Introduction
The family Cyprinidae is the most diverse of the freshwater fishes (Nelson, Reference Nelson1994; Fu et al., Reference Fu, Wu, Chen, Qu and Lei2003), with around 3002 species (Eschmeyer & Fong, Reference Eschmeyer and Fong2017) distributed almost worldwide except for Australia and Antarctica (Mayden, Reference Mayden, Winfield and Nelson1991). Within the family there are several important species with culinary, aquarium and biological uses, for example the common carp (Cyprinus carpio), goldfish (Carassius auratus) and zebrafish (Danio rerio) (Nelson, Reference Nelson2006); the latter is an excellent biological model used in genetics, embryology (Nelson, Reference Nelson2006), toxicology (Lele & Krone, Reference Lele and Krone1996; Arunachalam et al., Reference Arunachalam, Manickam, Manickam, Malaiammal and Mayden2013), molecular biology, neuroscience and other areas of biomedical research (Arunachalam et al., Reference Arunachalam, Manickam, Manickam, Malaiammal and Mayden2013).
Like the other cyprinids, Devario aequipinnatus has many advantages, such as low maintenance costs, low space requirements and a rapid reproductive cycle (Lele & Krone, Reference Lele and Krone1996), and it is also an important biological model for scientific research. However, knowledge of the reproductive biology of this species is scarce, especially regarding gonadal aspects.
The characterization of germ cells in their different stages as well as their distribution and frequency in the ovaries would allow us to infer the processes involved in ovarian development and type of spawn of the species (Lima et al., Reference Lima, Bernardino, Val-Sella, Fava-de-Moraes, Schemy and Borella1991). Histology is an important tool to acquire knowledge in reproductive biology (West, Reference West1990). According to Selman & Wallace (Reference Selman and Wallace1989), the growth of these cells occurs differently in various groups of fish, so an understanding of the development of the gametogenesis process will provide information on maturation and fertilization.
Thus, the present study aimed to characterize the ovarian cycle of D. aequipinnatus, describing morphologically oogenesis and the reproductive phases.
Materials and Methods
Sexually mature females of D. aequipinnatus (n = 70) were obtained from commercial fisheries in the Uberlândia region, MG, Brazil, then forwarded to the Neotropical Ichthyology Laboratory – LINEO, UNESP, Ilha Solteira, SP, Brazil where they remained for 30 days for acclimation. The animals were fed daily with Super Red commercial feed composed of 38% crude protein and 16% mineral material. The animals were kept in a 12 h light:12 h dark (12L:12D) photoperiod and the aquarium was cleaned daily with 10 to 20% water exchange. As a procedure to maintain water temperature and quality, chillers, aerators, thermostats and filters were used.
The animals were collected and euthanized with an alcohol solution containing benzocaine (0.2 g/l) according to the protocol approved by the Commission on Ethics in the Use of Animals (11/2013/CEUA-FEIS/UNESP). After anaesthesia, the animals had their mass (g), total and standard length (cm) measured. Posteriorly, an incision was made in the ventral region of the animal, exposing the abdominal cavity for removal of the ovaries.
The ovaries were collected and sectioned transversely in the medial region. The fragments were fixed in a solution of 4% paraformaldehyde and 2% glutaraldehyde in Sorensen phosphate buffer, pH 7.4 for 24 h. After fixation, samples were dehydrated in alcohol solutions with increasing concentrations, embedded in glycol–methacrylate historesin (Technovit 7100), sectioned at 3.0 μm on a microtome equipped with a glass blade (LEICA RM 2245) and stained with haematoxylin and eosin. For measurement of diameter of oocytes and photoprocessing, an optical microscope Zeiss equipped with camera AXIOCAM-MRc5 was utilized.
The classification of development of phases based on macroscopic and microscopic characteristics of ovaries and germ cells was performed according Brown-Peterson et al. (Reference Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-Barbieri2011).
Results
Devario aequipinnatus ovaries are paired organs fused in the caudal portion and irrigated by blood vessels located in the celomatic cavity, surrounded by conjunctive tissue (tunica albuginea) (Fig. 1a, b). The ovaries are formed of somatic and germ cells. The somatic cells are initially named the prefollicle (Fig. 2a, b); only after the formation of the ovarian follicle are they classified as follicle cells (Fig. 2c). The stages of oocyte development begin with the oogonia, the precursor cells of all the oogenesis process. In general, oogonia can proliferate into new cells (Fig. 2a) or differentiate into prophasic oocytes (Fig. 2b) that, at the end of this process, form the ovarian follicle (Fig. 2c) to end folliculogenesis. The germ cells undergo different stages of development until the liberation of oocytes into the ovarian lumen, i.e. ovulation.

Figure 1 Ovaries in D. aequipinnatus. (a) Ovaries inside the abdominal cavity (ac). (b) Ovaries: right (r) and left (l). Abbreviations: bv: blood vessels. Arrow: ovaries fused in caudal portion.

Figure 2 Folliculogenesis in D. aequipinnatus. (a) Cyst of oogonia. (b) Prophasic oocyte. (c) Formation of the ovarian follicle. Abbreviations: oc: oocyte; of: ovarian follicle; og: oogonia; fc: follicle cells; pfc: prefollicle cells. Stain: haematoxylin and eosin.
Stages of germ cells
At the end of the folliculogenesis stage, the germ cell begins primary growth or the previtellogenic stage, secondary growth or the vitellogenic stage and final maturation. Postovulatory follicles and atresic oocytes were also found.
Primary growth
Initial previtellogenic oocytes
These cells have a clear basophilic cytoplasm due to the intense proliferation of cellular organelles, a rounded nucleus with slight coloration and several nucleoli in the periphery. The follicle cells are squamous with a flattened nucleolus, scarce and separated from each other (Fig. 3a, b). Oocytes are still small when compared with the final development stage, with a diameter of 118.6 µm ± 20.5 µm, and can be observed in the Balbiani corpuscle that resemble fissures (Fig. 3b).
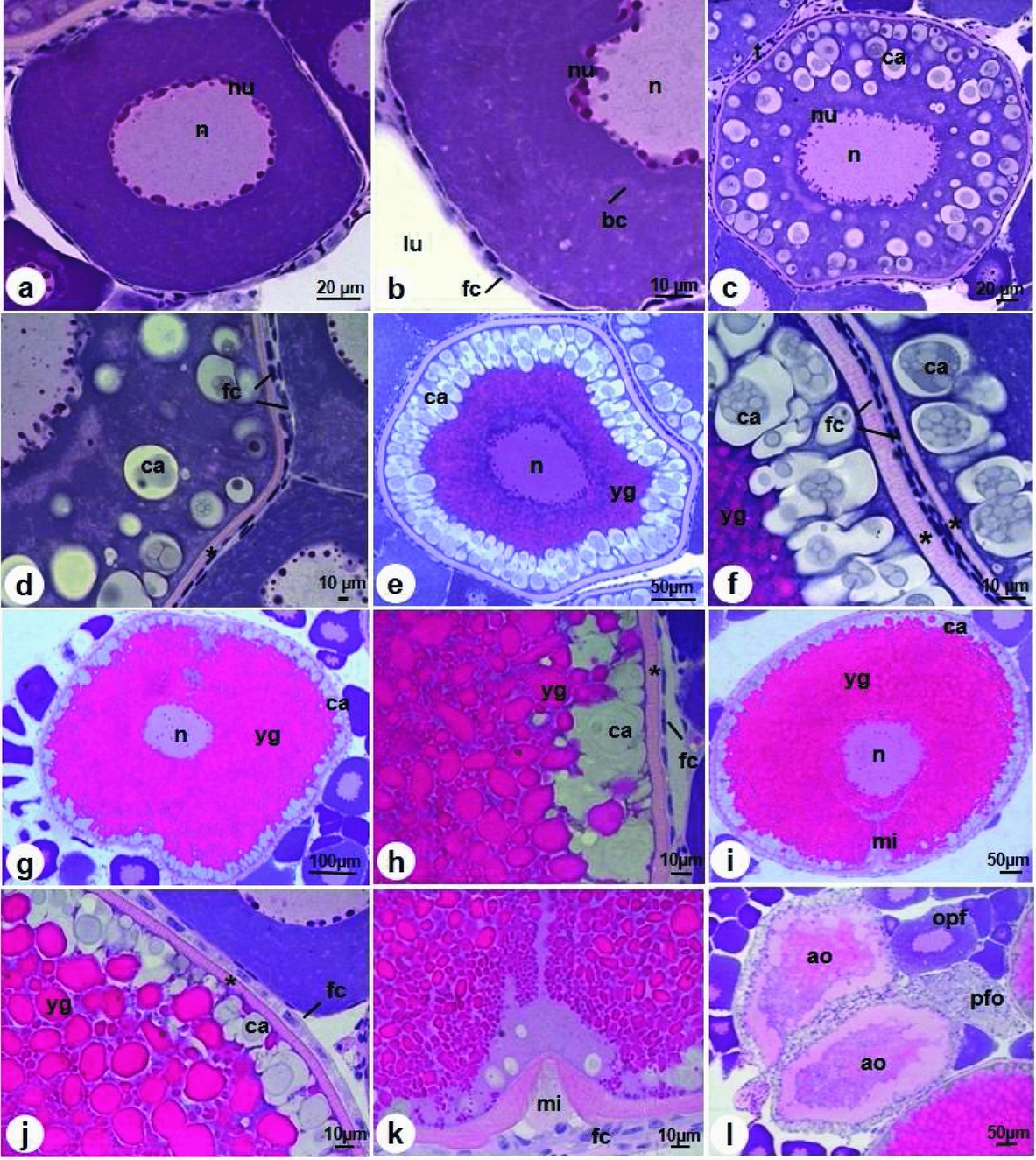
Figure 3 Oogenesis in D. aequipinnatus. (a) Initial previtellogenic oocyte with nucleoli (nu) in the periphery. (b) Squamous follicle cells (fc). (c) Final previtellogenic oocytes with cortical alveoli (ca) in cytoplasm. (d) Comparison of follicle cells between final previtellogenic and initial previtellogenic oocytes. (e) Initial vitellogenic oocyte with cortical alveoli in the periphery (ca) and yolk granules centralized. (f) Comparison of follicle cells and zona radiata between initial vitellogenic and final previtellogenic oocytes. (g) Final vitellogenic oocytes with the central nucleus (n) and accumulation of yolk granules (yg). (h) Squamous follicle cells. (i) Mature oocyte with migration of nucleolus (n) toward micropyle (mi). (j) Cuboidal follicle cells. (k) Detail of micropyle. (l) Atresic oocytes (ao) and postovulatory follicle (pfo). Abbreviations: bc: Balbiani bodies; opf: final previtellogenic oocyte; lu: ovarian lumen; t: theca. Asterisk: zona radiata. Stain: haematoxylin and eosin.
Final previtellogenic oocytes
As cellular development advances, the cytoplasm becomes less basophilic, with several nucleoli (Fig. 3c). In this stage, there is the appearance of many cortical alveoli, located along the cytoplasm (Fig. 3c). There is also the appearance of the zona radiata, which is a striated and acellular layer with pink coloration measuring 4.1 µm (Fig. 3d). Many squamous follicle cells are observed, but these are different from those seen in the previous step, as they are closer to each other (Fig. 3d). These oocytes measure around 246.4 µm ± 42.2 µm in diameter and are also observed in the theca cell layer (Fig. 3b).
Secondary growth
Initial vitellogenic oocytes
The cells increase in size according to stage, measuring around 421.1 ± 63.3 µm in diameter. The deposition of the yolk granules continues to occupy the center of the cytoplasm and, at the same time, the cortical alveoli are numerous, migrating to the cellular periphery (Fig. 3e). The follicle cells remain squamous and are closer to each other; the zona radiata measures 4.5 µm (Fig. 3f).
Final vitellogenic oocytes
In this stage, the nucleus is central, yolk granules fill the cytoplasm and the cortical alveoli are in the oocyte periphery (Fig. 3g). Oocytes increase in size (around 590.4 ± 108.3 µm in diameter) and nucleoli that were found in the periphery begin to occupy all the nucleus (Fig. 3g). The zona radiata thickens to 4.67 µm (Fig. 3h) and follicle cells remain squamous (Fig. 3h).
Final maturation
Mature oocytes
This is the last stage of development in which the cells can attain a maximum length of 721.1 ± 59.8 µm and width of 586.2 ± 57.2 µm. The yolk granules occupy all of the cytoplasm, the cortical alveoli remain in the periphery (Fig. 3i, j) and the zona radiata attains its maximum thickness of 6.81 µm (Fig. 3j). At this time, the follicle cells become cuboidal (Fig. 3j). This stage is marked by the migration of the germinal vesicle (nucleus) towards the micropyle (Fig. 3i, k).
Atresic oocytes
These oocytes become flaccid and can be found at any time during the reproductive cycle. They are characterized by disorganization of the cytoplasm, in which liquefaction of yolk granules and cortical alveoli occurs, and the zona radiata becomes thinner. The follicles cells are hypertrophic and have a thicker aspect (Fig. 3l). These oocytes will later be reabsorbed.
Postovulatory follicle
These follicles are formed by several follicle cells, theca cells and a basement membrane. After the release of oocytes in the ovulation process, these layers remain in the ovigerous lamellae, forming one layer because the ovarian lumen becomes irregular with blood vessels around it (Fig. 3l).
Reproductive phases
The morphology of the ovaries undergoes modifications throughout the development of germ cells, and thus oocytes in the previtellogenic, vitellogenic, mature, atresic and postovulatory follicle stages can be found. In this way, the reproductive phases are classified by germinal epithelium alterations associated with ovarian development, similar to the modifications that occur in the ovigerous lamellae.
Immature
The ovaries are thick, and ovigerous lamellae are in formation with little space between them (Fig. 4a). This phase is characterized by the predominance of initial previtellogenic oocytes (Fig. 4a), but oogonia in cysts are also observed (Fig. 4b). Few oogonia and prophasic oocytes are seen in this phase.

Figure 4 Ovaries in D. aequipinnatus in different reproductive phases. (a, b) Ovaries in immature phase with only previtellogenic oocytes (ipo) with organization of ovigerous lamellae (thick arrow). (b) Detail of initial previtellogenic oocytes (ipo) with nuclei (nu) in the nucleus periphery (n) and cysts of oogonia (c). (c–f) Ovaries in development phase. (c, d) Subphase of initial development. Ovaries with initial previtellogenic oocytes (ipo) and final previtellogenic oocytes (fpo). (e, f) Subphase of final development. Ovaries with initial previtellogenic oocytes (ipo); initial vitellogenic oocytes (ivo). Abbreviations: ca: cortical alveoli; fvo: final vitellogenic oocytes; lu: ovarian lumen; t: tunica albuginea; yg: yolk granules. Stain: haematoxylin and eosin.
Developing
For a more detailed understanding of this phase, we subdivided it into two subphases.
Early developing subphase
These ovaries are developing and have only primary growth oocytes, i.e. initial and final previtellogenic (Fig. 4a). This phase is marked by the appearance of cortical alveoli in the final previtellogenic oocytes (Fig. 4b).
Final developing subphase
In this subphase, secondary growth with initial vitellogenic oocytes is observed as well as final vitellogenic oocytes (Fig. 4e). Previtellogenic oocytes are also present (Fig. 4e). This phase is marked by the incorporation of yolk granules in the initial vitellogenic oocytes (Fig. 4f).
Spawning capable
In this phase, the ovary reaches it maximal development and occupies all space in the ovigerous lamellae. Mature oocytes are predominant with eccentric nucleus migrating to the periphery (Fig. 5b) but other types of oocytes and structures are also observed, such as initial and final previtellogenic oocytes (Fig. 5a), postovulatory follicles and atresic oocytes, which can be identified in any phase of development of ovary (Fig. 5b).

Figure 5 Ovaries in D. aequipinnatus in different reproductive phases. (a, b) Ovaries in spawning capable phase with mature oocytes with eccentric nucleus (mo); initial previtellogenic oocytes (ipo); final previtellogenic oocytes (fpo); atresic oocytes (ao). (c, d) Ovaries in regressing phase with mature oocytes (mo); initial previtellogenic oocytes (ipo); oocytes in different stages of atresia (ao); postovulatory follicle (pfo). (e, f) Ovaries in regenerating phase with previtellogenic oocytes (po); proliferation of oogonia (og); initial previtellogenic oocytes (ipo) and cysts with prophasic oocytes (c). Abbreviations: lu: ovarian lumen; n: nucleus. Arrowhead: postovulatory follicle (pfo). Stain: haematoxylin and eosin.
Regressing
In this phase, the ovaries become flaccid and there is more space between the ovigerous lamellae due the release of mature oocytes (Fig. 5c). The ovaries have previtellogenic oocytes, but mainly there are many atresic oocytes (Fig. 5c) and postovulatory follicles (Fig. 5d). This phase finishes the final reproductive cycle.
Regenerating
In this phase, the ovaries undergo restructuring of ovigerous lamellae (Fig. 5e) and produce a large quantity of nests and cysts with germ cells (Fig. 5f). Mitotic proliferation of oogonia occurs along with the formation of prophasic oocytes, which renew the reproductive cycle, and only initial previtellogenic oocytes predominate (Fig. 5e).
Discussion
In teleosts, the formation of ovigerous lamellae in the ovaries supports the development of germ cells. These ovigerous lamellae emit projections towards the stroma and present distinct morphological characteristics according to the stage of oocyte development (Ganeco et al., Reference Ganeco, Nakaghi, Urbinati, Neto and Vasques2001). These aspects of ovaries have been described for several groups, including Brycon orbignyanus (Ganeco et al., Reference Ganeco, Nakaghi, Urbinati, Neto and Vasques2001), Acestrorhynchus pantaneiro (Rodrigues et al., Reference Rodrigues, Querol and Braccini2005) and Pimelodus maculatus (Amorim, Reference Amorim2007). In D. aequipinnatus, the ovigerous lamellae also undergo modifications, becoming more developed along the oocyte growth.
Folliculogenesis begins with the proliferation of the oogonia, and the differentiation of the oocytes occurs later (Grier, Reference Grier2000, Reference Grier2012; Andrade et al., Reference Andrade, Bazzoli, Rizzo and Sato2001; Grier et al., Reference Grier, Uribe-Aranzabal and Parenti2007; Mazzoni et al., Reference Mazzoni, Grier and Quagio-Grassiotto2010). For D. aequipinnatus these modifications are observed and follow the pattern of other teleost orders, specifically Siluriformes and Labriformes (Quagio-Grassiotto et al., Reference Quagio-Grassiotto, Grier, Mazzoni, Nobrega and Amorim2011; Santos-Silva et al., Reference Santos-Silva, Siqueira-Silva, Silveira-Ninhaus and Silveira-Veríssimo2015).
In D. aequipinnatus, oogenesis was classified according to the morphological alterations of the cells, considering the nuclear, nucleolus, and cytoplasmic. Thus, these criteria can also be used for the classification of oogenesis stages in other species, such as Puntius conchonius, Ctenopharyngodon idella and Danio rerio (Çek et al., Reference Çek, Bromage, Randall and Rana2001; Glasser et al., Reference Glasser, Cauty, Mourot and Breton2003; Çakici & Üçüncü, Reference Çakici and Üçüncü2007). This way, the identification of primary, secondary and maturation growth stages in D. aequipinnatus is similar to other cyprinids.
During the previtellogenic stage, the zona radiata and the cortical alveoli are important structures that support ovarian development (Selman & Wallace, Reference Selman and Wallace1989; Patiño & Sullivan, Reference Patiño and Sullivan2002). These structures were observed in D. aequipinnatus and other representatives of Cypriniformes [e.g., Danio rerio (Yön et al., Reference Yön, Aytekin and Yuce2008, Connolly et al., Reference Connolly, Dutkosky, Heah, Sayker and Henry2014) and Cyprinus carpio (Shabanipour & Hossayni, Reference Shabanipour and Hossayni2010)], in which the zona radiata appears during the final previtellogenic stage, concomitantly with the cortical alveoli, which becomes thicker and more developed with advanced ovarian growth. The zona radiata plays an important role in the transport of substances for yolk synthesis (Celius & Walther, Reference Celius and Walther1998), such as proteins and lipids that are essential in the processes of vitellogenesis, maturation and hatching (Çakici & Üçüncü, Reference Çakici and Üçüncü2007).
The cortical alveoli are composed of proteins and carbohydrates and are responsible for the induration of the zona radiata (Tyler & Sumpter, Reference Tyler and Sumpter1996), and are a barrier to polyspermia (Wallace & Selman, Reference Wallace and Selman1981; Ohta et al., Reference Ohta, Iwamatsu, Tanaka and Yoshimoto1990; Rodrigues et al. Reference Rodrigues, Querol and Braccini2005) during fertilization. This is corroborated in D. aequipinnatus, in which these alveoli occupy the peripheral region of the cytoplasm and a greater increase occurs in its proportion as oogenesis progresses, as has been described for Serrasalmus maculatus (Quagio-Grassiotto et al., Reference Quagio-Grassiotto, Wildner and Guimarães-Bassoli2014).
The incorporation of the yolk granules into the cytoplasm occurs at the beginning of vitellogenesis such as Brycon orthotaenia (Gonçalves et al., Reference Gonçalves, Bazzoli and Brito2006); Metynnis maculatus, Megalancistrus parananus, Cichla kelberi, Satanoperca pappaterra (Martins et al., Reference Martins, Moura, Santos, Rizzo and Bazzoli2010) and Laetacara araguaiae (Santos-Silva et al., Reference Santos-Silva, Siqueira-Silva, Silveira-Ninhaus and Silveira-Veríssimo2015). Whereas in D. aequipinnatus, this incorporation occurs only at the end of previtellogenesis. Thus, granule proteins are an important energy reserve for the embryo and sustain its development (Rodrigues et al., Reference Rodrigues, Querol and Braccini2005; Amorim, Reference Amorim2007).
Resumption of the meiotic process initiates the maturation stage (Abascal & Medina, Reference Abascal and Medina2005), which is completed with fertilization (Quagio-Grassiotto et al., Reference Quagio-Grassiotto, Wilder and Ishiba2013). In this stage, the germ vesicle (nucleus) migrates towards the micropyle region (Honji et al., Reference Honji, Vaz-dos-Santos and Rossi-Wongtschowski2006; Quagio-Grassiotto et al., Reference Quagio-Grassiotto, Grier, Mazzoni, Nobrega and Amorim2011; Cassel et al., Reference Cassel, Camargo, Jesus and Borella2017a; Varela et al., Reference Varela, Ferreira, Da Cuna, Lo Nostro, Genovese and Meijide2017), as observed for D. aequipinnatus. However, for D. aequipinnatus, the presence of lipid droplets was not observed. This absence of lipid droplets has been reported for some marine species, including Aspitrigla obscura (Muñoz et al., Reference Muñoz, Sabat, Mallol and Casadevall2002), Liza aurata (Shabanipour & Heidari, Reference Shabanipour and Heidari2004) and Sciaenops ocellatus (Grier, Reference Grier2012).
The morphology of the D. aequipinnatus postovulatory follicles is similar to that described in the literature, being a remaining structure of the theca layer, follicular cells and basement membrane (Amorim, Reference Amorim2007; Quagio-Grassiotto et al., Reference Quagio-Grassiotto, Wilder and Ishiba2013) when mature oocytes were ovulated during the reproductive cycle (Morais et al., Reference Morais, Thomé, Santos, Bazzoli and Rizzo2016).
The atresic oocytes of D. aequipinnatus are also morphologically similar to other species, such as Danio rerio (Üçüncü & Çakici, Reference Üçüncü and Çakici2009), Prochilodus argenteus (Santos et al., Reference Santos, Thomé, Arantes, Sato, Bazzoli and Rizzo2008), Leporinus taeniatus (Thomé et al., Reference Thomé, Domingos, Santos, Martinelli, Sato, Rizzo and Bazzoli2012) and Astyanax altiparanae (Cassel et al., Reference Cassel, Chehade, Souza, Caneppele, Romagosa and Borella2017b). For D. aequipinnatus, observation of the atresia process was most commonly seen in oocytes at a more advanced stage of development, such as vitellogenesis (Üçüncü & Çakici, Reference Üçüncü and Çakici2009).
Based on the methodology proposed by Brown-Peterson et al. (Reference Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-Barbieri2011) and adapted in this study, Wildner et al. (Reference Wildner, Grier and Quagio-Grassiotto2013), Quagio-Grassiotto et al. (Reference Quagio-Grassiotto, Wilder and Ishiba2013) and Santos-Silva et al. (Reference Santos-Silva, Siqueira-Silva, Silveira-Ninhaus and Silveira-Veríssimo2015) also described the same reproductive phases for other orders, such as Siluriformes, Characiformes and Labriformes. Although this standardization has been developed for marine fish, this nomenclature has also proven to be appropriate for other species. However, for D. aequipinnatus, it was necessary to adapt the development phase to achieve a better understanding of the reproductive dynamics.
Thus, changes in the nuclear, cytoplasmic and follicular proportions arise as a result of an important underlying processes. These findings highlight the importance of studies addressing oocyte development to advance the understanding of reproduction (Marques et al., Reference Marques, Rosa and Gurgel2000). Thus, the cell modifications that occur between folliculogenesis and oogenesis are an important and understudied aspect of the reproductive biology of D. aequipinnatus. The favourable zootechnical characteristics of D. aequipinnatus make it a suitable biological model for another studies.
Acknowledgements
We would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (process no. 2014/12584–0) for financial support and to the Laboratório de Ictiologia Neotropical (LINEO), Universidade Estadual Paulista ‘Júlio de Mesquita Filho’ – UNESP. We would like thank Raphael da Silva Costa for providing photographs of animals.







