Introduction
Head and neck squamous cell carcinomas (SCCs) are cancers that originate in moist, mucosal surfaces lined by squamous cells. They are divided into categories based on the general area from which they originate. Commonly involved areas include the oral cavity, pharynx, larynx, salivary glands, paranasal sinuses and the nasal cavity. The most common types of head and neck SCC are found in the oral cavity, oropharynx, larynx and hypopharynx. Though relatively uncommon in the developed countries, oral cancer is globally the 8th most frequently occurring type among males and 14th in females. According to Global Cancer Incidence, Mortality and Prevalence (‘GLOBOCAN’) estimates, oral cavity cancers are one of the leading causes of death in less developed regions.1
Oral Squamous Cell Carcinoma (OSCC) accounts for 2–4 per cent of cancers worldwide, and up to 10–40 per cent in South Asia.Reference Markopoulos2 There is an increased prevalence of oral cancers in South Asian countries because of unhealthy habits of areca nut (supari) consumption. South Karachi (in Pakistan) has the highest age-standardised incidence rates of oral cavity cancers for both males and females, followed by India. The most common anatomical location worldwide is the tongue, while in South Asia it is the buccal mucosa.Reference de Camargo Cancela, Voti, Guerra-Yi, Chapuis, Mazuir and Curado3
Despite increasing awareness of the carcinogenic effects of the areca nut and smoking among the general population, the incidence of OSCC worldwide is still on the rise. It is suspected that this may be because of the increase in prevalence of human papillomavirus (HPV),Reference Pytynia, Dahlstrom and Sturgis4 which has been shown to be strongly associated with a certain subtype of OSCC.Reference Psyrri and DiMaio5
OSCC can be roughly divided into two types based on HPV status: HPV-negative OSCC, in which long-term exposure to tobacco and alcohol products leads to the development of malignancy; and HPV-positive OSCC, which starts with exposure to high-risk HPV, most often HPV 16, and develops independently of tobacco or alcohol exposure.Reference Mork, Lie, Glattre, Hallmans, Jellum and Koskela6 Chaturvedi et al. estimated that in the USA, by 2020, the incidence of HPV-positive oropharyngeal SCC will be greater than the incidence of cervical cancer, and by 2030, HPV will account for almost half of all head and neck cancers.Reference Chaturvedi, Engels, Pfeiffer, Hernandez, Xiao and Kim7 The increase in HPV-related oropharyngeal SCC is well documented not only in North America, but in Europe and Australia as well.Reference Chaturvedi, Engels, Pfeiffer, Hernandez, Xiao and Kim7, Reference Dayyani, Etzel, Liu, Ho, Lippman and Tsao8
HPV positive tumours present with a different molecular biology than HPV-negative tumours. The presence of viral oncogenes E6 and E7 is responsible for the degradation of p53 and Rb protein respectively, causing genetic instability, which in turn allows the tumour cells to replicate indefinitely. Normally, p53 senses damage that has occurred in the process of DNA replication, and once damaged DNA has been detected the cells are arrested in the G1 synthesis phase. At this point, these cells are either repaired or undergo apoptosis in order to prevent replication of faulty DNA. In a similar fashion, the Rb gene normally arrests the replication cycle in the G1 phase by forming a complex with E2 F.Reference Chung and Gillison9 As a result, HPV-positive tumours exhibit wild type p53 and downregulation of Rb protein, whereas HPV-negative tumours commonly have p53 mutations leading to decreased p16 and upregulation of Rb protein.
HPV related head and neck SCC accounts for 25 per cent of head and neck SCC cases. It exhibits a distinct biological behaviour, including an improved response to (chemo)radiation and survival, compared to HPV-negative head and neck SCC. Tumour HPV status has been used as a prognostic factor for overall and progression-free survival, and might be a predictive marker of response to treatment.Reference Chaturvedi, Engels, Pfeiffer, Hernandez, Xiao and Kim7 According to a randomised controlled trial, patients with HPV-positive cancer had better overall survival and progression-free survival than patients with HPV-negative cancer (p < 0.001 for both end points, by the log-rank test). The three-year rates of overall survival were 82.4 per cent (95 per cent confidence interval (CI), 77.2–87.6) in the HPV-positive subgroup and 57.1 per cent (95 per cent CI, 48.1–66.1) in the HPV-negative subgroup.Reference Ang, Harris, Wheeler, Weber, Rosenthal and Nguyen-Tan10
Given what we now know about HPV and OSCC, it is important to study their relationship, as well as the carcinogenicity and survival rates of HPV-positive SCCs.Reference Pytynia, Dahlstrom and Sturgis4 Previously, a study was carried out using the same patient dataset to understand HPV prevalence, and its effects on overall survival and disease-free survival.Reference Ali, Awan, Ghaffar, Salahuddin, Khan and Mehraj11 As five-year survival analysis is the ‘gold standard’ by which we judge the viability of any biomarker, a five-year survival analysis was scheduled for the future (the patients had just been enrolled in that study and the required data were unavailable at that time). While multiple studies observing the short-term survival rate of HPV-positive OSCC have been carried out, this study aimed to investigate the correlation of five-year survival with HPV positivity and other clinicopathological characteristics of OSCC patients at a tertiary care hospital in Karachi, Pakistan.
Materials and methods
In this retrospective case series, we enlisted patients diagnosed with OSCC from January 1991 to December 2004. The study included patients who had been diagnosed with primary OSCC, who had all undergone treatment at Aga Khan University Hospital, Karachi, Pakistan. Patients were excluded if they had not undergone surgery. This study was approved by the Ethical Review Committee at Aga Khan University Hospital. Consent was taken prior to enrolment in the study.
Data were collected by investigators, who reviewed individual medical charts and questionnaires to obtain the following information: age at time of diagnosis, gender, unhealthy habits (areca nut consumption, alcohol use and smoking) at any point in time, tumour status, site of tumour and presence of clinically palpable lymph nodes. All records were thoroughly reviewed, starting from the first visit until 60 months after the surgery. The investigators noted if the patient was alive, dead or lost to follow up at approximately five years after surgery.
Following our 2008 study, the patients’ HPV status and clinical characteristics were already known, and the same data were utilised in our study.Reference Ali, Awan, Ghaffar, Salahuddin, Khan and Mehraj11 The results were recorded by the study investigators.
The data were analysed using SPSS® statistical software, version 20. Potential associations between demographic, histological and clinical parameters and five-year survival were assessed. Univariate and multivariate analyses were performed to determine the significance of the results. Survival was estimated using Kaplan–Meier analysis and was reported in months. A Cox proportion hazard model was utilised to analyse the effect of several variables on survival. A p-value of less than 0.05 was considered statistically significant and all values were reported as two-sided.
Results
Patients’ characteristics
A total of 140 patients diagnosed with OSCC were included. In this study, OSCC referred to cancers of the oral cavity only, excluding oropharyngeal cancer. Ninety-five of the patients (67.9 per cent) were HPV-positive and 45 (32.1 per cent) were HPV-negative. Table I shows the general characteristics of the HPV-positive and HPV-negative patients. In the HPV-positive group, the majority of the patients were male (65.3 per cent), while in the HPV-negative group the majority were female (55.6 per cent). All patients underwent primary surgery to excise the tumours, and then, depending upon individual responses, patients underwent chemotherapy, radiotherapy or palliative treatment (Table I).
Table I. Patients’ demographic and clinical characteristicsReference Ali, Awan, Ghaffar, Salahuddin, Khan and Mehraj11

Data represent numbers (and percentages) of cases. HPV = human papillomavirus; PCR = polymerase chain reaction; N/A = not applicable; SCC = squamous cell carcinoma; AJCC = American Joint Committee on Cancer
Tables II and III show the survival status and the mean survival (in months) of patients with OSCC, respectively. The majority of patients (80 per cent) were older than 40 years. Age, gender and history of unhealthy habits were not found to be statistically significant in determining patient survival. Unhealthy habit history was defined as the use of smokeless tobacco (paan), smoking and/or areca nut consumption. History of tobacco use, smoking and areca nut consumption was positive in 108 patients (77.1 per cent), and these habits overlapped, with 40.7 per cent of the population having multiple unhealthy habits. Seventy per cent and 30 per cent of the patients had unhealthy habits in the HPV-positive and HPV-negative groups respectively (Table I). Furthermore, the type of treatment received by the patients was not significantly correlated with patients’ five-year survival. Mean survival was highest in patients who received surgery alone as compared to other treatment methods.
Table II. Association of clinicopathological variables with five-year survival

Data represent numbers (and percentages) of cases, unless otherwise indicated. N/A = not applicable; SCC = squamous cell carcinoma; AJCC = American Joint Committee on Cancer; HPV = human papillomavirus; PCR = polymerase chain reaction
Table III. Mean survival of OSCC patients

AJCC = American Joint Committee on Cancer; N/A = not applicable; HPV = human papillomavirus; PCR = polymerase chain reaction
The cheek was the primary site of malignancy in the majority of HPV-positive (n = 61) and HPV-negative (n = 25) patients. Of the tumours, 61.4 per cent were found on the cheek, followed by the tongue (38.6 per cent).
Tumour characteristics
Patients diagnosed with all stages of OSCC were reviewed. The majority of patients had stage II OSCC (31.4 per cent), followed by stage III (27.1 per cent), stage IV (22.1 per cent) and stage I (19.3 per cent). American Joint Committee on Cancer staging was statistically significantly correlated with overall survival in OSCC patients (p < 0.003). The patients were divided according to the histological classification of the tumour: the most common tumour was the moderately differentiated tumour (58.4 per cent), followed by well differentiated (39.3 per cent) and poorly differentiated (4.3 per cent) SCC (Table II).
No patients had been diagnosed with metastatic disease. Lymph nodes were also subjected to histopathological review. A total of 38 patients (27.1 per cent) had disease positive lymph nodes. Of these, 26 patients had N1 nodal staging, 9 patients had N2b, 3 patients had N2a, and 0 patients had N2c or N3 lymph nodes. The presence of these nodes was statistically significant in determining outcome after 60 months (p ≤ 0.001; Table II). These patients had a mean survival time of 37 months from the time of diagnosis.
Human papillomavirus positivity and survival
Polymerase chain reaction was performed to determine HPV status on all samples using general primers GP5 and GP6. This yielded a positive result for 95 patients (67.9 per cent). The HPV polymerase chain reaction findings were further specified according to the type: 85 patients were HPV 16 positive, while 2 were HPV 18 positive.Reference Ali, Awan, Ghaffar, Salahuddin, Khan and Mehraj11 Six patients were positive for HPV other than the 16 and 18 subtypes.
Fifty patients (52.6 per cent) who had an HPV-positive tumour had died by the 5-year mark, 30 patients (31.6 per cent) were alive and 15 patients (15.8 per cent) were lost to follow up (Table II). For patients who had an HPV-negative tumour, 19 patients (42.2 per cent) died, 17 patients (37.8 per cent) survived and 9 patients (20 per cent) were lost to follow up. The mean and median survival times for the HPV-positive patients were 44.3 and 60 months respectively. The mean and median survival times for the HPV-negative patients were 46.9 and 60 months respectively.
Figure 1 shows the Kaplan–Meier curve for patients with HPV-positive and HPV-negative tumours. Patients with HPV-negative OSCC had a better survival rate (0.47) compared to patients who were HPV-positive (0.38). However, the univariate analysis showed that HPV status in OSCC was not a statistically significant factor in determining five-year survival rate (p = 0.386).
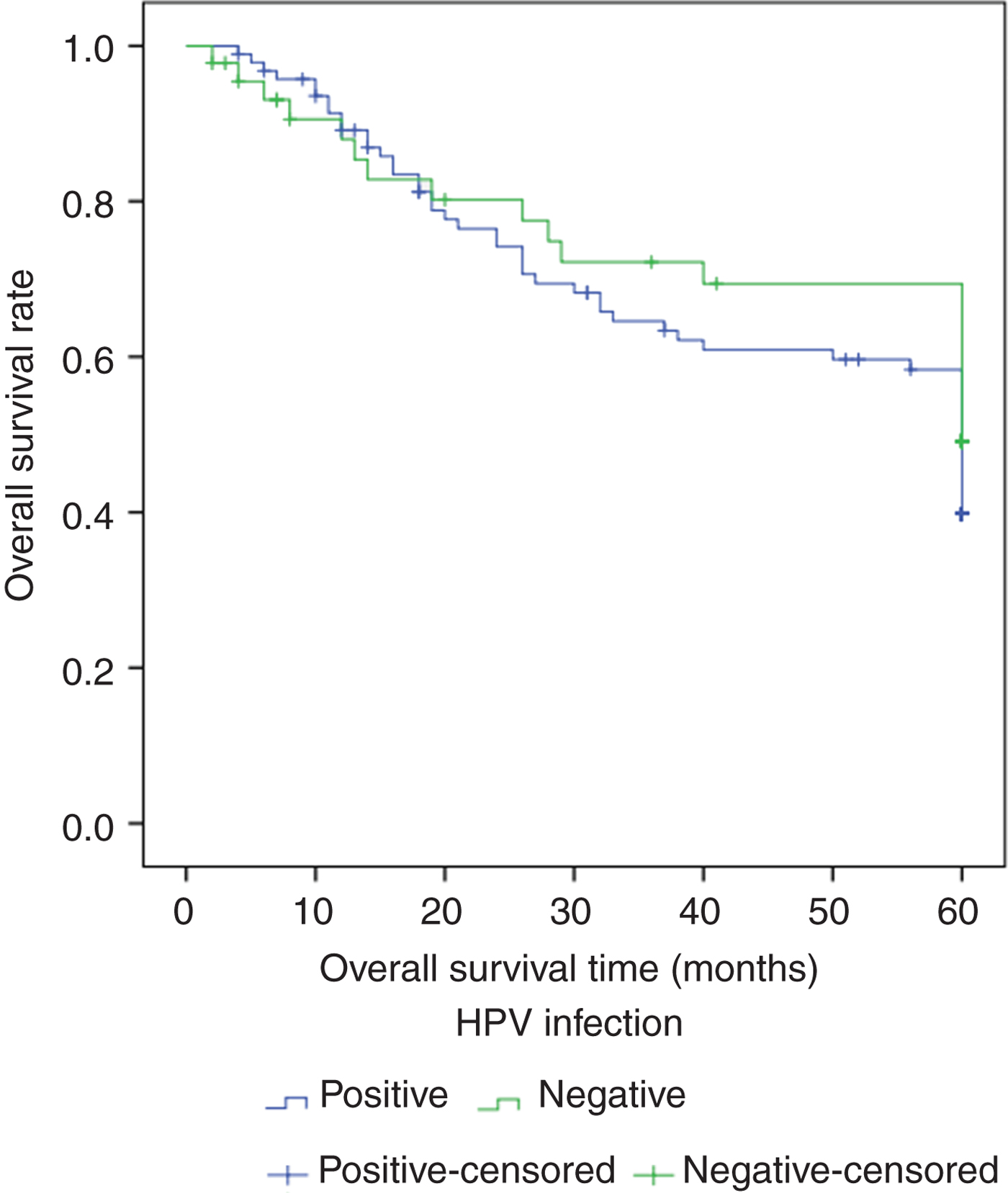
Fig. 1. Kaplan–Meier curve comparing five-year survival of human papillomavirus (HPV) positive and negative oral squamous cell carcinoma patients.
Table IV shows the findings of a multivariate analysis performed to correlate the survival percentage with tumour staging and nodal stage at the time of presentation. Using stage IV of the disease as a reference, and controlling for the nodal stage, the risk of death in stage I disease was 53 per cent lower as compared to those with stage IV disease. Similarly, the risk of death for disease stages II and III disease patients was 48 per cent and 55 per cent lower, respectively. When controlling for disease stage and considering stage 1 nodes as a reference, the risk of death was 55 per cent less for stage 1 nodes, almost unchanged for stage 2 nodes, but was more than doubled for stage 3 nodes, as outlined in Table IV.
Table IV. Multivariate analysis

*Used as the reference. HR = hazard ratio; CI = confidence interval; HPV = human papillomavirus; PCR = polymerase chain reaction; AJCC = American Joint Committee on Cancer
Discussion
The results of this study indicate no increase in survival in HPV-positive patients as compared to HPV-negative patients. In this study, we considered OSCC to include only cancers of the oral cavity, not oropharyngeal cancers. The finding shows that HPV positivity cannot be considered a good prognostic marker in patients with OSCC. These results are not in agreement with previous studies.Reference Umudum, Rezanko, Dag and Dogruluk12–Reference Benson, Li, Eisele and Fakhry15 According to Umudum et al., the spread of cancer in HPV-positive individuals is higher than in their counterparts, but overall survival is equal or better in HPV-positive individuals.Reference Umudum, Rezanko, Dag and Dogruluk12 Similarly, Gillison et al. concluded, after correcting for variables such as age and metastasis, that the survival rate was better in HPV-positive patients as compared to HPV-negative patients.Reference Gillison, Koch, Capone, Spafford, Westra and Wu13 The difference in our results could be due to other variables; for example, age at the time of detection, gender, patients’ unhealthy habits and the site involved.
In our study, male sex was significantly associated with HPV infection. Some other studies have reported males to have a higher incidence of HPV infection, and thus an increased risk for head and neck SCC,Reference Ritchie, Smith, Summersgill, Hoffman, Wang and Klussmann16 while others have not.Reference Krüger, Pabst, Walter, Sagheb, Günther and Blatt17 A study conducted by Zivile et al. investigating the prognostic factors for OSCCs showed worse survival in males as compared to female counterparts.Reference Zivile, Janina, Irina, Saulius, Raisa and Aliaksandr18 Our study, like several others,Reference Al-Rajhi, Khafaga, El-Husseiny, Saleem, Mourad and Al-Otieschan19, Reference Bryne, Koppang, Lilleng and Kjaerheim20 did not show any significant correlation between gender and survival.
• There is a high prevalence of human papillomavirus (HPV) associated oral squamous cell carcinoma (OSCC) in Pakistan
• In our previous study, overall and disease-free survival was better in HPV-positive than HPV-negative OSCC patients
• In this study, five-year survival was not significantly longer in HPV-positive than HPV-negative OSCC patients
Another variable that could influence survival rates in HPV-positive OSCC is age at the time of disease detection. In a study by Piccirillo et al., a significant rise in mortality was seen in patients with head and neck SCC after the age of 70 years.Reference Piccirillo, Lacy, Basu and Spitznagel21 Eighty per cent of our patients were aged over 40 years. Advanced age at the time of diagnosis may negatively affect prognosis. This may be due to a delay in starting treatment. In addition, older patients are more likely to have other co-morbidities, such as diabetes, heart or renal disease, and hypertension. These co-morbidities can hinder and prolong the healing process, and patients with these co-morbidities are known to have lower survival rates.Reference Piccirillo, Lacy, Basu and Spitznagel21, Reference Ribeiro, Kowalski and Latorre22 With advancing age, the number of risk factors increase as well. Increased exposure to and usage of carcinogenic substances (areca nut, tobacco, alcohol and so on), together with advancing age, may adversely affect the prognosis.
Apart from age and gender, the stage of malignancy at the time of presentation can also affect patient survival. The data show that the mortality rate increased steadily with disease stage (American Joint Committee on Cancer stages I–IV), from 33.3 to 64.5 per cent (Table II). The mean survival rate was lowest for stage 4 OSCC, and this trend was statistically significant for patient survival.
Historically, nodal status has been widely accepted as an important prognostic marker in patients with OSCC.Reference Ferlito, Rinaldo, Thomas Robbins, René Leemans, Shah and Shaha23–Reference Woolgar, Rogers, Lowe, Brown and Vaughan26 Positive nodes have been shown to decrease survival by almost 50 per cent.Reference Shingaki, Takada, Sasai, Bibi, Kobayashi and Nomura25–Reference Greenberg, Fowler, Gomez, Mo, Roberts and El Naggar27 This study also showed a statistically significant association between nodal involvement and survival (Table II).
Regarding the use of carcinogenic substances, in our study, more than two-thirds of patients had unhealthy habits. Areca nut and tobacco usage have been proven to be independent risk factors for oral cancers, with tobacco chewing being the strongest.Reference Ferlito, Rinaldo, Thomas Robbins, René Leemans, Shah and Shaha23 The increase in prevalence in recent years is not only because of tobacco chewing's addictive potential and the perception that it has stimulatory actions, but also because there is no social stigma attached to the usage of areca nut in the lower and middle income classes of Pakistan. Unhealthy habits involving tobacco consumption from not one but various sources, combined with a high prevalence of HPV, complicates the findings of our research, and this may be the reason for the discrepancy seen in our results as compared to other studies.
Lastly, there is an issue of compliance in our population. A lack of awareness, the absence of medical insurance and the resulting financial constraints mean that a lot of patients discontinue treatment or change doctors. This variability in patient treatment regimens may be having an unprecedented effect on disease prognosis.
Clinical relevance
Human papillomavirus associated OSCC has been linked to a better prognosis in many studies. Although the exact biology of this phenomenon is unknown, a few factors are thought to play a part. These include the fact that these tumours have no field cancerisation, there is good immune system response to certain viral-specific tumour antigens and they have an intact apoptotic reaction to radiation.Reference Tural, Elicin, Batur, Arslan, Oz and Serdengecti14 In addition, HPV-positive tumours occur most commonly in younger patients who are free of co-morbidities, and thus already have a better life expectancy. As a result, the prognosis of HPV-positive OSCC is positively influenced by these patient factors.Reference Preuss, Klussmann, Semrau and Huebbers28
The treatment regimens for HPV-positive and HPV-negative OSCC vary significantly. The HPV-positive patients undergo multiple radiotherapy sessions for improved survival, whereas HPV-negative patients undergo fewer radiotherapy cycles. Previous work has shown that HPV-induced OSCC responds better to radiation and to induction chemotherapy.Reference Fakhry, Westra, Li, Cmelak, Ridge and Pinto29 Furthermore, a meta-analysis of 30 published clinical trials revealed that HPV-positive OSCC patients had overall better disease-specific and progression-free survival.Reference Petrelli, Sarti and Barni30
As health insurance is rare in Pakistan, patients pay for all healthcare services with their own money, and these sessions are a financial burden. Moreover, additional radiotherapy is painful for the patients and carries many side effects. If determining the HPV status of OSCC patients becomes a routine measure, the treatment regimens could be modulated accordingly, and lead to fewer and more effective radiotherapy and chemotherapy sessions. This decrease in radiotherapy administration may not only save resources, but also prevent unwanted side effects and reduce the financial burden.
In conclusion, the results of this retrospective case series show a high prevalence of HPV-associated OSCC in the Pakistani population. However, we could not statistically prove that HPV positivity leads to longer survival for OSCC patients. Our findings are not in agreement with similar studies conducted elsewhere. A better understanding would be gained by conducting similar studies on a larger scale.
Acknowledgement
This study was supported by a grant from the University Research Council, Aga Khan University, Karachi, Pakistan (grant identification: 031018SURG).
Competing interests
None declared







