe swallowing of thin liquids. They also concluded that gender, tumour (T) stage, simultaneous neck dissection and vocal fold hypomobility affect post-operative swallowing rehabilitation in transoral robotic surgery patients.Reference Mendelsohn, Remacle, van der Vorst, Bachy and Lawson14
Length of hospitalisation has a considerable financial burden on the national healthcare system. The average hospital stay for the transoral robotic surgery group was shorter than in the open surgery group. However, this difference was not statistically significant in our study. The most important determinants for hospitalisation duration are the presence of a tracheostomy and feeding tube dependence. In the robotic surgery group, we were able to discharge patients from the hospital sooner because of the lack of a tracheostomy and shorter swallowing recovery times. This finding is in accordance with the current literature. Park et al. reported that the average hospital stay for the transoral robotic surgery and open surgery groups was 18.6 days and 24.9 days, respectively.Reference Park, Byeon, Chung, Choi and Kim10
Two patients in the open supraglottic laryngectomy group and one patient in the transoral robotic supraglottic laryngectomy group suffered aspiration pneumonia. This difference was not significant. One might expect a higher number of cases of aspiration pneumonia in the transoral robotic surgery group because of the absence of a tracheostomy, but this was not the case. Our finding may be a result of shorter swallowing function recovery times in this group. Bleeding is another important issue in transoral robotic surgery. However, in our relatively small number of cases, we did not encounter any bleeding following transoral robotic supraglottic laryngectomy.
The main limitation of our study is that it was not a prospective, randomised trial. In addition, the number of cases was relatively small. There is a need for larger series conducted in a prospective manner. Although our mean follow-up period is not short, it would be better to have five-year survival rates.
In this study, we evaluated the functional and oncological results of transoral robotic supraglottic laryngectomy and open supraglottic laryngectomy. In terms of the functional results, we showed that oral feeding time and mean hospitalisation duration were significantly shorter in the transoral robotic surgery group. No statistically significant differences were found for disease-specific survival and overall survival. These findings suggest that transoral robotic surgery offers similar oncological results to open surgery. Transoral robotic supraglottic laryngectomy is an oncologically and functionally safe procedure for T1, T2 and selected T3 tumours of the supraglottic larynx.
Competing interests
None declared






