Introduction
A large number of experimental studies on rodents have shown that the presence of the mother during lactation is crucial to prevent the incidence of negative and irreversible effects on the neurological and cognitive development of the offspring. Actually, adult rats exposed to maternal separations stress (MS) exhibit learning deficits, enhanced vulnerability to depression, increased anxiety and are at high risk of developing eating disorders in adulthood.Reference Ader 1 , Reference Hancock and Grant 23 It has also been documented that animals that experienced episodes of maternal separation during the neonatal period show a clear preference for ingesting palatable foods.Reference Dallman, Akana and Laugero 11 , Reference Silveira, Portella and Clemente 54 We ignore, however, if these deleterious effects are the consequence of decreased somatosensory contact, reduced nutrient supply or heat stress or result from the combination of these factors. Moreover, conflicting results exist concerning the exact nature of the long-term effects of MS. For example, separation from the mother and the rest of the offspring for 5 h daily between the 2nd and 6th day of lactation led to increased plasmatic levels of corticosterone in response to stressReference Biagini, Pich, Carani, Marrama and Agnati 4 separation for half an hour during 3 weeks produced the opposite effect.Reference Ogawa, Mikuni, Kuroda, Muneoka, Mori and Takahashi 41 These discrepancies could be explained by the numerous methodological approaches that have been used to study the long-term effects of MS, including different conditions of temperature and light during maternal separation.Reference Dsilna, Christensson, Gustafsson, Lagercrantz and Alfredsson 16 , Reference Lehmann, Stöhr and Feldon 34 , Reference Ruedi-Bettschen and Feldon 52 , Reference Simon, Pryce, Roff and Klemmack 55
An aspect that is often neglected when analyzing the effects of MS is the fact that, being nocturnal animals, rodents are essentially active during the dark phase of the dark/light cycle. Consequently, the maternal care of the offspring is greater in the light cycle than during the dark, because the dam does not change her biological rhythms and thus expends more time feeding the pups during the light cycle.Reference Simon, Pryce, Roff and Klemmack 55 Moreover, despite the existence of clear sex differences in behavior and other physiological functions, to the best of our knowledge, a systematic study on the effects of MS on both sexes has not been conducted. In the present study, we tested the hypothesis that maternal separation during the period of greatest contact between the mother and offspring in rodents, which occurs during daylight, promotes more intense physiological adjustments in the regulation of energy balance. Specifically, we assessed the effects of MS at different periods of the light and dark cycle on several parameters of feeding behavior in adult male and female rats. These parameters included spontaneous food ingestion, the behavioral satiety sequence (BSS), and food preference for carbohydrates, protein and fat.
Materials and methods
Animals
Wistar albino rats obtained from the Department of Nutrition of the Federal University of Pernambuco were kept under standard housing conditions with controlled temperature (20±2°C), a 12:12 h light:dark cycle (lights on at 6 am), and free access to water and food (Labina®, Purina, Brasil). Animal handling and care followed the recommendations of the Brazilian College of Animal Experimentation and were approved by the Ethics Committee for animal experimentation of the Federal University of Pernambuco (23076.022943/2008-71 Protocol). One day after birth, litters were adjusted at random to eight pups. The number of pups per litter is directly related to methodological requirement of the method per dam, maintaining a 1:1 male to female ratio. After weaning, animals were housed in groups of two animals per cage and fed standard chow (Labina®), until the end of the experiment.
Maternal separation stress
Beginning on postnatal day 1 (PND1), half of the pups from each litter (two male and two female rats), were separated from the dam for 6 h every day until PND14. Controls pups remained with their mothers throughout lactation (C). The experimental pups were moved to group cages with similar origin and with the temperature controlled to 30–32°C, and were kept in another room.Reference Plotsky and Meaney 49 To determine the differences induced by the separation from the dam at different periods of activity, which is associated with different levels of presence of the mother in the nest, maternal separation was carried out either during the light or the dark phase of the light/dark cycle forming the following experimental groups: maternal separation during the dark (MSD) and maternal separation during the light (MSL). Animals were further subdivided by gender so that each experimental group consisted of four male and four female rats. Control pups were not removed from their cages except for cleaning on PND7. After 14 days of life, the animals were kept with their mothers without any further manipulation until weaning.
Spontaneous food intake
The weight of each pup was recorded the day after birth and thereafter every 3–4 days. For the analysis of spontaneous food intake,Reference Orozco-Sólis, Lopes de Souza and Barbosa Matos 44 animals were separated into individual cages at 110 days of life. After 7 days of adaptation, during which the animals attained a stable pattern of feeding, food intake was monitored for the next 5 consecutive days at 12-h intervals by measuring the amount of pellet food provided at the onset of the light cycle and the amount of food remaining 12 h later.
BSS
At 150 days of age, animals were tested for the BSS following the procedure developed by Halford et al.Reference Harro, Merenakk, Nordquist, Konstabel, Comasco and Oreland 21 Animals were fasted for 5 h before they were supplied with food of a known weight. The BSS was recorded over 1 h by a video system coupled to a computer in a nearby room. Films were analyzed by a trained observer, who recorded the frequency and duration (seconds) of feeding, grooming and resting. Other parameters scored from the BSS were food intake and feeding rate, which was defined as the amount of ingested food (grams), per unit of time (minutes).
Feeding preference for macronutrients
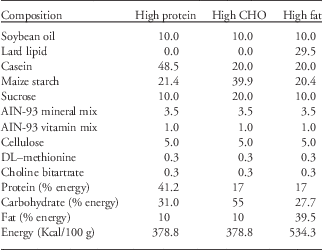
This analysis was performed at 180 days of life. After 7 days of adaptation in individual cages, rats were given access to three containers containing carbohydrates, lipids or protein diets (Table 1). The acrylic containers were fixed in a rectangular base fixed to the floor of the cage and kept in the box during the adaptation of the animal without any food. This allowed for a daily change in the position of the different diets among the containers to avoid the spatial learning factor. The intake (grams) of each diet was calculated as the difference between the amount of food provided at the onset of the light cycle and the amount of food remaining 24 h later. Food intake was measured daily for 5 consecutive days.
Table 1 Composition of self-selection foods (g/100 g diet)
Metabolite determinations
Animals were killed at 200 days of age, and serum from trunk blood was tested for high-density lipoprotein, glucose, total protein, total cholesterol and triglycerides by enzymatic methods (LABTEST®).
Statistical analysis
The results of food consumption during each 12-hour period of light/dark, macronutrient preference and behaviors during the behavioral sequence of satiety test were analyzed by a two-way ANOVA followed by Bonferroni’s multiple comparison test. Food consumption and feeding rate in the BSS, body weight (BW) and blood biochemical data were analyzed using one-way ANOVA followed by Bonferroni’s multiple comparison test. The level of statistical significance was set at P<0.05.
Results
Food intake in the dark and light periods
In the evaluation of the maternal separation effect (in the light cycle) on food intake in the dark and light periods, the two-way ANOVA indicated a significant interaction between gender and cycle [F(3,72)=5.24, P=0.002, Fig. 1a]. An important difference was found in relation to gender and food intake in the light and dark periods [F(3,72)=34.68, P<0.0001, Fig. 1b, two-way ANOVA)]. Post-hoc analysis indicated that MSL cycle significantly increases the food intake (Kcal/BW) of female rats compared with female controls (CF=8.2±0.2, n=10, v. SLF=9.9±0.3, n=10, Bonferroni post-tests, P<0.05).

Figure 1 Effect of maternal separation on the food intake of male and female rats in the light period (a), dark period (b) and total intake (Kcal/g) (c). Data are expressed as mean±s.e. of food intake during 12 h for 5 consecutive days up to 120 days of life. The animals were separated from their mothers from day 1 to day 14 of life during either the light or dark period. *P<0.05, **P<0.01, ***P<0.001, ### P<0.001 according to a two-way ANOVA for repeated measures with a Bonferroni multiple post-hoc test.
Comparing the caloric intake relative with BW revealed higher intake by female controls compared with male controls, only in the dark period (CF=23.3±0.5, n=10 v. CM=18.2 ±0.7, n=10, P<0.001, Bonferroni post-tests, Fig. 1a).
However, when comparing female and male rats separated from their mothers during the light period using Bonferroni post-tests, the food intake was higher for female rats during both the light (SLF=9.9±0.3, n=10 v. SLM=6.0±0.2, n=10, P<0.001) and dark periods (SDF=23±0.7, n=10 v. SDM=18.4±0.8, n=10, P<0.001, Fig. 1a). The results for animals that were separated from their mothers during the dark period of the light/dark cycle were different from those of rats separated during the light period. Lower calorie intake was observed during the light period for male rats separated during the dark period compared with male controls (CM=7.1±0.3, n=10 v. SDM=5.3±0.1, n=10, P<0.05), and no difference was observed for female rats. However, the food intake during the dark period of the cycle was reduced in female rats separated during the dark compared with female controls (CF=23.3±0.5, n=10 v. SDF=20.5±0.5, n=10, P<0.001, Bonferroni post-tests, Fig. 1b).
The two-way ANOVA revealed an effect of gender on food intake (Kcal/g BW) total [F(5,54)=19.16, P<0.001]. Post-hoc analysis revealed that male rats had lower food intake than female rats independently of maternal separation (CF=31.5±0.7, n=10 v. CM=25.3±0.9, n=10, P<0.001; SLF=32.8±0.9, n=10 v. SLM=24.4±1.0, n=10, P<0.001; SDF=29.7±0.6, n=10 v. SDM=24.4±1, n=10, P<0.001, Bonferroni post-tests, Fig. 1c).
Macronutrient preferences
The study on macronutrient preferences revealed a significant gender×nutrient interaction [F(3,84)=6.88, P<0.0001, Fig. 2]. For control groups, post-hoc analysis revealed that male rats had higher caloric intake from the protein diet than female rats (CF=4.1±0.7, n=8 v. CM=7.0±0.5, n=8, P<0.05, Bonferroni post-tests). However, the caloric intake from the carbohydrate diet (CF=15.7±0.7, n=8 v. CM=11.1±0.5, n=8, P<0.01, Bonferroni post-tests) and the lipid diet (CF=17.7±0.9, n=8 v. CM=12.8±0.6, n=8, P<0.01, Bonferroni post-tests) were significantly lower in male rats as compared with female rats (Fig. 2a).

Figure 2 Effect of maternal separation on feeding preferences for proteins, lipids and carbohydrates in male and female rats in adulthood (a and b) and on total intake (c). The animals were separated from their mothers from day 1 to day 14 of life (n=8) in either the light (18:00 to 00:00) or dark period (6:00 to 12:00).The food preference test was carried out at intervals of 12 h after 5 days of adaptation up to 180 days of age. Data are expressed as mean±s.e. # P<0.05, ## P<0.01, ### P<0.001, *P<0.05, ***P<0.001 according to a two-way ANOVA for repeated measures with a Bonferroni multiple comparison post-hoc test.
SLF rats exhibited increased calorie intake from protein compared with control rats (CF=4.1±0.7, n=8 v. SLF=7.1±0.5, n=8, P<0.05, Bonferroni post-tests), decreased intake from lipids (CF=17.7±0.9, n=8 v. SLF=13±1.1, n=8, P<0.001, Bonferroni post-tests) and no difference in carbohydrate intake (CF=15.7±0.7, n=8 v. SLF=14.2±0.8, n=8, P>0.05, Bonferroni post-tests, Fig. 2a). There was no difference in macronutrient intake for male rats separated during the same light period (protein: CM=7.0±0.5, n=8 v. SLM=4±0.8, n=8; carbohydrates: CM=11.1±0.5, n=8 v. SLM=10.4±0.4, n=8; lipid: CM=12.8±0.6, n=8 v. SLM=12.7±1.4, n=8, Fig. 2a).
The macronutrient intake for rats that were maternally separated during the dark period was different than that for rats separated during the light period. The separated females had lower lipid intake (CF=17.7±0.9, n=8 v. SDF=11.6±2.2, n=8, P<0.001, Bonferroni post-tests) and no difference (P>0.05) in carbohydrate and protein intake as compared with control rats (protein: CF=4.1±0.7, n=8 v. SDF=6.9±0.7, n=8; carbohydrate: CF=15.7±0.7, n=8 v. SDF=14.6±1.7, n=8, Bonferroni post-tests). There was no significant effect on macronutrient intake for males separated during the dark period compared with control males (protein: CM=7.0±0.5, n=8 v. SDM=4.4±0.6, n=8; carbohydrate: CM=11.1±0.5, n=8 v. SDM=12.4±0.6, n=8; lipids: CM=12.8±0.6, n=8 v. SDM=13.5±1.5, n=8, Fig. 2b).
When comparing the total calorie intake from the three nutrient sources, a one-way ANOVA revealed an effect of gender [F(5,42)=7.29, P<0.0001, Fig. 2c]. Control and separated male rats had lower calorie intake compared with female rats (CF=39.5±1.5, n=8 v. CM=30.9±0.6, n=8, P<0.01; SLF=34.4±1.9, n=8 v. SLM=27.1±1.8, n=8, P<0.05, Bonferroni’s multiple comparison test). No difference in caloric intake was observed between male and female rats separated during the dark period (CM=33.1±1.8, n=8 v. SDF=30.5±1.5, SDM=30.4±1.4, n=8, P>0.05, Fig. 2c, Bonferroni’s multiple comparison test).
BSS
A two-way ANOVA did not reveal significant effects of maternal separation in either period on feeding and grooming duration in the BSS test (Fig. 3a–3f). The two-way ANOVA revealed the effect of maternal separation [F(3,216)=16.31, P<0.0001] and the effect of light v. dark periods on resting behavior [F(3,216)=40.70, P<0.0001, Fig. 3a, 3b, 3d and 3e]. When the animals were separated from their mothers during the light period, the rest period began sooner than that for the controls, between periods 7 and 8 (P<0.001) (Fig. 3d and 3e). The male controls compared with the female ones were different for the same periods (7, P<0.01; 8, P<0.01, Bonferroni’s multiple comparison test, Fig. 3a and 3d).

Figure 3 Effect of manipulation by maternal separation on the behavioral sequence of satiety test on rats at 150 days of life. The animals were separated from their mothers from day 1 to day 14 of life (n=10). At 150 days, the animals were deprived for a period of 4 h. After deprivation, 30 g of diet was offered for 1 h and dietary behaviors such as cleanliness and rest were assessed. The transition point between the feed and rest behaviors was identified in the MC and SC groups (a and d); SLM and SLF (b and e) and SDF NO (c and f). Data are expressed as mean±s.e.
For resting behavior, the two-way ANOVA indicated a significant interaction of MSD cycle with periods [F(3,252)=2.212, P=0.0037]: maternal separation [F(3,252)=5.702, P=0.0009] and periods [F(3,252)=61.96, P<0.0001]. MSD cycle was associated with resting behavior during period 9 (P<0.01, Bonferroni’s multiple comparison test, Fig. 3a and 3c). There was no difference in resting behavior for female rats based on maternal separation.
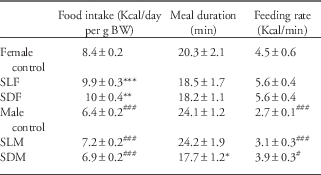
The one-way ANOVA revealed an effect of maternal separation on food intake in the BSS test [F(3,28)=44.24, P<0.0001, Table 2). Maternal separation in either the light or dark period increased food intake in female rats compared with female controls (CF=8.4±0.2, n=10 v. SLF=9.9±0.3, n=10, P<0.001, CF=10±0.4, n=10, P<0.01 v. SDF=11.4±0.5, P<0.01, Bonferroni’s multiple comparison test, Table 2). There was no effect of maternal separation on food intake in male rats. A post-hoc test revealed a decrease in food intake for male rats compared with female rats considering food intake relative to BW (Table 2).
Table 2 Effect of manipulation by maternal separation on the behavioral sequence of satiety test in rats at 150 days of life
Data are expressed as mean±s.e.
**P<0.01, ***P<0.001, # P<0.05, ### P<0.001 according to a two-way ANOVA for repeated measures with a Bonferroni multiple post hoc test.
For the rate of food intake (Kcal/min), a one-way ANOVA revealed an effect of gender (F(3,28)=11.4, P<0.0001, Table 2). The rate of food intake was higher in female controls compared with male controls (Table 2). Maternal separation had no effect on the rate of food intake (Table 2).
Body and adrenal weight and serum determinations
A one-way ANOVA revealed an effect of MSL period on BW at 180 days of life [F(3,36)=92.64, P<0.0001]. Male rats subjected to maternal separation had a higher BW than male controls (CM=439.2±21.3, n=10 v. SLM=499.4±61, n=10, P<0.01, Bonferroni’s multiple comparison test, Table 3). No difference was observed in the BW of the maternal separation groups between the female and male rats (Table 3).
Table 3 Effect of maternal separation on body weight and plasma levels of glucose, triglycerides (TG), high-density lipoprotein (HDL), total protein and cholesterol

Data are expressed as mean±s.e.
*P<0.05, ***P<0.001, ### P<0.001 according to a two-way ANOVA for repeated measures with a Bonferroni multiple post hoc test.
The one-way ANOVA revealed a significant effect of maternal separation in the dark period on serum biochemical tests [F(3,35)=6.39, P<0.05]. Female rats subjected to maternal separation exhibited a higher concentration of triglycerides in serum (CF=59.2±10.1, n=10 v. SDF=97.8±10.7, n=10, P<0.001, Bonferroni’s multiple comparison test, Table 3). No differences in serum determination in male rats (Table 3).
Discussion
The present study assessed the effects of a standard early maternal separation paradigm on the feeding behavior parameters in later life. Neonatal maternal separation is an animal model of a stressful experience in childhood. This study used the experience of repeated maternal separation during the first 2 weeks of life. However, the dynamics of interaction between the mother and offspring differ depending on the circadian cycle condition (light or dark). Pups receive more maternal care during the light period because the dam spends most of her time in the nest during this period.Reference Ader 1 During the dark phase, the dam spends most of her time on locomotor activity and feeding.Reference Ader 1
This study examined the possible factors programming feeding behavior later in life. We observed differences in feeding behavior parameters between offspring subjected to maternal separation in different periods of the circadian cycle. The results indicate that characteristics of mother–pup interactions can imprint various effects on feeding behavior in later life. Neonatal rats exhibit higher food intake during the light cycle until they are near weaning, when they begin the nocturnal pattern of food intake.Reference Halford, Wanninayake and Blundell 21 We suggest that this characteristic should be considered in animal models of maternal separation. However, most studies separate the mother and offspring during the light cycle. This could lead to not only a reduction in tactile contact but also in milk intake.
The maternal separation model was used here to consider the different effects of separation in the light and dark cycles. We characterized the food intake patterns starting from daily caloric intake. Because of the circadian rhythm of consumption in rodents (80% of eating takes place in the dark), we investigated the effect of maternal separation on food intake during the light and dark periods. To analyze the integrity of the mechanisms of satiety, we used the behavioral sequence of satiety test.Reference Blundell and Halford 6 Finally, we analyzed the distribution of nutrients consumed by rats and the effects of separation on these consumption patterns.
The results of this study showed that MSL cycle increased BW in later life only in male rats. Some studies found that maternally separated rats have a lower or normal BW just after weaning and that they thereafter (3 months) have the same or greater weight than the control rats.Reference Matthews, Wilkinson, Isoda, Beasley, Silverstein and Mobbs 39 , Reference Suchecki and Tufik 57 , Reference Tonjes, Hecht, Brautzsch, Lucius and Dörner 60 When separated from their mothers for 3 h, the pups showed no differences in BW gain and food intake during the first 2 weeks of life.Reference Plotsky and Meaney 49 In general, the studies in the literature do not show consistent effects of maternal separation on BW. These results are consistent with our findings for male rats that were subjected to maternal separation in the light cycle. One objective of this study was to evaluate the possible dissociation between factors involved in the maternal separation model in rodents. Separation from the mother during the light cycle leads to a decrease in both milk intake and tactile contact because mothers spend more time lactating the pups during the light cycle.Reference Ader 1 During the first 17 days of life, pups follow a diurnal pattern of food intake.Reference Hall and Rosenblatt 22 Although this deficit did not lead to a marked change in BW during the lactation period, it may have been sufficient to modulate the BW in later life, demonstrating that decreased food intake in early life can act as a programmer of overweight/obesity.
The 24-h food intake in kilocalories relative to BW was influenced not by maternal separation but by gender, with female rats eating more than male rats.Reference Orozco-Sólis, Lopes de Souza and Barbosa Matos 44 However, when food intake was analyzed separately in light and dark cycles, effects of maternal separation were observed in male and female rats.
The pattern of higher food intake during the dark cycle was not altered in any experimental group. However, we found that maternal separation in different cycles altered food intake during the light and dark periods. MSL period increased or reduced the food intake of female and male rats, respectively, during the light period, without altering intake in the dark. This result is consistent with that obtained in a study on rats submitted to early weaning, where food intake increased in the light period.Reference dos Santos Oliveira, de Lima, da Silva, da Silva, de Souza and Manhães-de-Castro 14
Although maternal separation altered food intake during the light and dark periods, these effects were not sufficient to modify daily food intake. However, these changes in food intake during the light phase can indicate that phenotypic adjustments in feeding rhythm during the light and dark periods can be influenced by maternal separation. In mammals, the circadian rhythm is generated by the pacemaker located in the suprachiasmatic nuclei of the hypothalamus (SCN).Reference Stephan and Zucker 56 At the molecular level, this pacemaker is controlled by a feedback network of transcriptional/translational genes Per1, Per2, Cry1, Cry2, Clock and BMAL1 in the SCN and peripheral clocks.Reference Bass and Takahashi. 3 This network interacts with peripheral cellular metabolic processes to produce an oscillation of 24 h in practically all cellular functions. In neonatal rats, changes in the timing of maternal care, restricted feeding or periodic maternal deprivation were reported to alter the pup circadian rhythms of certain behaviors and rPer1 and rPer2 circadian expression in the suprachiasmatic nuclei.Reference Ohta, Honma, Abe and Honma 42 , Reference Takahashi and Deguchi 58 , Reference Viswanathan 65 The periodic absence of the mothers in the nursing period reset the neonatal circadian clock and resulted in the phase reversal of behavioral rhythms measured after weaning.Reference Ohta, Honma, Abe and Honma 43 The phase reversal of rPer1 and rPer2 expression in the SCN of the deprived pups might result from stress-induced hormonal or neural signals in the paraventricular nucleus (PVN).Reference Ohta, Honma, Abe and Honma 42 The aggressions that affect the perinatal period can permanently alter the circadian factors.Reference Orozco-Solis, Matos and Lopes de Souza 46 For example, feeding with a low-protein diet during gestation and lactation induces a long-lasting disruption of the diurnal expression pattern of several genes involved in the regulation of food intake and energy metabolism.Reference Orozco-Solís, Matos and Lopes de Souza 45 Perinatal undernutrition abolished the circadian rhythm of cocaine- and amphetamine-regulated transcript and pro-opiomelanocortin (POMC) expression in the hypothalamus.Reference Orozco-Solís, Matos and Lopes de Souza 45 One factor to be considered in perinatal stress is the absence of the oscillation of the clock gene in the suprachiasmatic nucleus and the role of the adrenal gland as a pacemaker.Reference Torres-Farfan, Mendez, Abarzua-Catalan, Vilches, Valenzuela and Seron-Ferre 61
When animals were submitted to MSD period of the light/dark cycle in this study, in addition to the same alterations observed for separation during the light period, there was also a reduction in the nocturnal food intake of female rats. Experiments on ovariectomized rats demonstrated that estrogen replacement decreases food intake, especially during the light period.Reference Varma, Meguid, Hammond and Gleason 63 This finding suggests that the anorexic effect of estrogen may be specific for the light phase.Reference Varma, Meguid, Hammond and Gleason 63 Estrogen receptors are expressed in the suprachiasmatic nucleus,Reference Vida, Hrabovszky, Kalamatianos, Coen, Liposits and Kalló 64 and they can modify the excitability and neurotransmission of neurons.Reference Fatehi and Fatehi-Hassanabad 17 Estrogen replacement increases the neuronal activity of the SCN, especially during the light phase, reducing food intake.Reference Takamata, Torii, Miyake and Morimoto 59
This study showed that maternal separation did not change the structure of satiety expression, but only modified the parameter and microstructural constituents of this event, particularly the feeding rate. The feeding rate (Kcal/min) was higher in females than in males. Studies have reported that the increase in food intake may be linked to increased levels of hypothalamic neuropeptide Y (NPY) promoted by maternal separation.Reference Husum, Termeer, Mathé, Bolwig and Ellenbroek 27 , Reference Jiménez-Vasquez, Mathé, Thomas, Riley and Ehlers 28 This peptide is a potent orexigenic factorReference Yokosuka, Kalra and Kalra 70 that can be regulated by glucocorticoids.Reference Ponsalle, Srivastava, Uht and White 50 , Reference Yokosuka, Kalra and Kalra 70
In the test of satiety, we observed effects of maternal separation on food intake after fasting. The increase in corticosterone levels associated with fasting may be required to increase the expression of hypothalamic NPY mRNA.Reference Makimura, Mizuno, Isoda, Beasley, Silverstein and Mobbs 37 – Reference Ponsalle, Srivastava, Uht and White 50 Furthermore, fasting was not observed to have a significant influence on food intake in male rats. In a study that examined the expression of hypothalamic peptides in response to a 48 h starvation period in male rats subjected to maternal separation, no changes in the corticosterone levels or NPY expression were observed in adult life.Reference Kim, Lee, Choi, Lee and Jahng 31 However, increased expression of the anorexigenic neuropeptide POMC was observed.Reference Kim, Lee, Choi, Lee and Jahng 31 In this study, male rats had a shorter meal duration when subjected to MSD cycle. This characteristic is associated with the profile of advancement of satiety.Reference Blundell and Halford 6 This result can also be associated with the increased expression of the POMC peptide because this peptide reduces meal size.
The other model of neonatal manipulation, early weaning, has effects similar to those of maternal separation, with high anxiety and high levels of corticosterone secretion in response to stress.Reference Kikusui, Nakamura, Kakuma and Mori 30 Rats subjected to early weaning later showed a smaller meal size and an acceleration of satiety in response to fasting in adult life.Reference dos Santos Oliveira, de Lima, da Silva, da Silva, de Souza and Manhães-de-Castro 14 In another perinatal manipulation, the supply of a low-protein diet during gestation and lactation induced a delay in satiety and increased meal duration.Reference Orozco-Sólis, Lopes de Souza and Barbosa Matos 44 These studies indicate the potential effects of manipulations during the vulnerable developmental period on the control mechanisms of satiety.
The present study is the first to show a relationship between maternal separation in the light or dark phases of the circadian cycle on macronutrient self-distribution in adult life. Most studies have instead evaluated the intake of palatable food or diets high in fat or carbohydrates.Reference Gibson 19 – Reference Maniam and Morris 38
This study showed that maternal separation induced changes in the selection of nutrients depending on gender. Protein intake increased in male rats and decreased in female rats, and only female rats reduced their lipid intake. Differences in the effects of perinatal manipulations between the sexes are well documented.Reference Darnaudery and Maccari 12 , Reference de Jongh, Geyer, Olivier and Groenink 13 , Reference Ward and Renz 68 A pioneering study showed that male rats chose a glucose solution, whereas female rats preferred a saccharin solution.Reference Valenstein, Kakolewski and Cox 62 Rats genetically manipulated to develop obesity and exposed to an obesogenic environment showed a reduction in food intake accompanied by high levels of leptin, a result not observed in female rats.Reference Ruedi-Bettschen and Feldon 52 The maternal separation induced behavior related to anorexia nervosa in adolescent female rats and not during adulthood, contrary to observations for male rats.Reference Hancock and Grant 23
Maternal separation also promoted a preference for protein at the expense of fat in female rats. The influence of gonadal hormones on regulatory processes such as the control of food intake and BW has been documented since the 1970s.Reference Wade 66 , Reference Wade 67 In female rats, the main findings are that a gain in BW occurs after the removal of the ovaries, and a substantial increase in food intake and meal size and a concomitant increase in BW are observed compared with gonadally intact female rats.Reference Blaustein and Wade 5 These effects are because of the reduction in circulating the estrogen levels consequent to ovariectomy, and they can be prevented or reversed by appropriately timed treatment with estradiol.Reference Wade 66 The pre-ovulatory increase in plasma estradiol concentration is associated with a transient decrease in food intake during estrus in cycling rats.Reference Blaustein and Wade 5 , Reference Brown-Grant, Exley and Naftolin 7 , Reference Drewett 15 , Reference Kennedy and Mitra 29 During proestrus, just after the peak in estradiol secretion, food intake decreases relative to diestrus levels.Reference Kennedy and Mitra 29 This decrease in energy intake, along with a correlated increase in energy expenditure,Reference Presl, Horsky, Herzmann, Mikulás and Henzl 51 results in a net BW decrease during proestrus.Reference Kennedy and Mitra 29
Decreased maternal care increases plasma corticosterone and E2 levels, cell proliferation in the ovarian follicles and the duration of the estrus cycle and differentially regulates the expression of ER-a and ER-b in the ovaries of the offspring during adulthood.Reference Amorim, Damasceno and Perobelli 2 Several studies have indicated that estradiol leads to an enhanced responsiveness of the hypothalamus–pituitary–adrenal (HPA) axis to a stressor that may be partly due to the impairment of negative glucocorticoid feedback.Reference Burgess and Handa 8 , Reference Carey, Deterd, de Koning, Helmerhorst and de Kloet 9 , Reference Ferrini, Lima and De Nicola 18 , Reference Patchev, Hayashi, Orikasa and Almeida 47 , Reference Peiffer and Barden 48 The strong expression of glucocorticoid receptors in the PVN, especially in the parvocellular subdivision,Reference Morimoto, Morita, Ozawa, Yokoyama and Kawata 40 is thought to be mainly involved in the negative feedback of corticosterone on the HPA axis.Reference Weiser and Handa 69 Interestingly, intracerebroventricular infusion of dexamethasone (a glucocorticoid receptor agonist) stimulates food intake and BW gainReference Zakrzewska, Cusin and Stricker-Krongrad 71 while decreasing peripheral glucose uptake.Reference Cusin, Rouru and Rohner-Jeanrenaud 10 In view of this evidence, we suggest that the female rats in this study reduced their fat intake to maintain a normal BW.
In summary, this study indicates possible phenotypic adjustments in feeding behavior promoted by maternal separation during the first 2 weeks of lactation. These adjustments differ depending on the period of the circadian rhythm (light or dark) in which the separation is carried out. We observe that separation during the dark cycle leads to more severe adjustments, indicating a dissociation between the presence of the mother and milk intake as factors that induce adjustments in feeding behavior.
Acknowledgements
The authors thank the research group Neuroplasticity and Behavior and its professor leader Sandra Lopes de Souza.
Financial Support
This work totally supported by FACEPE (Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco.
Conflicts of Interest
None.
Ethical Standards
The experimental procedures were approved by the Ethical Committee of Health Centre of the Federal University of Pernambuco, Brazil (23076.022943/2008-71) and followed the Guideline for the Care and Use of Laboratory Animals.








