Animal studies have shown that prenatal maternal stress (PNMS) can reprogram the function of the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes and can affect behavior in the offspring, although the effects are variable depending on the species examined, the nature and timing of the stress, and the sex and age range of the sample examined (Glover, O'Connor, & O'Donnell, Reference Glover, O'Connor and O'Donnell2010). More recently, human studies have also found long-term differences in basal cortisol levels or cortisol reactivity in children exposed to prenatal mood or stress compared to controls (Brennan et al., Reference Brennan, Pargas, Walker, Green, Newport and Stowe2008; Entringer, Kumsta, Hellhammer, Wadhwa, & Wust, Reference Entringer, Kumsta, Hellhammer, Wadhwa and Wust2009; Grant et al., Reference Grant, McMahon, Austin, Reilly, Leader and Ali2009; Gutteling, de Weerth, & Buitelaar, Reference Gutteling, de Weerth and Buitelaar2004, Reference Gutteling, de Weerth and Buitelaar2005; Huizink et al., Reference Huizink, Bartels, Rose, Pulkkinen, Eriksson and Kaprio2008; O'Connor et al., Reference O'Connor, Ben-Shlomo, Heron, Golding, Adams and Glover2005; Van den Bergh, van Calster, Smits, van Huffel, & Lagae, Reference Van den Bergh, van Calster, Smits, van Huffel and Lagae2008; Yehuda et al., Reference Yehuda, Engel, Brand, Seckl, Marcus and Berkowitz2005). Higher and lower basal cortisol levels, as well as increased cortisol response to stress, have been documented in exposed offspring of both humans and nonhuman mammals (Glover et al., Reference Glover, O'Connor and O'Donnell2010). Similarly, PNMS may alter the function of the HPG axis. In animal models, PNMS inhibits sex-specific sexual behaviors that are dependent on gonadal hormone secretion. For example, in male rats, PNMS inhibits mounting, intromissions, and ejaculations (Anderson et al., Reference Anderson, Fleming, Rhees and Kinghorn1986) that are dependent on testicular testosterone. Moreover, PNMS is associated with reduced plasma testosterone in adulthood in males (Anderson, Fleming, Rhees, & Kinghorn, Reference Anderson, Fleming, Rhees and Kinghorn1986). In female rats, PNMS is associated with higher postnatal testosterone levels and polycystic ovaries (Herrenkohl, Reference Herrenkohl1979, Reference Herrenkohl1986; Vomsaal et al., Reference Vomsaal, Quadagno, Even, Keisler, Keisler and Khan1990), a condition associated with high levels of circulating androgens, such as testosterone. In human female infants, prenatal exposure to stress is associated with masculinized anogenital distance, a marker of excessive prenatal androgen exposure (Barrett et al., Reference Barrett, Parlett, Sathyanarayana, Liu, Redmon, Wang and Swan2013), and girls exposed to the Chernobyl disaster in utero had higher testosterone levels in adolescence compared to age-matched controls (Huizink et al., Reference Huizink, Bartels, Rose, Pulkkinen, Eriksson and Kaprio2008).
Human studies also show that cortisol and testosterone levels tend to be positively correlated at rest in amniotic fluid in utero (reflecting fetal levels; Sarkar, Bergman, Fisk, O'Connor, & Glover, Reference Sarkar, Bergman, Fisk, O'Connor and Glover2007) and in peripheral blood samples during adulthood (Liening, Stanton, Saini, & Schultheiss, Reference Liening, Stanton, Saini and Schultheiss2010), suggesting that in a nonstressed state, the HPA and HPG axes mutually upregulate one another during both prenatal and postnatal life. In contrast, stress during pregnancy may alter postnatal associations between testosterone and cortisol. This is reported in human offspring by associating in utero levels of cortisol and testosterone as proxies for stress (Bergman, Glover, Sarkar, Abbott, & O'Connor, Reference Bergman, Glover, Sarkar, Abbott and O'Connor2010), and in animal offspring using a variety of prenatal stressors, which suggest that PNMS may deregulate the typical interactions between the HPA and HPG axes (Bergman et al., Reference Bergman, Glover, Sarkar, Abbott and O'Connor2010; Kapoor, Dunn, Kostaki, Andrews, & Matthews, Reference Kapoor, Dunn, Kostaki, Andrews and Matthews2006; Kapoor & Matthews, Reference Kapoor and Matthews2011; Ward & Weisz, Reference Ward and Weisz1980, Reference Ward and Weisz1984). In an individual who has not been exposed to high levels of PNMS, the HPA and HPG axes may have a tendency to upregulate themselves in a reciprocal fashion at acute or low levels of stress; in contrast, they tend to downregulate themselves at chronic or high levels of stress (Bergman et al., Reference Bergman, Glover, Sarkar, Abbott and O'Connor2010; Kapoor et al., Reference Kapoor, Dunn, Kostaki, Andrews and Matthews2006; Kapoor & Matthews, Reference Kapoor and Matthews2011; Ward & Weisz, Reference Ward and Weisz1980, Reference Ward and Weisz1984). High levels of PNMS may therefore impact this “typical” relationship by causing dissociation between HPA and HPG function. For example, PNMS has been shown to affect cortisol response in exposed children, though not always in a predictable fashion (i.e., exposure to PNMS can increase or decrease cortisol response depending on the age of the individual and the nature of the stressor; Tollenaar, Beijers, Jansen, Riksen-Walraven, & de Weerth, Reference Tollenaar, Beijers, Jansen, Riksen-Walraven and de Weerth2011). The relationship between cortisol response and basal testosterone levels in PNMS-exposed children may be an indicator of underlying mechanisms disrupted by PNMS. However, it is difficult to design PNMS studies in humans, due to obvious ethical constraints.
Disasters are natural experiments that allow us to study the potential effects of PNMS in humans. Project Ice Storm is the world's first and longest prospective study of PNMS from a natural disaster, the January 1998 Quebec ice storm. Project Ice Storm was initiated soon after a series of ice storms struck southern Quebec in January 1998 knocking out electricity for more than 3 million people for as long as 6 weeks. At the time of the crisis, Environment Canada and the Insurance Bureau of Canada counted the ice storm as the worst and most costly natural disaster in Canadian history. The extent of objective hardship from the ice storm (specific events experienced by the women during the crisis such as days without power, financial loss, etc.) was randomly distributed, with no association with socioeconomic status. On June 1, 1998, we mailed questionnaires to women whose doctors identified them as being pregnant during the ice storm or who became pregnant within 3 months of the storm. The questionnaires assessed the levels of both objective hardship (the severity of their exposure) and subjective distress (the degree of posttraumatic stress-like symptoms), experienced in relation to the ice storm. The preconception-exposed group was included as it has been well documented that maternal stress experienced within months and up to a year prior to conception can have immediate and long-term postnatal effects on offspring development, in humans and animals alike (Nuckolls, Kaplan, & Cassel, Reference Nuckolls, Kaplan and Cassel1972; Ryzhavskii et al., Reference Ryzhavskii, Sokolova, Fel'dsherov, Uchakina, Sapozhnikov and Malysheva2001; Shachar-Dadon, Schulkin, & Leshem, Reference Shachar-Dadon, Schulkin and Leshem2009; Witt et al., Reference Witt, Cheng, Wisk, Litzelman, Chatterjee, Mandell and Wakeel2014; Zaidan & Gaisler-Salomon, Reference Zaidan and Gaisler-Salomon2015).
In our PNMS studies of mothers exposed to a natural disaster during pregnancy, high objective hardship is associated with more externalizing problems in the children (including, but not limited to, aggressive behavior), although an even stronger association is found with high subjective distress (King, Dancause, Turcotte-Tremblay, Veru, & Laplante, Reference King, Dancause, Turcotte-Tremblay, Veru and Laplante2012). Further, experimental animal models have also shown a link between PNMS and aggressive behavior in the exposed offspring in a variety of species (e.g., mice, piglets, and voles), though the timing and magnitude of that effect differed between species (Kinsley & Svare, Reference Kinsley and Svare1988; Kranendonk et al., Reference Kranendonk, Hopster, Fillerup, Ekkel, Mulder and Taverne2006; Marchlewska-Koj, Kruczek, Kapusta, & Pochron, Reference Marchlewska-Koj, Kruczek, Kapusta and Pochron2003). In humans, high testosterone/cortisol ratios have been shown to predict aggressive behavior in adolescent boys and girls (Platje et al., Reference Platje, Popma, Vermeiren, Doreleijers, Meeus, van Lier and Jansen2015). The relationship between cortisol and testosterone may be particularly involved in regulating levels of overt and interpersonal aggression in both nonhuman primates and humans, with a significant positive relationship between basal testosterone levels and overt aggression in subjects with low cortisol levels or reactivity but not in subjects with high cortisol levels or reactivity (Archer, Reference Archer2006; Hermans, Putman, Baas, Koppeschaar, & van Honk, Reference Hermans, Putman, Baas, Koppeschaar and van Honk2006; Platje et al., Reference Platje, Popma, Vermeiren, Doreleijers, Meeus, van Lier and Jansen2015; Popma et al., Reference Popma, Vermeiren, Geluk, Rinne, van den Brink, Knol and Doreleijers2007; Putman, Hermans, & van Honk, Reference Putman, Hermans and van Honk2004; Sapolsky, Reference Sapolsky2005; van Honk, Peper, & Schutter, Reference van Honk, Peper and Schutter2005).
Thus, disrupted HPA–HPG interactions due to PNMS could explain behavioral effects observed during childhood and adolescence, such as the expression of aggressive behavior, known to be dependent on those endocrine systems. As such, peripheral levels of cortisol and testosterone, as measured by salivary sampling, may represent potent and proximal moderators of the neuroendocrine effects of PNMS on aggressive levels during childhood and adolescence. Yet no previous study has examined the relationship between PNMS, HPA–HPG interactions, and levels of aggressive behavior in human children. Herein we examined the relationship between cortisol response to a stressor and basal testosterone levels in a sample of children whose mothers were exposed to sudden-onset stress from a natural disaster: the 1998 Quebec ice storm (King & Laplante, Reference King and Laplante2005). First, we aimed to determine whether PNMS moderates the relationship between basal testosterone and cortisol response. Second, we tested the behavioral relevance of the association between testosterone and aggressive symptoms, as moderated by cortisol response. We hypothesized that PNMS (both objective hardship and subjective distress) would lead to dissociation between testosterone–cortisol measures, and that in turn, this dissociation would be associated with higher levels of aggressive behavior.
Materials and Method
Participants
Within 5 months of the 1998 ice storm, mothers were identified by their physicians who agreed to mail Project Ice Storm consent forms and questionnaires to them. Mothers were eligible if at least 18 years old; French-speaking and living in the Montérégie region southeast of Montreal, Québec, Canada; and were pregnant or became pregnant within 3 months of the ice storm. Additional details regarding the identification and recruitment of participants is reported elsewhere (Laplante, Zalazo, Brunet, & King, Reference Laplante, Zelazo, Brunet and King2007). More than 200 women returned the recruitment questionnaire, of whom 178 agreed to further contact. The recruitment questionnaires were mailed on June 1, 1998. Prospective follow-up assessments of the mothers and their children were scheduled at several ages after a postal questionnaire at 6 months postpartum, including a face-to-face assessment of the children at age 11½ years that included brain imaging.
Not all children agreed to, or were eligible for, the brain imaging (e.g., due to orthodontics) and so did not have the testosterone and cortisol samplings, and others were lacking behavioral assessments. As such, the analyses are from a subset of 11½-year-olds (n = 59 children; 31 boys, 28 girls) with a complete set of hormonal and psychosocial measurements. Children in this sample were exposed to the peak of the ice storm (January 9, 1998) during the 3-month preconception period (n = 18), the first (n = 13), second (n = 14), or third (n = 14) trimester. The work described has been carried out in accordance with the Code of Ethics of the World Medical Association, approved by the Douglas Mental Health University Institute Research Ethics Board, and informed consent (from mothers) and assent (from children) was obtained from all participants.
Measures
Objective hardship and subjective distress (June 1998)
The Storm32 questionnaire, with items from four categories of objective disaster exposure (threat, loss, change, and scope), was used to measure objective hardship. Because each natural disaster presents unique experiences to the exposed population, questions pertaining to each of the four categories must be tailor-made; the Storm32 questionnaire was therefore designed specifically for Project Ice Storm, as previously described (Laplante, Brunet, Ciampi, & King, Reference Laplante, Brunet, Schmitz, Ciampi and King2008). Scores for each of the four categories ranged from 0 to 8. A total objective hardship score was obtained by summing the scores of the four categories. Higher scores reflect greater hardship. In order to assess the reliability of the women's recall of events experienced during the ice storm, a sample of 59 women completed the questionnaire 6 years later. Cronbach's αs were 0.88 for the total score, and 0.89 for scope, 0.82 for loss, 0.91 for change, and 0.60 for threat.
A validated French version (Brunet et al., Reference Brunet, St.-Hilaire, Jehel and King2003) of the 22-item Impact of Events Scale—Revised (IES-R; Weiss & Marmar, Reference Weiss and Marmar1997), which assesses the severity of current (preceding 2 weeks) posttraumatic stress symptoms (hyperarousal, intrusive thoughts, and avoidance), was used to measure the subjective distress experienced in reaction to the ice storm. The total score was used in the analyses, and higher scores reflect greater distress. The IES-R is one of the most widely used self-report measures in the trauma literature. The factor structure of the IES-R, as well as reliability and validity, are well supported in the literature (Beck et al., Reference Beck, Grant, Read, Clapp, Coffey, Miller and Palyo2008) and in Project Ice Storm (Brunet, St.-Hilaire, Jehel, & King, Reference Brunet, St.-Hilaire, Jehel and King2003). The internal consistency of the French adaptation was very good with Cronbach's αs of 0.93 (total score), 0.86 (intrusion), 0.86 (avoidance), and 0.81 (hyperarousal).
Hormonal measures (age 11½)
Saliva samples for cortisol and testosterone were collected by passive drool from the subjects at their 11½-year assessments, when we first began acquiring structural magnetic resonance imaging (MRI) of their brain and body. We intend to analyze the MRI data as part of a longitudinal analysis that includes later visits of the children at 16½ and 18½ years; the MRI data were not analyzed as part of this study. Hormonal response to MRI scanning is a well-demonstrated phenomenon, with several hormones, including salivary cortisol and testosterone levels, increasing both during simulation (practice) and actual MRI scanning (Eatough, Shirtcliff, Hanson, & Pollak, Reference Eatough, Shirtcliff, Hanson and Pollak2009). To avoid the scanning effect, the sample for testosterone was collected approximately an hour (M = 62 min, SD = 30) before the stressor (start of the first scan sequence). To assess cortisol response, the saliva samples for cortisol were collected before (PRE), just after (POST), and 45 min after (POST45) our 60-min-long neuroimaging session (see Figure 1). On average, the first cortisol sample was obtained 9 min (SD = 8 min) before the start of the first scan sequence. The average time interval between cortisol sample collections is presented in Table 1. Our prestressor cortisol sample does not appear to be biased by a potential anticipatory increase in cortisol, as the prescan cortisol baseline levels were no different from baseline levels collected by participants on the same day of the week and time at their homes 1 week later, t (56) = 1.114, p = .270.
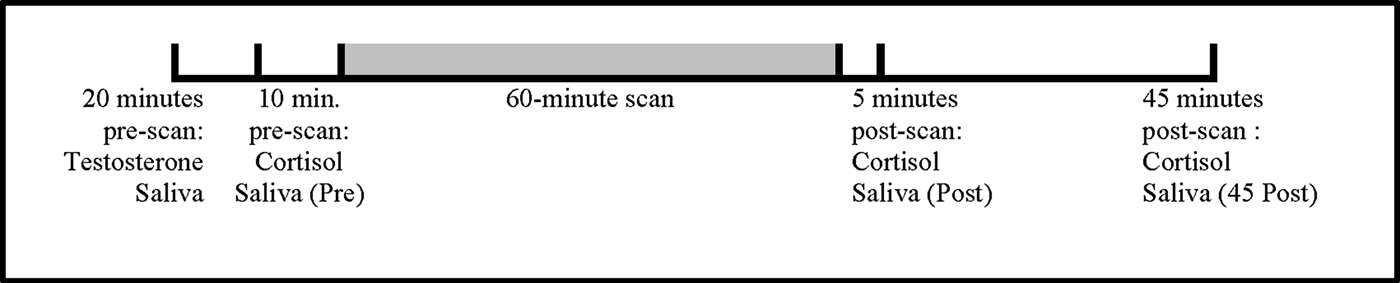
Figure 1. Timeline illustrating the timing of testosterone and cortisol sampling relative to the 60-min magnetic resonance imaging scans.
Table 1. Sample characteristics and t tests between boys and girls: Mean (SD) of sample

Note: Aggressive behaviors were measured with the Child Behavior Checklist. No differences were detected between males and females on any measure. IES-R, Impact of Events Scale—Revised. MRI, magnetic resonance imaging. SES, socioeconomic status. aTestosterone and cortisol values are presented in raw values in this table; values were log transformed prior to conducting the analyses.
All steroid hormones easily cross the blood–brain barrier when not bound to proteins such as the sex hormone-binding globulin or alpha-fetoprotein (Khan-Dawood, Choe, & Dawood, Reference Khan-Dawood, Choe and Dawood1984; Worthman, Stallings, & Hofman, Reference Worthman, Stallings and Hofman1990). Salivary sampling measures the free, biologically active portions of circulating hormonal levels relevant to studies of hormone– behavior associations (Khan-Dawood et al., Reference Khan-Dawood, Choe and Dawood1984; Worthman et al., Reference Worthman, Stallings and Hofman1990). In addition, because cortisol and testosterone levels have been shown to follow diurnal patterns in response to the pulsatile release of adrenocorticotropic hormone and gonadotropin-releasing hormone (GnRH), particularly in boys (Brambilla, Matsumoto, Araujo, & McKinlay, Reference Brambilla, Matsumoto, Araujo and McKinlay2009; Stanczyk, Reference Stanczyk2006), we controlled for collection time by limiting the period of hormonal sampling and MRI scanning to the early afternoon. To rule out any effects of seasonal variation on hormonal levels (Choi et al., Reference Choi, Lee, Choi, Chung, Seo and Lim2014), we tested for any curvilinear effects of month of sampling on the testosterone–cortisol relationship (see Statistical Analyses).
Commercial enzyme-linked immunoassays (cortisol: Salimetrics kit#1-3002 Research; testosterone: Salimetrics kit#1-2402 Research) were used to determine testosterone and cortisol levels. Inter- and intra-assay coefficients were 13.5% and 5.2%, respectively, for testosterone and 1.9% and 7.7%, respectively, for cortisol.
There are several ways to characterize the cortisol response to a stressor, but it is common to calculate the area under the curve (AUC) calculated from serial cortisol measurements over time (Khoury et al., Reference Khoury, Gonzalez, Levitan, Pruessner, Chopra, Basile and Atkinson2015). Because AUC is thought to represent a better index of the stress response than isolated cross-sectional measures of cortisol, the integrated AUC values relative to ground were calculated using a slight modification to the formula in Fekedulegn et al. (Reference Fekedulegn, Andrew, Burchfiel, Violanti, Hartley, Charles and Miller2007), by dividing by the time lapse between the first and last saliva collection: AUC = ([Cortisolpre + Cortisolpost] × [Timepost – Timepre 2] + [Cortisolpost + Cortisolpost45] × [Timepost45 – Timepost2] / (Timepost45 – Timepre). In this equation, averages of paired cortisol values were multiplied by the time interval of each pair, the resulting values added together and then divided by the total time interval (from first to last cortisol sampling). Division by the total time lapse from pre to post45 was done to account for individual differences in the latency between the first and last saliva collections.
Aggression levels (age 11½)
The Child Behavior Checklist (CBCL) is a widely used parental report tool that includes information on specific behaviors exhibited by the child within the previous 6 months (Bordin et al., Reference Bordin, Rocha, Paula, Teixeira, Achenbach, Rescorla and Silvares2013). CBCL aggressive behavior standardized T scores were calculated from reports completed by mothers within 1 month of the hormonal collection. These subscales demonstrate excellent psychometric properties, with high internal consistency for the aggression subscale (Cronbach's alpha = 0.91; Bordin et al., Reference Bordin, Rocha, Paula, Teixeira, Achenbach, Rescorla and Silvares2013).
Socioeconomic status (SES)
SES was measured in the recruitment questionnaire using the four-factor Hollingshead scale (Hollingshead, Reference Hollingshead1973), one of the most frequently used measures of SES. It is calculated from maternal and paternal education and occupation levels. Hollingshead scores are scored such that the lower the number, the higher the SES.
Procedures
Shortly following the ice storm, physicians who perform deliveries in the four hospitals in the Montérégie region were contacted. Those who agreed to collaborate indicated the number of eligible women in their practice. Each doctor received the appropriate number of envelopes, which contained the questionnaires (including Storm32, IES-R, and demographics) and a postage-paid return envelope addressed to the researchers. The packets were mailed from the doctors’ offices on June 1, 1998. Six months after each woman's due date, she received a postal questionnaire that included questions about the pregnancy outcomes. Mothers and children have been assessed several times since. An assessment was scheduled at age 11½ years that included the MRI described here. Upon arrival of the children at the scanning site, initial saliva samples for testosterone and cortisol were collected prior to the MRI. In addition, additional saliva samples for cortisol were collected immediately after the MRI as well as 45 min post-MRI, and frozen at –20 °C until assayed. The assay was completed less than 4–5 months after the collection. Within 6 months of the scan, mothers completed the CBCL. In total, our analyses included 59 participants.
Statistical analyses
Prior to data transformation, all variables were examined with regard to variance and outliers, and any data point greater than 2 SD from the mean was excluded from further analyses (n = 1). Independent samples t tests were used to test for differences in PNMS between mothers of boys and of girls, hormone levels, aggressive behaviors, as well as the other potential covariates: saliva collection time, month of sampling, child's age at MRI, Hollingshead SES, and mother's age at the child's birth. The positive skew on testosterone, cortisol AUC, and subjective distress measures was normalized using the natural logarithmic transformation. Correlations were calculated among PNMS, hormone levels, secondary outcomes, and the potential covariates, except for month of sampling. Since the seasonal effect is measured as month of assessment, curvilinear analyses between season and hormonal levels are better suited at testing the hypothesis, as we would expect Months 1 and 12 (January and December) to have closer hormonal levels (as seen with a curvilinear effect), rather than to be at the extremes (as seen with a linear effect). All subsequent analyses were run using the PROCESS macro (www.processmacro.org) for SPSS version 22 (IBM). PROCESS uses an ordinary least squares or logistic regression-based path analytic framework for estimating two- and three-way interactions in moderation models along with conditional effects and regions of significance when probing interactions (Hayes, Reference Hayes2009; Hayes & Preacher, Reference Hayes and Preacher2010). To graph the moderation effect, the predictor's conditional effects for moderator levels set to the 10th and 90th percentiles were computed using the regression on the full sample to better represent the association to the outcome at low and high moderator levels. The formulas for the conditional effects, their standard errors, and their significance levels are provided in Hayes (Reference Hayes2013). The Bonferroni correction was used to control for multiple comparisons.
Primary predictor and outcome: Prenatal stress and the testosterone–cortisol relationship
To assess whether the degree of objective hardship and/or subjective distress moderated the relationship between basal testosterone and cortisol AUC, a simple moderator model was used (PROCESS Model 1; see Figure 2). Basal testosterone levels were entered as the outcome variable, objective hardship or subjective distress as the moderating variable, and cortisol AUC as the predictor variable, with the hypothesis that objective hardship and/or subjective distress would disrupt the typical positive relationship between testosterone and cortisol AUC. When testing subjective distress as a moderating variable, objective hardship was entered as a covariate.
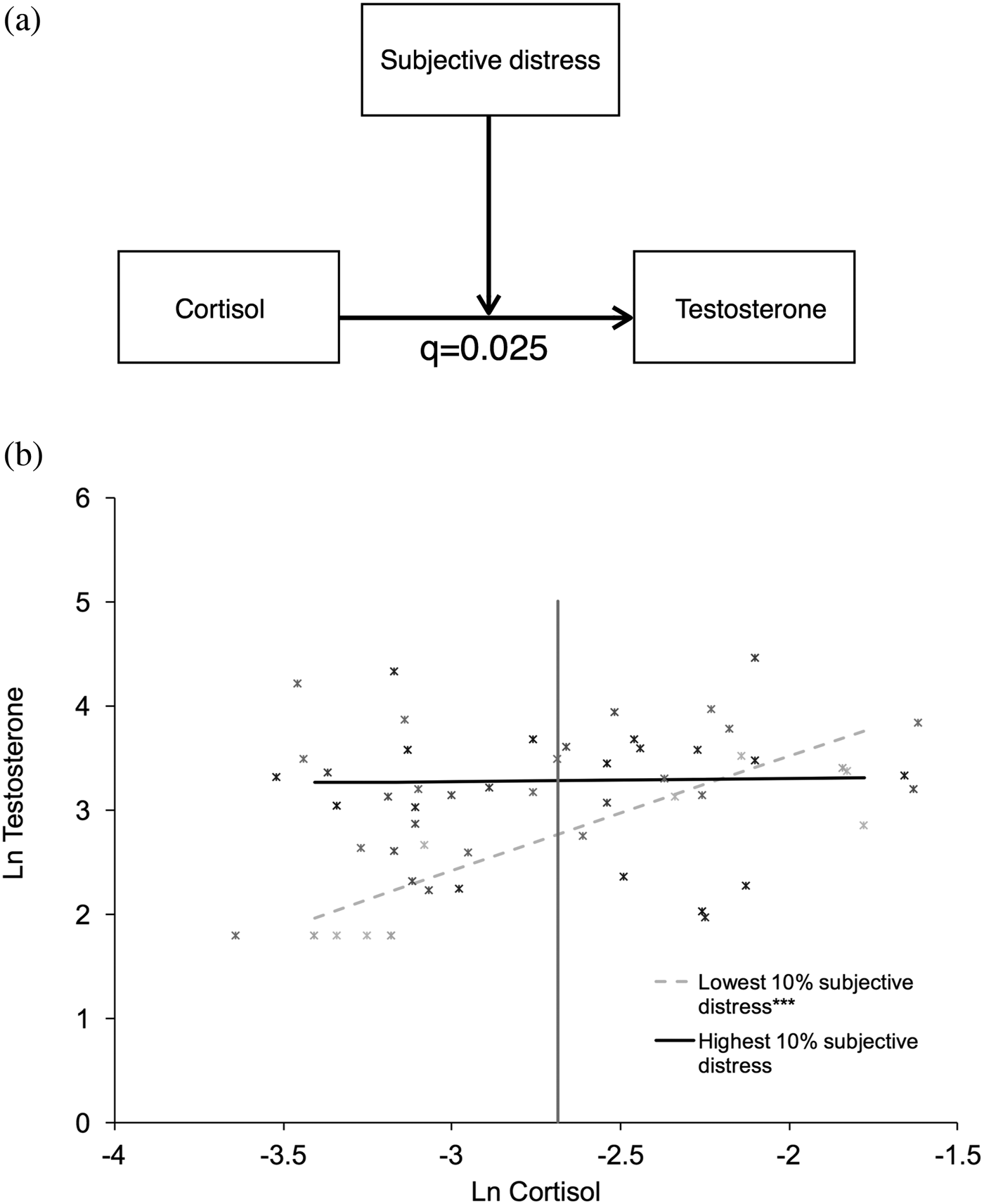
Figure 2. Maternal subjective distress moderates the relationship between basal testosterone and cortisol response. (a) Maternal subjective distress moderated the relationship between basal testosterone levels and cortisol area under the curve (AUC) at 11½ years of age (B = –0.3338, SE = 0.1283, p = .012, adjusted p: q = .025). (b) The association between salivary testosterone and cortisol AUC levels at different levels of maternal subjective distress for each participant is shown. Darker markers represent higher maternal subjective distress. The regression lines represent the top and bottom 10th percentiles of subjective distress exposure as an example of the conditional effect of cortisol AUC on testosterone. At lower subjective distress (lowest 10%; dotted gray line), cortisol AUC is positively correlated to testosterone, and this relationship is significant (conditional effect of cortisol AUC on testosterone at lowest 10% subjective distress = 1.101; SE = 0.249; p < .001). At higher subjective distress levels (highest 10% displayed as an example; black line), the effect of cortisol AUC on testosterone is not significant (conditional effect of cortisol AUC on testosterone at highest 10% subjective distress = –0.026; SE = 0.257; p = .919). The region of significance for cortisol levels is to the left of the vertical line, indicating a significant, positive association between subjective prenatal maternal stress and testosterone at cortisol AUC levels below –2.685, but not above.
Secondary outcome: Aggression levels
To determine the clinical relevance of the basal testosterone–cortisol AUC relationship, we tested the relationship between these hormones and aggressive behavior. We hypothesized that cortisol AUC could be a moderator of the relationship between testosterone and aggressive behavior. Simple moderation analyses were run to test this hypothesis (PROCESS Model 1: see Figure 3).

Figure 3. Cortisol response moderates the relationship between basal testosterone and aggressive behavior. (a) Cortisol area under the curve (AUC) moderated the association between basal testosterone levels and aggressive behavior (B = –3.3626, SE = 1.1599, p = .006, adjusted p: q = .011). (b) The association between salivary testosterone and aggressive behavior at different levels of cortisol AUC for each participant is shown. Darker markers represent increasing cortisol reactivity. The regression lines represent the top and bottom 10th percentiles in cortisol AUC as an example of the conditional effect of testosterone on aggressive behavior. At higher (highest 10%; solid line) cortisol levels, testosterone is negatively significantly associated with aggressive behavior (conditional effect of testosterone on aggression at highest 10% of cortisol AUC = –4.109; SE = 1.440; p = .006). At lower cortisol AUC levels (lowest 10%; dotted gray line), testosterone is not significantly associated with aggressive behavior (conditional effect of testosterone on aggression at lowest 10% cortisol AUC = 1.366; SE = 1.004; p = .180). The region of significance for testosterone is to the left of the vertical line and indicates that that when testosterone levels are below 2.546, there is a significant, positive association between cortisol and aggression. Note that testosterone and aggression values were transformed using the natural logarithm.
Regression covariates
Because hormonal levels and diurnal patterns of secretion have been shown to differ between males and females (Bale, Reference Bale2011; Dabbs, Reference Dabbs1991; Kirschbaum, Wust, & Hellhammer, Reference Kirschbaum, Wust and Hellhammer1992), sex was included as a covariate in all analyses. Because testosterone and cortisol both demonstrate diurnal patterns of release and secretion (Matchock, Dorn, & Susman, Reference Matchock, Dorn and Susman2007), collection time was also included as a covariate in all analyses. Because children with low SES backgrounds tend to exhibit more emotional dysregulation than children living in high SES contexts (Raizada & Kishiyama, Reference Raizada and Kishiyama2010; Raver, Reference Raver2004), and because it is correlated with aggressive behavior (see Results), we also included SES as a covariate in all models. Finally, maternal age at the child's birth was correlated with aggressive behavior, so it was added to all models for consistency across models. Again, objective hardship was included in all models.
Sensitivity analyses for timing effects of disaster exposure in pregnancy
Although underpowered to run a three-way interaction with timing of PNMS exposure as a moderator due to the sample sizes within each trimester, we conducted exploratory sensitivity analyses for testing potential effects of gestational exposure, by running the moderation analyses and removing subjects from each trimester group (preconception, first, second, and third) one at a time.
Results
Sample characteristics
Sample characteristics are listed in Table 1. There were no differences in objective hardship or subjective distress levels between the mothers of boys and of girls. There were also no sex differences in the levels of testosterone or levels of cortisol pre, post, or 45 min postscan, as expected in children at this age (see Discussion for more details). Similarly, no sex differences were present in the mean CBCL T scores for aggressive symptoms, collection times, or month of sampling. There was, however, an effect of scanning on measures of cortisol response, such that cortisol levels significantly varied from one time point to the next within individuals (F = 8.927, p < .001), showing a nonsignificant increase from pre- to postscan collection times, with a significant drop in cortisol 45 min after the scan.
Bivariate associations
Correlations between predictors, outcomes, and potential covariates can be found in Table 2. Testosterone and cortisol response were found to have a significant positive association. In addition, cortisol response was associated with SES, such that lower SES was associated with higher cortisol AUC. Higher subjective distress was found to be associated with more aggressive behaviors. Finally, older mothers were found to have children with more aggressive behaviors. There were no curvilinear associations between season of sampling and either testosterone or cortisol levels.
Table 2. Correlation matrix between outcomes, predictors, and covariates

Note: N = 59. IES-R, Impact of Events Scale—Revised. MRI, magnetic resonance imaging. SES, socioeconomic status. *p < .05. **p < .01. Hollingshead SES is coded such that the lower the number the higher the SES.
Primary predictors and outcomes: PNMS and the testosterone–cortisol relationship
To make sure that there were no multicollinearity issues among our predicting variables, variance inflator factors were computed. No values were greater than 1.626, indicating that there is no multicollinearity problem in our analysis.
After controlling for covariates, subjective distress was found to be a significant moderator of the relationship between basal testosterone and cortisol AUC in 11½-year-old children (B = –0.3338, standard error [SE] = 0.1283, p = .012, adjusted p value = .025). The association is displayed graphically in Figure 2, which presents regression lines for the 10th and 90th percentiles of subjective distress as illustrations. Post hoc examinations of this interaction revealed that, at lower subjective distress levels, cortisol AUC was positively and significantly correlated with testosterone (conditional effect of cortisol AUC on testosterone at the 10th percentile in subjective distress = 1.101; SE = 0.249; p < .001). At higher subjective distress levels, the association between cortisol AUC and basal testosterone was no longer significant (conditional effect of cortisol AUC on testosterone at the 90th percentile in subjective distress = 0.026; SE = 0.257; p = .919). Thus, higher subjective distress was associated with a negative shift in the positive basal testosterone–cortisol AUC relationship such that hormonal measures became dissociated from each other, starting at a log-transformed IES-R score of 2.2649 (original scale: 8.6302). The vertical line in Figure 2 represents the region of significance for cortisol levels, indicating that when cortisol AUC was greater than log-transformed level –2.685, the positive association between subjective PNMS and testosterone was no longer significant. In contrast to subjective distress, objective hardship levels did not significantly moderate the testosterone–cortisol relationship.
Sensitivity analyses testing potential timing effects did not change the overall direction of results; however, statistical significance was lost when removing the second-trimester exposed group from the analysis (Table 3).
Table 3. Sensitivity analyses tested whether sequential exclusion of preconception, first, second, or third trimester exposed groups altered the statistical significance of the moderation models. The first model tested the association between child cortisol responsivity and basal testosterone moderated by maternal subjective distress associated with the ice storm. The second model tested the association between basal testosterone on aggression moderated by cortisol responsivity. The coefficients remained largely unchanged, although statistical significance was lost when the children exposed to the ice storm during the second trimester were excluded from the first model, and when the third trimester exposed were excluded from the second model.

aIndicates that exclusion of the trimester weakened the model such that a loss of statistical significance was observed.
Secondary outcome: Aggression levels
To make sure that there were no multicollinearity issues among our predicting variables for the current model, variance inflation factors were computed. In this analysis, no values were greater than 1.487, indicating that there is no multicollinearity problem. CBCL Aggressive Behavior T scores ranged from 50 to 65, with a mean SD of 52.8±3.7, all within the normative range for children in this age group (Bordin et al., Reference Bordin, Rocha, Paula, Teixeira, Achenbach, Rescorla and Silvares2013).
As shown in Figure 3, after controlling for covariates, cortisol AUC was found to moderate the association between basal testosterone and aggressive behaviors (B = –3.3626, SE = 1.1599, p = .006, adjusted p value = .011). Post hoc examinations of this interaction showed that, at higher cortisol response, testosterone was negatively associated with aggressive behaviors (conditional effect of testosterone on aggressive behavior, at the 90th percentile in cortisol AUC = –4.109; SE = 1.440; p = .006), with the highest aggression scores in subjects with high cortisol response but lower testosterone levels. However, no significant relationship was present between testosterone and aggression at lower cortisol response (conditional effect of testosterone on aggressive behavior, at the 10th percentile in cortisol AUC = 1.366; SE = 1.004; p = .180); the actual level of cortisol at which testosterone was no longer significantly associated with aggression was log-transformed level –2.489. The vertical line in Figure 3 indicates the region of significance for testosterone levels: there was a significant, positive association between cortisol AUC and aggression only when testosterone levels were below 2.546.
Sensitivity analyses testing potential timing effects did not change the overall direction of results; however, statistical significance was lost when removing children exposed during the third trimester from the analysis, becoming marginally significant (Table 3).
Discussion
Results from this study show that higher levels of maternal subjective distress assessed 5 months after the ice storm predicted a dissociation in basal testosterone–cortisol AUC measures in 11½-year-old children, even when controlling for the degree of disaster-related hardship. As predicted, increasing levels of PNMS are associated with both higher and lower hormonal levels; that is, a nonlinear relationship exists between levels of PNMS and absolute hormonal values. As a result, children exposed to higher PNMS levels show increasing dissociation (i.e., lack of association) between testosterone and cortisol levels. This is in contrast with children exposed to lower PNMS levels, who show a positive correlation between their testosterone and cortisol values at age 11. These effects of subjective PNMS are significant even when controlling for objective levels of hardship from the ice storm, suggesting that distal factors such as prenatal maternal distress may carry persistent effects on postnatal HPA and HPG function several years later. Season and time of day of sampling, and child sex, were not associated with hormone levels or aggression. The sensitivity analyses suggest that the effects of maternal distress on vulnerability of the HPA and HPG axes span the entire prenatal period, but that the second and third trimesters may be particularly vulnerable periods.
As expected, subjective PNMS was a more important moderator of the basal testosterone–cortisol AUC relationship than objective hardship, and this finding is similar to that of Adler and Achenbach (Reference Adler and Achenbach2001), which showed that subjective social status (as opposed to objective indicators) was more directly associated with biological markers such as cortisol habituation. Thus, disaster-related prenatal maternal subjective distress, in addition to being a stronger predictor of externalizing symptoms (King et al., Reference King, Dancause, Turcotte-Tremblay, Veru and Laplante2012), was shown here to have a moderating effect on neuroendocrine associations in the human offspring, distinct from the objective severity of stress exposure. We have repeatedly found that objective hardship and subjective distress are each related to different physical and behavioral outcomes in this cohort (Cao, Laplante, Brunet, Ciampi, & King, Reference Cao, Laplante, Brunet, Ciampi and King2014; Cao-Lei et al., Reference Cao-Lei, Massart, Suderman, Machnes, Elgbeili, Laplante and King2014, Reference Cao-Lei, Dancause, Elgbeili, Massart, Szyf, Liu and King2015; Dancause et al., Reference Dancause, Laplante, Oremus, Fraser, Brunet and King2011, Reference Dancause, Laplante, Fraser, Brunet, Ciampi, Schmitz and King2012; Dancause, Veru, Andersen, Laplante, & King, Reference Dancause, Veru, Andersen, Laplante and King2013; King et al., Reference King, Dancause, Turcotte-Tremblay, Veru and Laplante2012; Laplante et al., Reference Laplante, Barr, Brunet, Galbaud du Fort, Meaney, Saucier and King2004; St.-Hilaire et al., Reference St.-Hilaire, Steiger, Liu, Laplante, Thaler, Magill and King2015; Turcotte-Tremblay et al., Reference Turcotte-Tremblay, Lim, Laplante, Kobzik, Brunet and King2014; Walder et al., Reference Walder, Laplante, Sousa-Pires, Veru, Brunet and King2014). In our complete sample at the time of recruitment, objective hardship (Storm32) and subjective distress (IES-R) were only weakly correlated (r < .30; King & Laplante, Reference King and Laplante2015) as they continued to be in the current sample (r = .215, p = .102), supporting the idea that subjective distress and objective hardship capture unique aspects of the human stress experience.
One strength of Project Ice Storm is that the stressor distributed hardship to the population in a quasi-random fashion; there are no associations between our objective hardship score and any demographic variables. This cannot, however, be said of maternal subjective distress levels as individuals are not randomly assigned to respond to a disaster with posttraumatic stress-like symptoms. As such, our study cannot determine the extent to which the statistical “effects” of our subjective stress variable on the testosterone–cortisol association reflect true causal effects stemming from the external or intrauterine environment, or from genetic connections between mother and child, or from the postnatal parenting style.
We also observed a strong positive correlation between basal testosterone and cortisol AUC in children exposed to lower levels of prenatal maternal subjective distress, while dissociation between these measures was present at higher prenatal maternal subjective distress levels. The level of subjective distress at which the cortisol–testosterone association becomes nonsignificant is an IES-R score of 7.8 (untransformed). IES-R scores can range from 0 to 88, with a liberal screening cutoff score for possible posttraumatic stress disorder of 22, and more stringent cutoff recommended with a score of 32. Our results suggest that a slight increase in levels of maternal hyperarousal, avoidance, and/or intrusive thoughts and images can have significant implications for the HPA–HPG associations in their fetus in later life.
These observations are consistent with previous evidence of HPA–HPG crosstalk and reciprocal regulation under varying levels of stress. Children exposed to very low levels of subjective PNMS exhibit the positive correlation between testosterone and cortisol response typically seen in rodents exposed to low-level stress conditions (Rivier & Rivest, Reference Rivier and Rivest1991; Viau, Reference Viau2002; Viau, Soriano, & Dallman, Reference Viau, Soriano and Dallman2001). This occurred despite the fact that we did not observe a significant increase in cortisol response from pre- to postscan; however, the prescan cortisol baseline levels were no different from the baseline levels collected by participants on the same day of the week and at the same time at their homes 1 week later. This suggests that the lack of MRI-induced cortisol response was not due to an anticipatory increase in cortisol at baseline on the day of the scan as one might expect. This further suggests that the endocrine profile in this cohort is disrupted (in this case, blunted) by prenatal stress, similar to previous findings linking PNMS to disrupted neuroendocrine function in the offspring (Glover et al., Reference Glover, O'Connor and O'Donnell2010; Sandman, Wadhwa, Chicz-DeMet, Dunkel-Schetter, & Porto, Reference Sandman, Wadhwa, Chicz-DeMet, Dunkel-Schetter and Porto1997). Despite this disruption, the expected positive association between basal testosterone and cortisol AUC was seen in the individuals exposed to low PNMS, but was disrupted at high PNMS.
Potential mechanisms of the dose-dependent effect of PNMS on HPA–HPG interactions can be drawn from animal models. Rodents exposed to low-level stress were found to exhibit reciprocal upregulation of their HPA and HPG axes. In that context, adrenocorticotropic hormone (ACTH) was previously found to stimulate GnRH release as well as the adrenal production of testosterone (Rivier & Rivest, Reference Rivier and Rivest1991), and testosterone has been observed to stimulate corticotropin-releasing factor (CRF) mRNA expression and arginine-vasopressin mRNA expression in the brain, increasing the pituitary's sensitivity to CRF (Viau, Reference Viau2002; Viau et al., Reference Viau, Soriano and Dallman2001). In contrast, in rats exposed to conditions of chronic or higher level stress, the HPA and HPG axes have been found to enter a state of mutual downregulation with less sensitivity to external feedback mechanisms. In high-stress situations, increased CRF release was previously linked to decreases in GnRH (Rivier & Rivest, Reference Rivier and Rivest1991), while increased testosterone secretion was linked to decreased CRF sensitivity at the level of the pituitary gland (Viau, Reference Viau2002; Viau et al., Reference Viau, Soriano and Dallman2001). In addition, the high corticosteroid levels typical of elevated stress levels have been found to be associated with decreases in luteinizing hormone (Rivier & Rivest, Reference Rivier and Rivest1991), while excessively high testosterone levels may lower ACTH levels (Viau et al., Reference Viau, Soriano and Dallman2001). Further, high corticosteroid levels were previously observed to decrease the sensitivity of the testes to luteinizing hormone (Rivier & Rivest, Reference Rivier and Rivest1991), and sustained high levels of testosterone may lower the sensitivity of the adrenal glands to ACTH (Viau, Reference Viau2002).
Although reciprocal HPA–HPG interactions have not been as well delineated in humans, stress and high doses of glucocorticoids have been shown to generally disrupt all aspects of HPG function in humans, rodents, and nonrodent mammals (Rivier & Rivest, Reference Rivier and Rivest1991; Tilbrook, Turner, & Clarke, Reference Tilbrook, Turner and Clarke2000). Although speculative, given that the level of subjective PNMS moderated the associations between testosterone and cortisol AUC, it should also be considered that the aforementioned mechanisms could be present in human mothers. Specifically, mothers who experienced low levels of subjective distress may have experienced the typical positive upregulation between the HPA and HPG axes, whereas mothers who experienced high subjective distress may have experienced a downregulation between the axes. Given that cortisol and testosterone are important for fetal development, such alterations to the typical prenatal hormone milieu may explain associations between PNMS and the endocrine and behavioral outcomes in exposed children 11½ years later. However, a confirmation of these hypotheses awaits future and more extensive studies of HPA–HPG crosstalk in humans.
It follows that the placenta may also play a crucial role in regulating the crosstalk between maternal and fetal HPA–HPG function. For example, the fetal enzymes necessary for metabolizing maternal cortisol (11-β-hydroxysteroid dehydrogenase type 2) and testosterone (aromatase) show a transient increase in their activity under conditions of acute or low-level maternal stress or anxiety, but also demonstrate decreased activity under conditions of chronic or high-level maternal stress (O'Donnell, O'Connor, & Glover, Reference O'Donnell, O'Connor and Glover2009; Weisz, Brown, & Ward, Reference Weisz, Brown and Ward1982). Although much of this research is based on animal studies, there is preliminary evidence that similar hormonal responses to exogenous stress (coming from the mother or from environmental toxins) may also be observed in the human placenta (Canton, Scholten, Marsh, de Jong, & Van den Berg, Reference Canton, Scholten, Marsh, de Jong and Van den Berg2008; O'Donnell et al., Reference O'Donnell, O'Connor and Glover2009). As such, in conditions of acute or lower stress, the fetal contribution to HPA/HPG programming may increase (a potentially adaptive response), while in conditions of chronic or higher stress, it is the maternal contribution to HPA/HPG that may increase, potentially disrupting the regulation of these neuroendocrine systems in the offspring. The aforementioned mechanisms could explain the positive correlation between testosterone and cortisol AUC at lower levels of maternal subjective distress and the dissociation between testosterone and cortisol AUC at high levels of maternal subjective distress observed in this sample.
Finally, our data support the dual-hormone hypothesis of aggression that posits that cortisol and testosterone interact to influence aggressive behaviors (Mehta & Josephs, Reference Mehta and Josephs2010; Montoya, Terburg, Bos, & van Honk, Reference Montoya, Terburg, Bos and van Honk2012). We found cortisol AUC to be a significant moderator of the impact of testosterone on aggressive behaviors such that, with higher cortisol response, lower basal testosterone levels were associated with more aggressive behaviors. This adds to a growing literature showing that the relationship between basal testosterone and aggression is moderated by cortisol. Unfortunately, the interactions between testosterone and cortisol and how they predict aggression are not clear. Whereas the majority of studies find that higher basal testosterone is associated with more aggression at low cortisol levels even in nonclinical populations (Montoya et al., Reference Montoya, Terburg, Bos and van Honk2012; consistent with the pattern we observed with lower levels of cortisol response seen in Figure 2), others have found that aggression is predicted by high cortisol and high testosterone (Denson, Mehta, & Tan, Reference Denson, Mehta and Tan2013; Welker, Lozoya, Campbell, Neumann, & Carre, Reference Welker, Lozoya, Campbell, Neumann and Carre2014) or have found no association (Mazur & Booth, Reference Mazur and Booth2014; Scerbo & Kolko, Reference Scerbo and Kolko1994). Our data bring a further inconsistency, that low basal testosterone is predictive of higher aggression in individuals with higher cortisol response to an acute stressor. Some of these inconsistencies of these interactions in the literature may arise from different methodologies (e.g., associations between the hormones, ratios, resting vs. reactive cortisol, and age of participants), as well as personality characteristics (Pfattheicher, Reference Pfattheicher2017).
Still, it is also possible that our findings capture different types of youth aggression, which may in turn be associated with different hormonal profiles. For example, individuals with high testosterone and low cortisol response may display calculated, planned aggressive behaviors more often associated with psychopathy and antisocial personality disorders (Dabbs, Jurkovic, & Frady, Reference Dabbs, Jurkovic and Frady1991; Popma et al., Reference Popma, Vermeiren, Geluk, Rinne, van den Brink, Knol and Doreleijers2007; Yu & Shi, Reference Yu and Shi2009). In contrast, individuals with high cortisol response tend to display reactive aggression (i.e., aggression in response to provocation; Denson et al., Reference Denson, Mehta and Tan2013; Dismukes, Johnson, Vitacco, Iturri, & Shirtcliff, Reference Dismukes, Johnson, Vitacco, Iturri and Shirtcliff2015; Gerra et al., Reference Gerra, Zaimovic, Avanzini, Chittolini, Giucastro, Caccavari and Brambilla1997; Han, Miller, Cole, Zahn-Waxler, & Hastings, Reference Han, Miller, Cole, Zahn-Waxler and Hastings2015). These studies also show, however, that after provocation, testosterone and cortisol were positively coupled. We do not have poststress testosterone levels and therefore cannot test the results of these prior studies in our cohort. However, high cortisol levels may induce such a state of reactive aggression (secondary to high stress levels) that the role of testosterone in modulating aggression may be comparatively reduced. There is some evidence to support this hypothesis; for example, at high cortisol levels, the expected association between testosterone and lack of empathy is not present (Zilioli, Ponzi, Henry, & Maesripieri, Reference Zilioli, Ponzi, Henry and Maestripieri2015). An important caveat is that most of the evidence above refers to older adolescent or young adult samples, and thus has limited generalizability to our cohort.
Regardless of the type of aggressive behavior, one potential neural mechanism relating these hormone systems to aggression is the corticolimbic network. Cortisol and testosterone are steroid hormones that are regulated by, and have negative feedback on, the hypothalamus and pituitary (i.e., HPA and HPG axes). Moreover, cortisol binds primarily to glucocorticoid receptors and testosterone to androgen receptors in brain regions implicated in aggressive behaviors, particularly corticolimbic networks that include the prefrontal cortex and amygdala (de Almeida, Cabral, & Narvaes, Reference de Almeida, Cabral and Narvaes2015; Sar, Lubahn, French, & Wilson, Reference Sar, Lubahn, French and Wilson1990; Sarrieau et al., Reference Sarrieau, Dussaillant, Agid, Philibert, Agid and Rostene1986; Simerly, Chang, Muramatsu, & Swanson, Reference Simerly, Chang, Muramatsu and Swanson1990). Steroid hormones can result in amygdala hyperactivation and prefrontal cortex hypoactivation (orbitofrontal cortex, ventromedial prefrontal, and anterior cingulate cortex), to reduce descending inhibitory tone and stimulate aggressive behaviors (de Almeida et al., Reference de Almeida, Cabral and Narvaes2015). Steroid hormones, their precursors, and their metabolites can also modulate inhibitory neurotransmitter systems in these brain regions, including GABAA receptors and 5-HT1A receptors (de Almeida et al., Reference de Almeida, Cabral and Narvaes2015). Stress, and cortisol secretion in particular, interacts with the serotonergic system. Serotonergic precursors can stimulate cortisol secretion, and high levels of cortisol can reduce the production of tryptophan. Moreover, low serotonin levels associated with high testosterone/cortisol ratio can predict aggressive behavior (de Almeida et al., Reference de Almeida, Cabral and Narvaes2015; Montoya et al., Reference Montoya, Terburg, Bos and van Honk2012). Adverse maternal experiences during pregnancy (i.e., anxiety and severe life event stress) suggest impaired prefrontal cortical function in the offspring (Buss et al., Reference Buss, Entringer, Reyes, Chicz-DeMet, Sandman, Waffarn and Wadhwa2009, Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012; Entringer et al., Reference Entringer, Buss, Shirtcliff, Cammack, Yim, Chicz-DeMet and Wadhwa2010). In summary, our data suggest that PNMS interferes with basal testosterone, and cortisol response, to in turn predict aggressive behaviors. However, further research is required to determine the mechanisms that may explain these interactions.
The sensitivity analyses testing potential timing effects suggest that although the effects of maternal subjective distress on the vulnerability of the HPA and HPG axes and at least one behavioral consequence span the entire prenatal period, the second and third trimesters may be particularly vulnerable periods. The second trimester may be a particularly vulnerable period for PNMS to disrupt the relationship between basal testosterone and cortisol response (since the model is weakened without the second trimester exposed), being the time in adrenal gland development when the production of cortisol begins (Chamoux, Otis, & Gallo-Payet, Reference Chamoux, Otis and Gallo-Payet2005). In contrast, the behavioral consequences in relation to these hormones may be particularly vulnerable to third-trimester exposure, potentially due to changes in neurodevelopment. For example, maternal depression measured at 26 weeks of pregnancy is associated with changes in functional connectivity of networks involved in emotional regulation, including the amygdala to prefrontal cortical regions (Qiu et al., Reference Qiu, Anh, Li, Chen, Rifkin-Graboi, Broekman and Meaney2015). We are currently collecting resting-state functional MRI data to investigate whether core networks are disrupted in our sample compared to a control cohort. Of course, the interpretation of our timing effects must be considered with reservation, due to the small sample size and the statistical method used.
Strengths and limitations
One limitation is the absence of a control group, which precludes comparisons between unexposed and exposed children. Nevertheless, the careful and timely assessments of both the mother's objective hardship exposure to the ice storm and her degree of subjective distress allowed for testing dose–response relationships within PNMS. We also acknowledge that only salivary levels of testosterone and cortisol were measured, whereas the interactions between the HPA and HPG axes rely on both peripheral and central feedback systems. Regarding hormonal analyses, a single measurement of testosterone was obtained in this study, which could be susceptible to diurnal or seasonal variability. There is, however, evidence that testosterone levels remain stable up to an entire year in humans (Dabbs, Reference Dabbs1991; Granger, Shirtcliff, Booth, Kivlighan, & Schwartz, Reference Granger, Shirtcliff, Booth, Kivlighan and Schwartz2004; Sellers, Mehl, & Josephs, Reference Sellers, Mehl and Josephs2007), and we included both time of day and month of measurement as covariates. Although there was no significant increase in cortisol from pre- to postscan, this is probably not due to the prescan cortisol being sampled too close to the stressor; we found no difference between prescan cortisol and levels measured at home 1 week later, suggesting that the lack of increase was not due to an anticipatory increase in cortisol levels just prior to the scan.
An important limitation is the lack of pubertal assessment at 11½ years, the age at which the hormonal collection was done in the context of this study. However, since then, we have collected pubertal staging at ages 13½ and 15½ years, and have found that the majority of girls in our sample (~80%) had their first period after the age of 11½ (mean age of 11.97; Duchesne, Liu, Jones, Laplante, & King, Reference Duchesne, Liu, Jones, Laplante and King2017). We ran exploratory analyses using self-reported Tanner staging at 13½ years as our best estimate of pubertal maturation at 11½ (acknowledging the limitations of this approach given the vast individual variability in temporal progression through puberty, but assuming that pubertal status does not regress across time) and did not find any moderation by Tanner stage in either model (data not shown). The lack of sex differences in testosterone or cortisol levels, as observed in the current sample, is consistent with previous studies of children and adolescents in the earlier stages of puberty (August, Tkachuk, & Grumbach, Reference August, Tkachuk and Grumbach1969; Quaiser-Pohl, Jansen, Lehmann, & Kudielka, Reference Quaiser-Pohl, Jansen, Lehmann and Kudielka2016; Tsai, Seiler, & Jacobson, Reference Tsai, Seiler and Jacobson2013). However, it will be important for future studies to consider how testosterone–cortisol associations might interact with gold standard physician-reported Tanner staging.
One may wonder whether examining the HPG axis is relevant even in children who are prepubertal or in the earliest stages of puberty, as may have been the case with some of the participants in our study. In this context, it is important to note that the capacity for HPG activation is fully functional in mammals even prior to the normal onset of puberty (Lutz, Rampacek, Kraeling, & Pinkert, Reference Lutz, Rampacek, Kraeling and Pinkert1984; Urbanski & Ojeda, Reference Urbanski and Ojeda1987). At any given age, even in prepubertal mammals, external stressors and chemical manipulations can drive the GnRH secretory system (Ebling & Cronin, Reference Ebling and Cronin1998; Lincoln & Wu, Reference Lincoln and Wu1991; Plant, Gay, Marshall, & Arslan, Reference Plant, Gay, Marshall and Arslan1989; Viguie, Caraty, Locatelli, & Malpaux, Reference Viguie, Caraty, Locatelli and Malpaux1995). There is also evidence to suggest that the HPG axis is becoming increasingly activated over the course of several years, leading to gradually higher testosterone levels from early (i.e., Tanner 1–3) to late (i.e., Tanner 4–5) puberty (Rilling, Worthman, Campbell, Stallings, & Mbizva, Reference Rilling, Worthman, Campbell, Stallings and Mbizva1996), with no clear cutoff point where the HPG can be defined as completely “inactive,” even during earlier pubertal stages. In sum, HPG axis function may be much more significant than originally thought, even during middle childhood, a period thought to be linked to HPG quiescence. For example, there is evidence of sporadic follicular development in girls throughout infancy and childhood under the influence of follicle-stimulating hormone even prior to the physical changes of puberty (Crofton et al., Reference Crofton, Evans, Groome, Taylor, Holland and Kelnar2002). In relation to this, the lack of sex differences in testosterone levels is not entirely surprising, given that individuals in our cohort were likely in various stages of pubertal development at the time of the hormonal sampling. Other studies also report a lack of sex differences in testosterone levels, and in some cases the median testosterone level is even higher in prepubertal girls than prepubertal boys (August et al., Reference August, Tkachuk and Grumbach1969; Quaiser-Pohl et al., Reference Quaiser-Pohl, Jansen, Lehmann and Kudielka2016; Tsai et al., Reference Tsai, Seiler and Jacobson2013).
A final limitation is our sample size, which was restricted by the number of youths who were eligible for, and then accepted, to undergo the MRI scans; the sample size of 59 is limited in statistical power. Despite these limitations, this study has many strengths, including the use of a research paradigm utilizing a sudden-onset, natural disaster stressor, whose levels of hardship were evenly distributed across socioeconomic groups, the independence of the severity of exposure from maternal psychosocial characteristics, and the speed of recruitment and measurement of stress levels following the disaster.
Conclusions
These results suggest that high levels of subjective maternal distress during pregnancy can lead to sustained alterations in HPA–HPG interactions in human offspring more than one decade after exposure. Moreover, maternal states during stressful events in pregnancy may be particularly important in programming HPA–HPG interactions, given that the neuroendocrine effects were seen with maternal distress but not objective hardship. Further, HPA–HPG interactions predicted current levels of aggression in 11½-year-olds. Taken together, these findings support the notion that children exposed to even moderate levels of subjective PNMS show meaningful changes in behavior related to a long-term disruption in their neuroendocrine programming.








