Introduction
Transportation of cattle is a necessary part of the production cycle that occurs during significant bovine life events including sorting, weaning, processing, and slaughter (Swanson and Morrow-Tesch, Reference Swanson and Morrow-Tesch2001; Schwartzkopf-Genswein and Grandin, Reference Schwartzkopf-Genswein, Grandin and Grandin2014). Transportation is a stressor that predisposes calves to the development of bovine respiratory disease (BRD). At least 7% of the cost of cattle production can be attributed to BRD (Griffin, Reference Griffin1997). The financial costs associated with BRD-induced shipping fever are extremely high. The US Department of Agriculture estimates that the cattle industry loses 1 billion USD each year from shipping-associated BRD (NAHMS, 2013). Most of the approaches to respiratory disease management in cattle are limited to vaccination and antibiotics to decrease disease prevalence and severity (Penny, Reference Penny2015). The physiological changes that occur during transportation begin with dehydration (Galyean et al., Reference Galyean, Lee and Hubbert1981), lack of feed intake (Cole et al., Reference Cole, Camp, Rowe, Stevens and Hutcheson1988), tissue damage (Murata et al., Reference Murata, Shimada and Yoshioka2004), fume inhalation (Wong et al., Reference Wong, Sun, Keith, Kweon, Foster, Schauer and Witten2003, Reference Wong, Sun, Lantz and Witten2004), and physical and psychological stress. These changes can result in immune system inhibition from prolonged exposure to stressful stimuli. Inflammation due to cytokine and acute phase protein responses are consistent components of BRD progression. Inflammation from BRD within the lung significantly affects performance through decreases in average daily gain, dry matter intake, and feed conversion (Gifford et al., Reference Gifford, Holland, Mills, Maxwell, Farney, Terrill, Step, Richards, Robles and Krehbiel2012). The additional increases in inflammation can result in increased pyrexia- and cachexia-related energy demand, which leads to protein resorption from skeletal muscle.
Though there is evidence for decreased immune function and increased inflammation, little intervention is performed in cattle prior to transportation (Duff and Galyean, Reference Duff and Galyean2007). Most, if not all, of conventional on-arrival metaphylaxis or vaccination, or both, occurs within the first weeks after arrival (Richeson et al., Reference Richeson, Beck, Gunter, Gadberry, Hubbell and Jones2006; White et al., Reference White, Amrine and Goehl2015).
A recent diagnostic laboratory investigation revealed that during the three-year period from 2009–2011, the prevalence of antimicrobial resistance in submitted isolates of Mannheimia haemolytica increased from 5 to 35% (Lubbers and Hanzlicek, Reference Lubbers and Hanzlicek2013). Consumer concern about resistance in meat products contributed to the development of organic and all-natural product niche markets (Sofos, Reference Sofos2008). Though selection for resistance has been documented in respiratory pathogens after antimicrobial treatment, changes in common foodborne pathogens have not been consistently found to be different (Fox et al., Reference Fox, Reinstein, Jacob and Nagaraja2008). To reduce antimicrobial usage, valid methods that can be used to identify the cattle most susceptible to disease while in the feed yard are needed. Some early detection behavior methods have been proposed to remotely detect cattle that are displaying abnormalities in social behavior, activity, and location in the pen (e.g. feed bunk or water trough) that indicate the presence of respiratory disease, without the need for human interaction (White et al., Reference White, Amrine and Goehl2015). Previous research studies have also examined the use of trends in physiologic and metabolic parameters after the cattle arrive at their destination. There is no single biomarker that indicates BRD risk upon arrival and has been proven useful as a mitigation strategy tool to be solely effective in decreasing BRD incidence.
The objective of this review is to highlight the biomarkers or analytes in recent literature that have been evaluated at transportation. Using the biomarkers outlined in this review, compiling a quantitative trend of increasing and decreasing biomarkers may be the best possible method for indicating problematic groups of cattle, and the use of non-antimicrobial methods to help decrease BRD incidence for incoming cattle requires investigation. For this review, relevant transportation effects on BRD risk are depicted in Fig. 1; the figure illustrates the complex connections within the cascade of events and biomarker changes that result in clinical respiratory disease. The purpose of this review is to highlight biomarker changes that occur after transportation that may be useful in formulating a predictive model for BRD.
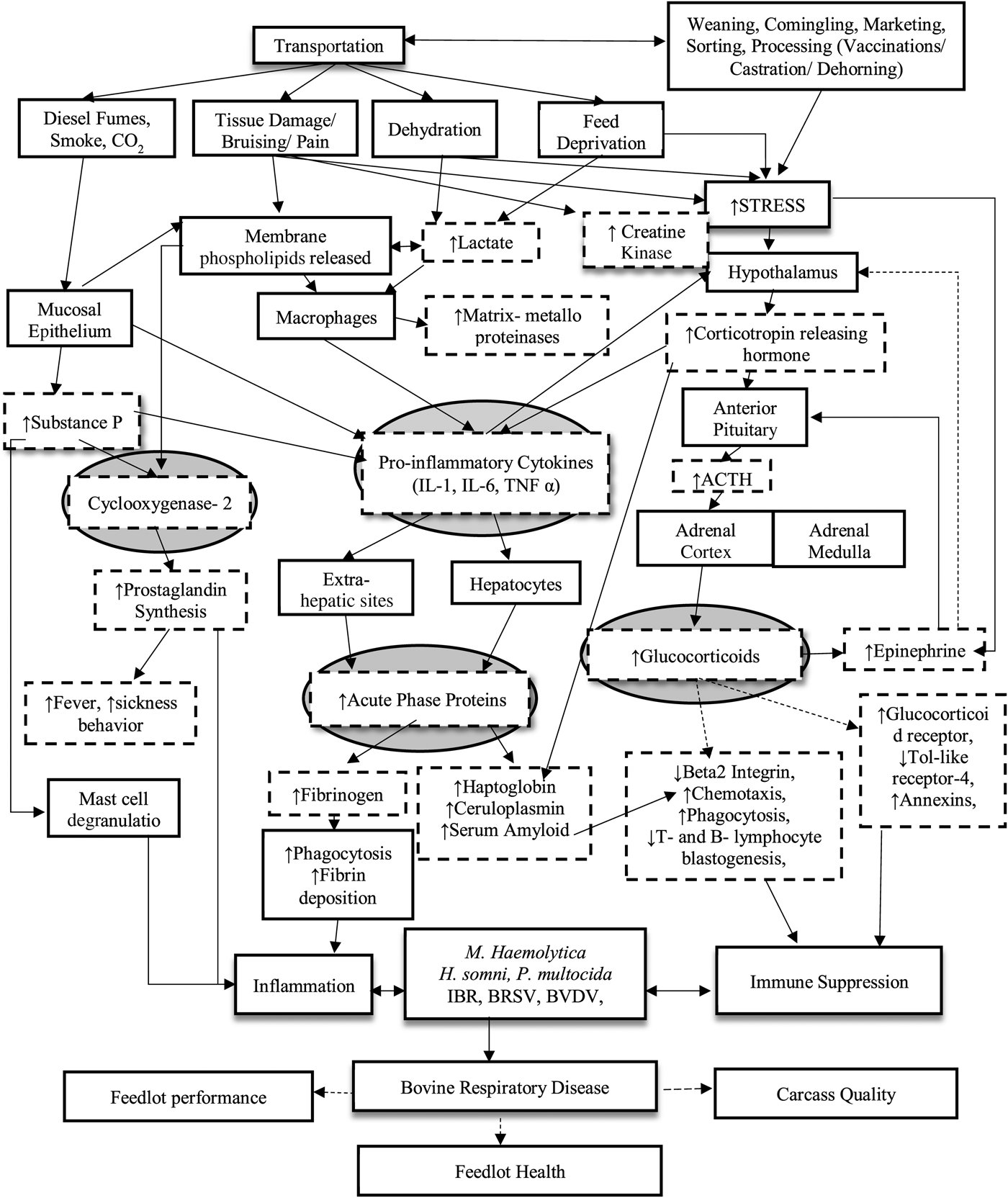
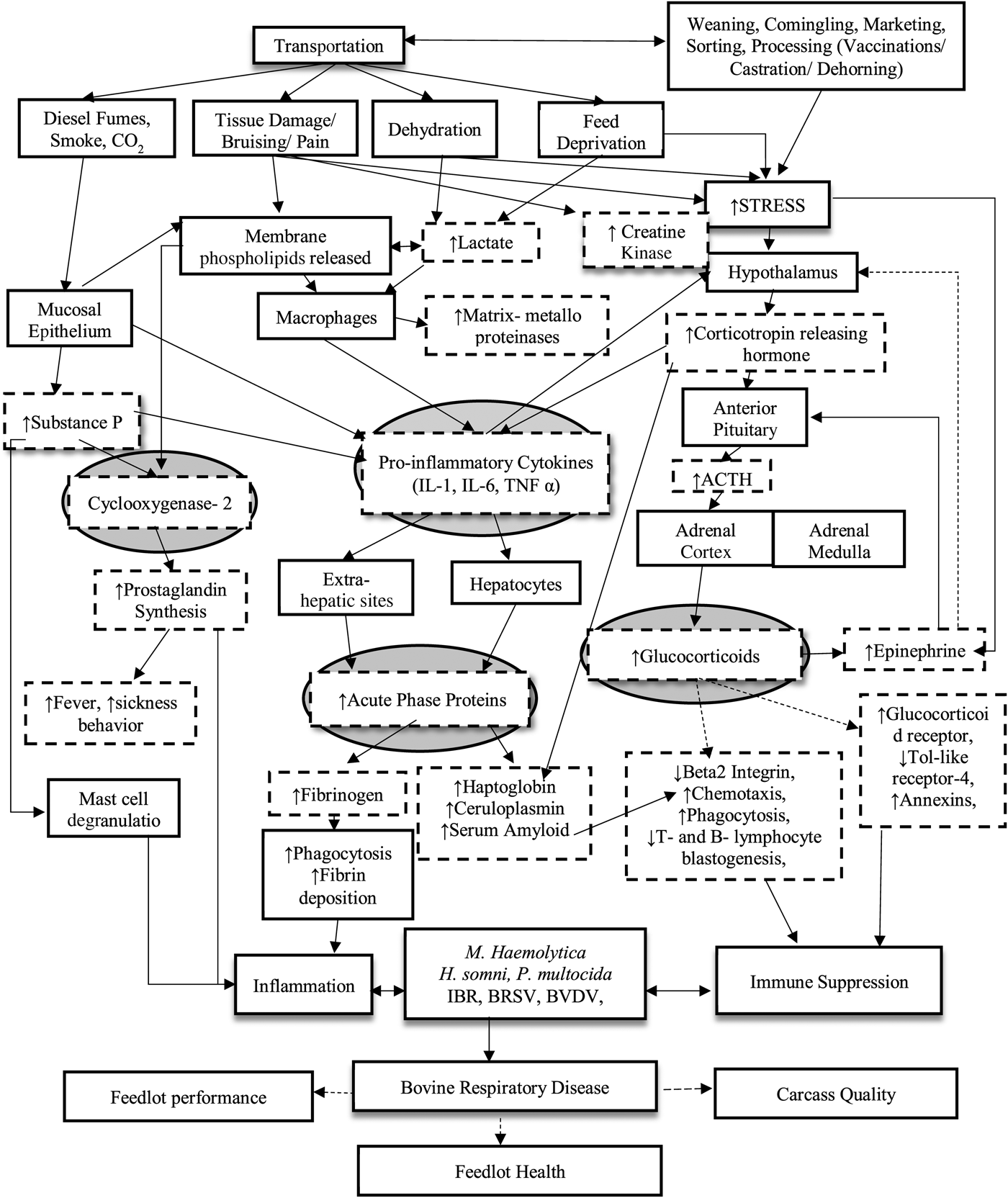
Fig. 1. Effects of transportation on bovine respiratory disease outcomes. This is a flow diagram depicting the intricacies of the role transportation has on increasing the risk of bovine respiratory disease in the feed yard. Arrows connect the hierarchy of cascading effects incited by transportation starting with the visually observed and progressing to the animal's underlying physiology that results in respiratory disease outcomes. Boxes or arrows with a dash line represent changes monitored after transport.
Deleterious effects of transportation
Truck transport effects
At the cellular level, tissue damage results in the release of the membrane phospholipid bilayer and incites an inflammatory cascade at the site of injury through the conversion of lipid membrane into prostaglandins and other metabolites (Enyedi et al., Reference Enyedi, Jelcic and Niethammer2016). During road transport, the truck environment and truck-associated parameters can have characteristics with an underlying predisposition to cause tissue damage, discomfort, and added stress. The truck design, stocking density, driver, road quality, and ventilation and ambient temperature of the hauling container are important factors to consider during an assessment of transport conditions (Broom, Reference Broom2008). The pre-transport loading process can be the most stressful part of the trip, and the stress response then normalizes while the cattle are in transit (Pettiford et al., Reference Pettiford, Ferguson, Lea, Lee, Paull, Reed, Hinch and Fisher2008). Loading stress can be associated with cattle that have high temperaments or calm temperaments (Burdick et al., Reference Burdick, Carroll, Randel, Willard, Vann, Chase, Lawhon, Hulbert and Welsh2011). During loading or transit, movement of the cattle can increase the chances for soft tissue injury. Rough roads and improper handling can cause carcass bruising, which is detrimental to animal welfare and is an additional loss in the product at the packer (Huertas et al., Reference Huertas, Gil, Piaggio and Van Eerdenburg2010). If the cattle are not destined for slaughter, the bruising nature of the shipment process can affect behavior and investigation is needed to tie into effects on cattle acclimation and BRD. This trauma combined with the distance traveled may be associated with the animal's responses to the transportation event. Compared with shorter distances, travel distances greater than 40 miles from the slaughterhouse are associated with a statistically significant increase in bruising risk (Jarvis et al., Reference Jarvis, Messer and Cockram1996). The increases in bruising can be monitored physiologically using increases in plasma creatine kinase concentrations. One study found that increases in creatine kinase occurred in veal calves during transport, but there was no correlation with the amount of muscle bruising at harvest (Grigor et al., Reference Grigor, Cockram, Steele, Mcintyre, Williams, Leushuis and Van Reenen2004).
After arrival at the destination, normal cattle behavior is altered as cattle recuperate. Immediately after transport at high temperatures (≥32.2 °C), heifers spend more time at the hay feeder; they also lie down more often 1 to 2 days after transport, compared with control heifers (Theurer et al., Reference Theurer, White, Anderson, Miesner, Mosier, Coetzee and Amrine2013). These findings are consistent with the loss of body weight in the form of rumen gastrointestinal contents and the physical exertion required to stand the entire transport period. A split nine-hour (h) transport, 12-h rest and nine-h transport evaluated animal activity during the resting periods after each transport compared to non-transport controls. In each resting period after transport, bulls spent significantly less time lying down compared with their non-transported counterparts (Earley et al., Reference Earley, Drennan and O'Riordan2013). In other studies, this is supported and likely has a behavior component due to sex. Non-transported bulls spent significantly more time in recumbency than bulls that were transported. Results from other observational studies have indicated that on arrival and following transport, young bulls spent less time acclimating or exploring the new home pen and more time interacting with each other (Cafazzo et al., Reference Cafazzo, Magnani, Cala, Razzuoli, Gerardi, Bernardini, Amadori and Costa2012).
The transport truck's compartment environments are not equal; there are compartment-associated differences in average daily gain and disease risk (White et al., Reference White, Blasi, Vogel and Epp2009). Cattle in the forward sections or transported in spaces with less than 15 head have reduced odds of treatment. Likely due to more animals comingling. The vibration of the vehicle while in motion can affect the animals during transportation. The greatest vibration occurs on gravel roads. When cattle assume a position perpendicular to the direction of travel, they experience significantly less vibration (Gebresenbet et al., Reference Gebresenbet, Aradom, Bulitta and Hjerpe2011). Vibration originating predominantly from the vertical axis of the trailer is dependent on tire pressure (Stevens and Camp, Reference Stevens and Camp1979). Truck design characteristics and road conditions cannot be readily changed but there are parameters that should be controlled. The driver's behavior during transport affects the cattle's orientation (Cockram and Spence, Reference Cockram and Spence2012). Driver tendencies when handling the truck can be defined by the need to accelerate or brake to quickly in certain situations, but changes in rapid motion are less frequent during highway transit. There is a lower probability of sudden changes during highway driving versus city driving. The role of the driver in the prevention of animal discomfort is important and easy to address.
Fumes
During the transportation process, cattle are exposed to potentially irritating diesel fumes. Fumes can consist of organic compounds (e.g. aldehydes, benzenes, and polycyclic aromatic carbons) that are disruptive to the mucosal epithelium (Wierzbicka et al., Reference Wierzbicka, Nilsson, Rissler, Sallsten, Xu, Pagels, Albin, Osterberg, Strandberg, Eriksson, Bohgard, Bergemalm-Rynell and Gudmundsson2014). Wong et al. (Reference Wong, Sun, Keith, Kweon, Foster, Schauer and Witten2003), found that exposure of rats to diesel exhaust can cause neurogenic pulmonary responses that can perpetuate lung inflammation. Diesel fume disturbance and disruption of the respiratory mucosal system can be a large contributor to respiratory impairment and subsequent disease (Riedl and Diaz-Sanchez, Reference Riedl and Diaz-Sanchez2005). Other heavy metals (e.g. vanadium) that are released during exhaust excretion of diesel fuels cause inhibition of the innate defenses of the respiratory epithelium (Klein-Patel et al., Reference Klein-Patel, Diamond, Boniotto, Saad and Ryan2006). Fumes and their effects on respiratory disease are difficult to study in cattle and are best interpreted through extrapolation of research findings from other mammalian species. No published data has been generated for cattle in conventional transport scenarios to indicate that fumes enter the trailer that cattle are housed in during transport. This is a point that requires investigation and future research.
Dehydration and temperature
Increased values for PCV and total protein concentrations are evidence of the dehydration that occurs during shipping (Jarvis et al., Reference Jarvis, Messer and Cockram1996). Dehydration is directly related to disease due to the disruption in the mucociliary apparatus function of the respiratory epithelium (Ackermann et al., Reference Ackermann, Derscheid and Roth2010). The ambient temperature in the transportation environment is a potential cause of dehydration. When the temperature is outside the animal's normal critical limits, energy expenditure increases to maintain body temperature homeostasis. Most trailers that are hauling cattle depend on movement to provide ventilation; a stationary position results in stagnant conditions. Quantification of the trailer's temperature climate is difficult to interpret when one temperature logger is used; multiple loggers should be used to obtain more accurate information (Goldhawk, Reference Goldhawk, Crowe, Gonzalez, Janzen, Kastelic, Pajor and Schwartzkopf-Genswein2014a, Reference Goldhawk, Crowe, Janzen, Gonzalez, Kastelic, Pajor and Schwartzkopf-Genswein2014b). However, there are some management benefits from even limited logger information. Time of year and ambient conditions are key inputs for decisions and associated risks during transport (Goldhawk, Reference Goldhawk, Crowe, Gonzalez, Janzen, Kastelic, Pajor and Schwartzkopf-Genswein2014a, Reference Goldhawk, Crowe, Janzen, Gonzalez, Kastelic, Pajor and Schwartzkopf-Genswein2014b). In-transit temperatures >30°C have adverse effects on thermoregulation. Heifers shipped at a high ambient temperature (>32°C) had significant behavioral changes, weight loss, and peripheral vasoconstriction-associated nasal and rectal temperature decreases (Theurer et al., Reference Theurer, White, Anderson, Miesner, Mosier, Coetzee and Amrine2013). On the reversal, cold stress draws limited attention, however cattle coat acclimation to cold weather as well as truck preparation for cold weather by plugging specific holes to prevent cold wind is critical (Broom, Reference Broom2008).
Weight and distance
The most significant aspect of transport for consideration is transporting cattle long-distances without stops for food, water, and rest (Schwartzkopf-Genswein and Grandin, Reference Schwartzkopf-Genswein, Grandin and Grandin2014). An unavoidable loss of body weight (i.e. shrink) occurs during transport. This effect usually occurs due to the lack of feed and water, and increased defecation and urination. Marques et al. (Reference Marques, Cooke, Francisco and Bohnert2012), found that food deprivation in transport is the major factor that accounts for lost performance. They transported steers and heifers for 24 h and allowed one period of rest at 12 h with no access to food or water, and they had a separate group non-transported that was restricted from ration and water. These treatments had statistically significant decreases in body weight ADG, compared with control animals who were rested and received rations. Addressing the question of access to food and water during transport, the effectiveness of a rest stop with access to food and water for avoiding the performance losses during transport was examined by Cooke (Reference Cooke, Cappellozza, Guarnieri and Bohnert2013a, Reference Cooke, Guarnieri, Cappellozza and Bohnert2013b). The results for use of a two-h rest stop at the half-way point of a 1290-km journey with ad libitum hay and water access were not different from the results for straight-through transport. Only the non-transported control animals had a greater feed to gain and average daily gain values. Even when transporting calves less than 4 weeks of age for a duration of 19 h, there is no benefit from a one-h feeding stop (Knowles, Reference Knowles1999; Knowles et al., Reference Knowles, Brown, Edwards, Phillips and Warriss1999). This finding is supported by other results that calves regain their original body weight by 24 to 72 h post-transport (Knowles et al., Reference Knowles, Warriss, Brown, Edwards, Watkins and Phillips1997). The losses occur during a wide time interval. Most of the gut contents are lost during the first 24 h of transport, but the greatest rate of loss occurs within the first 12 h (Knowles, Reference Knowles1999; Knowles et al., Reference Knowles, Brown, Edwards, Phillips and Warriss1999). Overall shrink is directly tied to transportation despite offerings of food or feeding, the event results in losses.
Shrink is a measure of performance, but it is also associated with the health of cattle that are transported. Loss of bodyweight in lighter weight cattle may be connected to increased morbidity and mortality, but increased hot carcass weight and average daily gain during the post-transport period (Cernicchiaro, Reference Cernicchiaro, White, Renter, Babcock, Kelly and Slattery2012a, Reference Cernicchiaro, White, Renter, Babcock, Kelly and Slattery2012b). For the body weight loss variable, there were similar outcomes for morbidity, mortality, hot carcass weight, and average daily gain when distances traveled were compared (Cernicchiaro, Reference Cernicchiaro, White, Renter, Babcock, Kelly and Slattery2012a, Reference Cernicchiaro, White, Renter, Babcock, Kelly and Slattery2012b). Cernicchiaro et al. found that procurement of cattle from specific regions of the USA correlated with increased post-transport morbidity. Other study findings indicated there were no correlations between the shrink that results from short- or long-term transportation and the development of respiratory disease (Ribble, Reference Ribble, Meek, Shewen, Guichon and Jim1995a, Reference Ribble, Meek, Shewen, Jim and Guichon1995b). Ribble et al. (Reference Ribble, Meek, Shewen, Guichon and Jim1995a, Reference Ribble, Meek, Shewen, Jim and Guichon1995b) found a strong correlation between comingling calves from different sources and the subsequent development of BRD. These conflicting results indicate the multifactorial nature of BRD. Though these studies may have been small in number and lacked the appropriate power, the work is still beneficial. However, it is highly likely that comingling, age, arrival weights, and pre-transport management strategies are associated with disease risk.
Biomarkers of stress
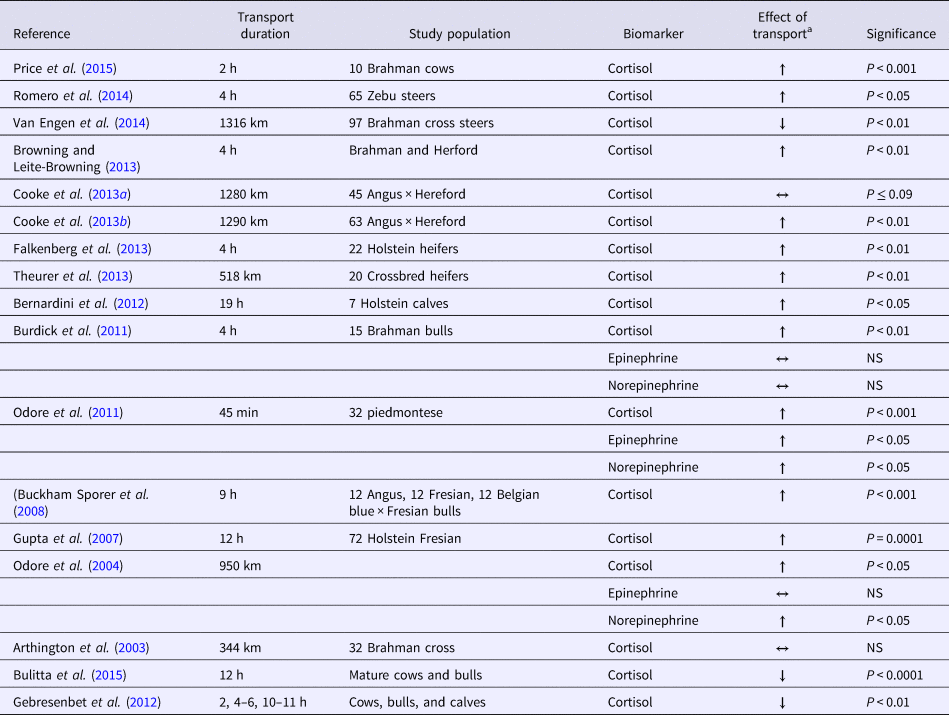
Components of the bovine endocrine system and non-endocrine physiological parameters have been used to quantify the level of stress (Arthington et al., Reference Arthington, Eicher, Kunkle and Martin2003). Neural-hormonal biomarkers of interest have been identified (i.e. ACTH, cortisol, catecholamines, and iodothyronines). The rapid release and degradation of these hormones and the natural, circadian rhythm release during the day present a challenge to the use of these markers for assessing transport stress (VanCauter et al., Reference Vancauter, Leproult and Kupfer1996).
Hypothalamus pituitary adrenal (HPA) axis
Glucocorticoids (specifically cortisol) are released during stressful situations, but responses are delayed due to transcriptional downstream effects from cytoplasmic receptor complexes that interact with DNA. Glucocorticoid interference can inhibit signaling mechanisms (e.g. Toll-Like Receptor-4) during dendritic cell maturation; these signaling mechanisms are critical for the innate defense response and lipopolysaccharide recognition (Rozkova et al., Reference Rozkova, Horvath, Bartunkova and Spisek2006). Other DNA transcription effects increase during glucocorticoid upregulation of the gluconeogenic capacity of the liver to use amino acids and fatty acid mobilization for energy creation (Hall, Reference Hall2006). In addition to metabolic functions, cortisol has inhibitory effects on inflammation, vascular permeability to white cells, and the immune system (i.e. primarily for lymphocyte reproduction) (Hall, Reference Hall2006). Although not studied extensively in cattle, the effects of circadian rhythms are important for the interpretation of cortisol levels over periods of time; there are variations in normo-physiologic secretions throughout the day (VanCauter et al., Reference Vancauter, Leproult and Kupfer1996).
The HPA axis should be considered during the investigation of transportation effects on cattle. Sustained stressor events result primarily in corticosteroid and aldosterone release (von Borell, Reference Von Borell2001). Cortisol is the main corticosteroid measured in studies of the stress response (Table 1). Cortisol can be used as a biomarker that is linked with behavior responses in cattle; anxiety is indicated by less time ruminating and increased vocalization (Bristow and Holmes, Reference Bristow and Holmes2007). Cortisol identification methods include measurement in plasma, hair, and the fecal contents of calves during transport (Mostl et al., Reference Mostl, Maggs, Schrotter, Besenfelder and Palme2002; Arthington et al., Reference Arthington, Eicher, Kunkle and Martin2003; Marti et al., Reference Marti, Wilde, Moya, Heuston, Brown and Schwartzkopf-Genswein2017). Results for beef bulls indicated that 4.5 h after the start of a nine-h transportation event there was a 321% increase in cortisol concentrations, compared with a 24-h baseline sample that was taken before transportation began (Buckham Sporer et al., Reference Buckham Sporer, Weber, Burton, Earley and Crowe2008). Other groups and reviews confirm that the cortisol increase that occurs during transportation can be used as a marker of stress (Knowles, Reference Knowles1999; Knowles et al., Reference Knowles, Brown, Edwards, Phillips and Warriss1999; Odore et al., Reference Odore, Badino, Re, Barbero, Cuniberti, D'Angelo, Girardi, Fraccaro and Tarantola2011). Ruling out a novel environment as a cause of stress is important. Arrival at an original starting point location versus a naïve arrival location had minimal effects on cortisol levels (Browning and Leite-Browning, Reference Browning and Leite-Browning2013).
Table 1. Summary of the scientific literature examining the changes in stress biomarkers during cattle transportation
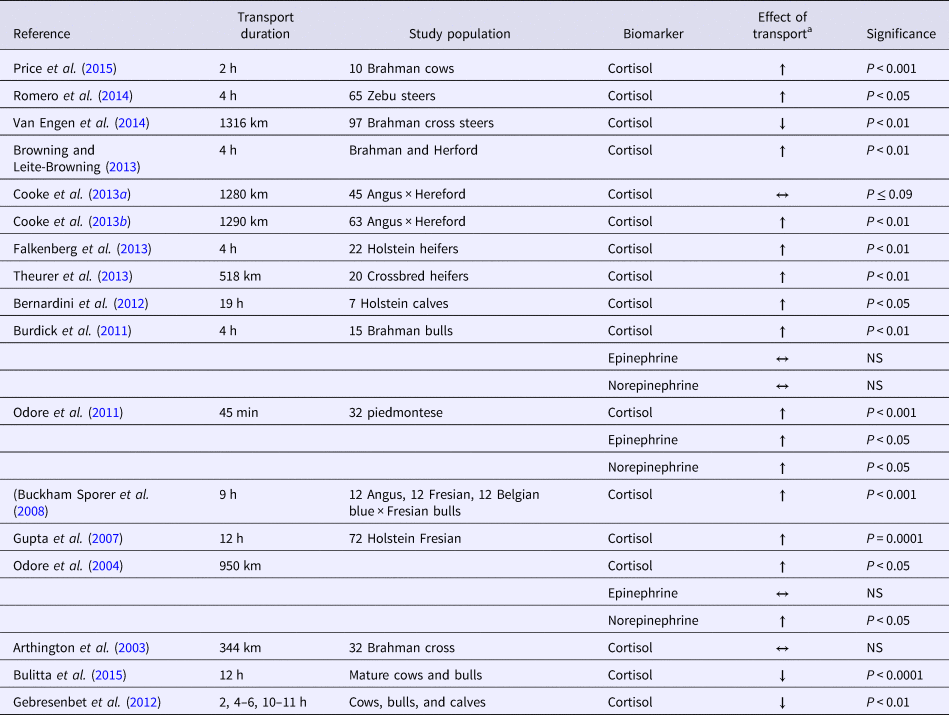
a All arrows are in reference of transported animal's stress biomarkers response in comparison with either baseline value or a non-transported control group.
Effects revealed after longer transport distances can potentially cause a decrease in the cortisol response that may be due to the extensive fasting or exhaustion associated with the journey (Van Engen et al., Reference Van Engen, Stock, Engelken, Vann, Wulf, Karriker, Busby, Lakritz, Carpenter, Bradford, Hsu, Wang and Coetzee2014). Supporting results indicated that increased transport time correlated with a decrease in the level of plasma cortisol concentration (Gebresenbet et al., Reference Gebresenbet, Wikner, Bobobee, Maria and Villarroel2012; Bulitta et al., Reference Bulitta, Aradom and Gebresenbet2015). This change could be caused by the animal's acclimation to transport. However, attributing the cortisol response only to transportation-related variables excludes other variables associated with the cortisol response. Cattle handling and processing variables may also contribute to the stress response. In addition to animal handling, the effects of the individual animal's temperament should be considered when cortisol is examined (Burdick et al., Reference Burdick, Carroll, Hulbert, Dailey, Willard, Vann, Welsh and Randel2010). Regardless of differences in animal handling style, temperament can confound the outcome. Conclusions have greater validity when temperament is not a factor.
Counterintuitively with the HPA axis, corticotropin-releasing hormone can have alternate effects from cortisol. Corticotropin-releasing hormone increases the inflammatory response of macrophages in vitro and incites increased production of inflammatory cytokines (Agelaki et al., Reference Agelaki, Tsatsanis, Gravanis and Margioris2002). ACTH did not increase prostaglandin levels in pregnant cows (Geary, Reference Geary2012). This result suggested that specific pathways of inflammation can be affected by physiologic fluctuations in CRH, ACTH, and cortisol. Regardless of circulating cortisol levels in response to stressors, it is important to note that there is a complex relationship between glucocorticoid synthesis and development of BRD (Senthilkumaran et al., Reference Senthilkumaran, Clark, Abdelaziz, Bateman, Mackay, Hewson and Caswell2013). Annexin A1 is a protein found in epithelial secretions. Concentrations of annexin A1 correlate with circulating cortisol concentrations. However, calves that do not develop BRD while in the feed yard have increased Annexin A1 concentrations, compared with calves that do develop BRD (Senthilkumaran et al., Reference Senthilkumaran, Clark, Abdelaziz, Bateman, Mackay, Hewson and Caswell2013). This finding emphasizes the complex associations between glucocorticoids, stress, and BRD.
Fight or flight
A short startle response induces the hypothalamic adrenal medullary system (i.e. the fight or flight response) and causes an increased sympathetic tone. Similar to cortisol, epinephrine and norepinephrine responses are correlated with animal temperament (Table 1). Compared with calm bulls, temperamental bulls have elevated epinephrine levels following transportation (Burdick et al., Reference Burdick, Carroll, Hulbert, Dailey, Willard, Vann, Welsh and Randel2010). Two studies found that transportation alone increased norepinephrine (Odore et al., Reference Odore, D'Angelo, Badino, Bellino, Pagliasso and Re2004) and epinephrine (Odore et al., Reference Odore, D'Angelo, Badino, Bellino, Pagliasso and Re2004; Aktas et al., Reference Aktas, Ozkanlar, Karakoc, Akcay and Ozkanlar2011), but temperament was not included in the analyses of the results of either of these studies. The short half-lives of epinephrine and of norepinephrine negatively affect the value of these parameters as biomarkers for the identification of stress.
Iodothyronines
Tri-iodothyronine (T3) has many and diverse functions in tissues; T3 is a metabolically important hormone. The effects of periods of stress due to handling and transport on changes in T3 levels have been investigated. The results indicated that there was an increase in T3 concentrations after transport and after handling events, but there were no differences between the post-transport and post-handling T3 concentrations (Mitchell et al., Reference Mitchell, Hattingh and Ganhao1988). Mitchell et al (Reference Mitchell, Hattingh and Ganhao1988) suggested that the transport effect was confounded by the need to handle the animals after transport and that the effect of handling, not transport, was the cause of the T3 increase. Further research on post-transport T3 levels is needed, but one plausible hypothesis is that increased metabolism mobilizes nutrients during the fasting that occurs during transport.
Metabolic changes
Cortisol and catecholamines control metabolism in the liver and are included in pathways important for processing carbohydrates (gluconeogenesis), proteins, and lipids (lipolysis) (Gifford et al., Reference Gifford, Holland, Mills, Maxwell, Farney, Terrill, Step, Richards, Robles and Krehbiel2012). Investigations of specific hematological outcomes associated with circulating energy modifications are of interest because of the shrink and fasting effects of transport. Non-esterified fatty acids, glucose, and albumin levels in blood were elevated, and beta-hydroxybutyrate levels in blood were decreased, after transport (Earley and O'Riordan, Reference Earley and O'Riordan2006; Earley et al., Reference Earley, Fisher and O'Riordan2006; Earley et al., Reference Earley, Murray and Prendiville2010; Cafazzo et al., Reference Cafazzo, Magnani, Cala, Razzuoli, Gerardi, Bernardini, Amadori and Costa2012). Other results indicate there is an increase in beta-hydroxybutyrate following transportation, which is relevant to a metabolic fasting state (Bernardini et al., Reference Bernardini, Gerardi, Peli, Costa, Amadori and Segato2012). Increases in these elements of a blood panel are consistent with the effects of cortisol during the stress response. Breed differences in cortisol response may account for the significant glucose increase in Brahmans compared with Herefords (Browning and Leite-Browning, Reference Browning and Leite-Browning2013). Although glucose increases, the return to homeostasis occurs within 24 h of the transport event (Earley et al., Reference Earley, Murray and Prendiville2010). Lipid mobilization also occurs during the shrink and fasting states of transportation. Mobilization is linked with increased non-esterified fatty acid levels in circulating blood after transportation (Cooke, Reference Cooke, Cappellozza, Guarnieri and Bohnert2013a, Reference Cooke, Guarnieri, Cappellozza and Bohnert2013b). Non-esterified fatty acid increases are linked to catecholamine release (Agnes et al., Reference Agnes, Sartorelli, Picotti, Arrigoni and Locatelli1990). A reduction in non-esterified fatty acid metabolism occurred in cattle transported for 1290 km when there were two, two-h stops with feed and water (i.e. at each 430 km). However, this intervention reduced but did not eliminate the increase in the non-esterified fatty acid response (Cooke, Reference Cooke, Cappellozza, Guarnieri and Bohnert2013a, Reference Cooke, Guarnieri, Cappellozza and Bohnert2013b).
Metabolic markers can be used for more than indicators of fasting. Lactate concentrations can be significantly elevated after transport and correlated with transport time (Chacon et al., Reference Chacon, Garcia-Belenguer, Villarroel and Maria2005). Lactate is a marker for transport-associated muscle fatigue; longer distances and increased standing times can cause increased lactic acid production due to oxygen depletion and the inability to re-phosphorylate ADP (Sahlin, Reference Sahlin1986). The circulating blood level of creatine kinase has also been studied as a marker of muscle damage. Most investigators have found elevated creatine kinase levels upon arrival (Van de Water et al., Reference Van De Water, Verjans and Geers2003; Grigor et al., Reference Grigor, Cockram, Steele, Mcintyre, Williams, Leushuis and Van Reenen2004; Earley et al., Reference Earley, Murray and Prendiville2010; Bernardini et al., Reference Bernardini, Gerardi, Peli, Costa, Amadori and Segato2012). However, the results of another study indicated that there were no significant changes in creatine kinase following transport (Cafazzo et al., Reference Cafazzo, Magnani, Cala, Razzuoli, Gerardi, Bernardini, Amadori and Costa2012). Increases in creatine kinase can be used as an indicator for exertion associated with transport. However, fluctuations in creatine kinase levels do not always result in significant effects and can vary based on the trip- and animal-associated differences.
Pain biomarker
Results from a castration trial indicated that the neuropeptide substance P (i.e. a derivative of the nociception response) can be used as a biomarker (Coetzee et al., Reference Coetzee, Lubbers, Toerber, Gehring, Thomson, White and Apley2008). Other implications of the peptide in physiology include effects on mast cell degranulation and migration of macrophages to the lung epithelium during acute respiratory infection (Ramirez-Romero et al., Reference Ramirez-Romero, Brogden, Gallup, Sonea and Ackermann2001). Substance P has been examined during transportation events. Increases have been documented on arrival and at 4 h after arrival, compared with baseline levels (Theurer et al., Reference Theurer, White, Anderson, Miesner, Mosier, Coetzee and Amrine2013), and at 24 h post initiation of transportation (Van Engen et al., Reference Van Engen, Stock, Engelken, Vann, Wulf, Karriker, Busby, Lakritz, Carpenter, Bradford, Hsu, Wang and Coetzee2014). Discovery and expansion of the pain biomarker library in cattle are crucial for the understanding of welfare issues.
Immune system translation
Cellular profiles
Stress and inflammatory responses can mediate immune system signaling through changes in the peripheral blood cellular profile. Transportation is accountable for a stress-induced neutrophilia (Earley and O'Riordan, Reference Earley and O'Riordan2006; Earley et al., Reference Earley, Fisher and O'Riordan2006). There are two different understandings of neutrophil functionality after transportation. Indications have been made for a reduction of neutrophil oxidoreductase activity at arrival and increase 4 h after arrival (Murata et al., Reference Murata, Takahashi and Matsumoto1987). Others report reductions in phagocytic neutrophil intensity at 48 h post-transit (Burdick et al., Reference Burdick, Carroll, Randel, Willard, Vann, Chase, Lawhon, Hulbert and Welsh2011). Linking these changes has been investigated at an expression level. The increasing circulating neutrophil genetic expression level suggests an increase in antibacterial function. Neutrophilia upregulation and the corresponding bacteria-killing properties occur through increases in tissue remodeling matrix metalloproteinase 9, and decreased expression of the apoptotic marker FAS and extravasation cell marker L selectin. (Buckham Sporer et al., Reference Buckham Sporer, Weber, Burton, Earley and Crowe2008). Neutrophil phagocytosis and oxidative burst capacity increase, and beta 2 integrin (essential for extravasation out of the vasculature) decreases after transport (Hulbert et al., Reference Hulbert, Carroll, Burdick, Randel, Brown and Ballou2011). Transport leads to neutrophils that are activated in circulation but with a diminished ability to enter tissue, and a neutrophilia occurs. Hulbert and colleagues also found that upregulation of phagocytosis creates resistance to a microbial threat in calm-disposition cattle compared with temperamental cattle. The numbers of neutrophils expressing a betaglycan gene correlated with decreased lung lesions and pulmonary adhesion, when the gene expression was elevated 7 days after transportation (Eitam et al., Reference Eitam, Vaya, Brosh, Orlov, Khatib, Izhaki and Shabtay2010). However, the increased betaglycan results were not statistically significant following a nine-h transportation (Buckham Sporer et al., Reference Buckham Sporer, Burton, Earley and Crowe2007). Betaglycan is of primary interest because it binds to TGFβ. TGFβ is involved in the development of lung pathology that results in fibrosis of lung parenchyma (Bartram and Speer, Reference Bartram and Speer2004). Betaglycan has potential as a genetic marker for BRD in calves that are arriving in the feed yard and its measurement may be a tool that can be used to identify calves that would eventually succumb to the effects of chronic lung fibrosis.
Lymphocytes are the main adaptive immune functioning cells in the bovine. Transportation events are associated with decreases in the numbers of peripheral circulating lymphocytes (Earley and O'Riordan, Reference Earley and O'Riordan2006; Earley et al., Reference Earley, Fisher and O'Riordan2006). These events have also been associated with decreased lymphocyte blastogenesis (Murata, Reference Murata1989). Despite decreasing lymphocyte counts after transport, the level of circulation of natural killer cells is positively correlated with the increasing post-transport levels of cortisol (Ishizaki and Kariya, Reference Ishizaki and Kariya2010). A decrease in the ratio of lymphocytes to granulocytes is accounted for by an increase in circulating granulocytes (Kang et al., Reference Kang, Lee, Piao, Kwak, Gu, Yun, Kim, Ahn, Kim, Kim, Kim, Ko, Ha and Baik2017). Post-transport lymphocyte changes in expression caused significant upregulation of the heat shock proteins 70A1A and 60, which has a cytoprotective effect (Eitam et al., Reference Eitam, Vaya, Brosh, Orlov, Khatib, Izhaki and Shabtay2010). Lymphocytes were taken from transported Holstein-Friesian bulls, cultured, and stimulated with concanavalin A; the decrease in IFNγ production suggested there was a decrease in immune function (Gupta et al., Reference Gupta, Earley and Crowe2007). Other findings have suggested that IFNγ is not inhibited in specific T cell populations upon stimulation; in some cases, it is enhanced by transportation when cells are stimulated in vitro (Van Engen et al., Reference Van Engen, Platt, Roth, Stock, Engelken, Vann, Wulf, Busby, Wang, Kalkwarf and Coetzee2016). Vaccination within the receiving period results in variability in performance outcomes, and positive and negative effects. The result of a comparison between cell-mediated immunity on arrival and at 7 days after use of an on-arrival vaccination also supports the hypothesis of a maintained immune function response (Van Engen et al., Reference Van Engen, Platt, Roth, Stock, Engelken, Vann, Wulf, Busby, Wang, Kalkwarf and Coetzee2016). The results suggest that transport does not have a substantial prolonged effect on a parenterally induced immune response. Immune function impairment associated with shipping stress could be confined to the lung and increases the likelihood of shipping fever. However, it is likely that stress is a result of prolonged and multifactorial events and transport alone is not sufficient to decrease immune function.
Cytokines
Inflammatory cytokine signals are released during reactions to infection, tissue injury, and stress. Lipopolysaccharide challenge in whole blood 48 h after transport indicated there was a statistically significant 40% increase in tumor necrosis factor (TNF) α expression, followed by a statistically significant 59% decrease at 96 h, compared with pre-transport baseline values (Carroll et al., Reference Carroll, Arthington and Chase2009). TNF in serum alone has also been found to increase after transportation (Van Engen et al., Reference Van Engen, Stock, Engelken, Vann, Wulf, Karriker, Busby, Lakritz, Carpenter, Bradford, Hsu, Wang and Coetzee2014). An increase in proinflammatory cytokines (e.g. IL 6 and TNF α) can be attributed to a novel environment and can be modulated for 15–30 days after arrival (Razzuoli et al., Reference Razzuoli, Olzi, Cala, Cafazzo, Magnani, Vitali, Lacetera, Archetti, Lazzara, Ferrari, Costa and Amadori2016). The release of corticotropin-releasing hormone is linked to the release of circulating immune system proinflammatory cytokines (Hulbert et al., Reference Hulbert, Carroll, Burdick, Randel, Brown and Ballou2011). Description of different cytokine profiles is warranted, but there is currently no single cytokine that is a valid indicator of shipping fever outcomes. Combining cytokine profiles with cortisol or epinephrine changes could give an indication of calf disease status or risk.
Acute phase proteins
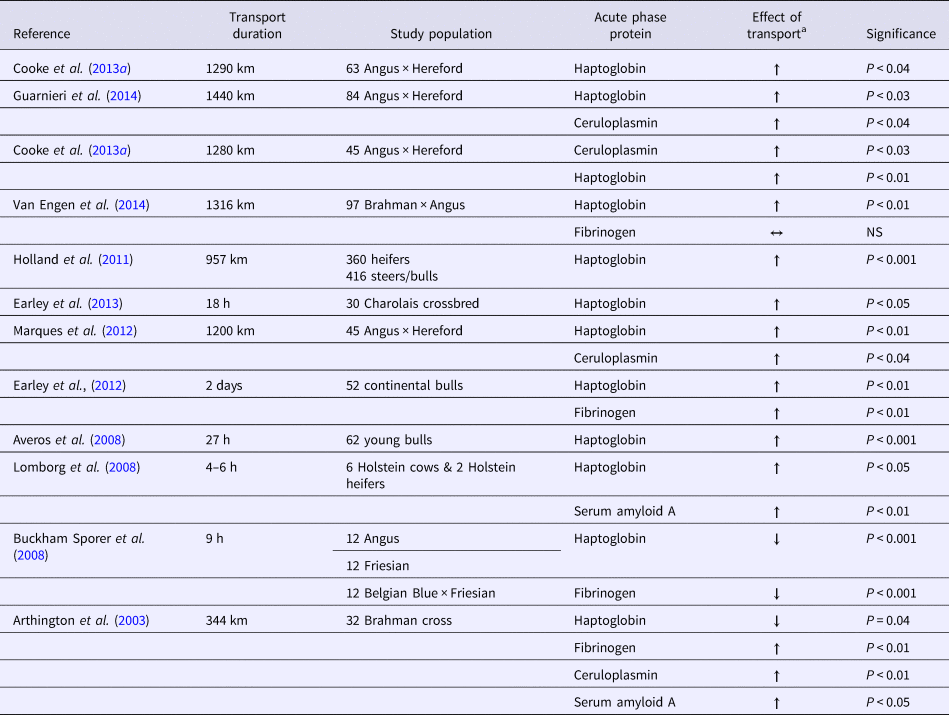
Acute phase proteins have been suggested as markers of post-transportation stressor events (Arthington et al., Reference Arthington, Eicher, Kunkle and Martin2003). There is a crucial need to develop a valid method that can be used to identify cattle requiring or that will require prophylactic treatment for BRD. Acute phase proteins released from hepatocytes are potential markers, but they have poor functionality as identifiers for specific diseases (Tothova et al., Reference Tothova, Nagy and Kovac2014). They do provide indications of fulminant and subclinical inflammation and when used together may indicate that a disease process is imminent (Ceron et al., Reference Ceron, Eckersall and Martinez-Subiela2005). Haptoglobin, serum amyloid A, ceruloplasmin, and fibrinogen are the proteins routinely investigated for cattle. The investigation of acute phase proteins as markers for potential BRD has value. However, the results of a limited meta-analysis indicated that they have no diagnostic accuracy for, and are not valid indicators of, BRD (Abdallah et al., Reference Abdallah, Hewson, Francoz, Selim and Buczinski2016). When the prevalence of the disease is low, acute phase proteins have poor utility as a standalone diagnostic tool (Seppa-Lassila et al., Reference Seppa-Lassila, Eerola, Orro, Hartel, Simojoki, Autio, Pelkonen and Soveri2017).
Circulating haptoglobin released from hepatocytes binds free hemoglobin and reduces oxidative damage (Murata et al., Reference Murata, Shimada and Yoshioka2004). Calves inoculated with respiratory bacterial pathogens had marked increases in the acute phase protein haptoglobin; this protein is a potential diagnostic tool for detection of pulmonary inflammation in calves (Hanthorn et al., Reference Hanthorn, Dewell, Dewell, Cooper, Wang, Plummer and Lakritz2014). Haptoglobin has limited merit for detection of BRD on arrival to determine whether targeted prophylactic treatment should be used (Holland et al., Reference Holland, Step, Burciaga-Robles, Fulton, Confer, Rose, Laidig, Richards and Krehbiel2011). Calves with detectable haptoglobin values had significantly higher severity scores and rectal temperatures at the first, second, and third treatments and were more likely to be treated sooner after arrival (Holland et al., Reference Holland, Step, Burciaga-Robles, Fulton, Confer, Rose, Laidig, Richards and Krehbiel2011). The release of haptoglobin is a response to exogenous corticotropin-releasing hormone; this result suggests that it is connected to stress events (Cooke et al., Reference Cooke, Carroll, Dailey, Cappellozza and Bohnert2012). Long transportation events lead to a significant increase in the release of haptoglobin (Table 2). Haptoglobin has poor utility as a biomarker that can be used to distinguish between chronic and acute inflammation (Bannikov et al., Reference Bannikov, Hinds, Rajala-Schultz, Premanandan, Rings and Lakritz2011). Use of haptoglobin and matrix metalloproteinase complexes may improve the validity of haptoglobin's use as a biomarker. Matrix metalloproteinases are secreted from numerous immune cells (e.g. macrophages).
Table 2. Summary of the scientific literature examining the changes in acute phase proteins during cattle transportation
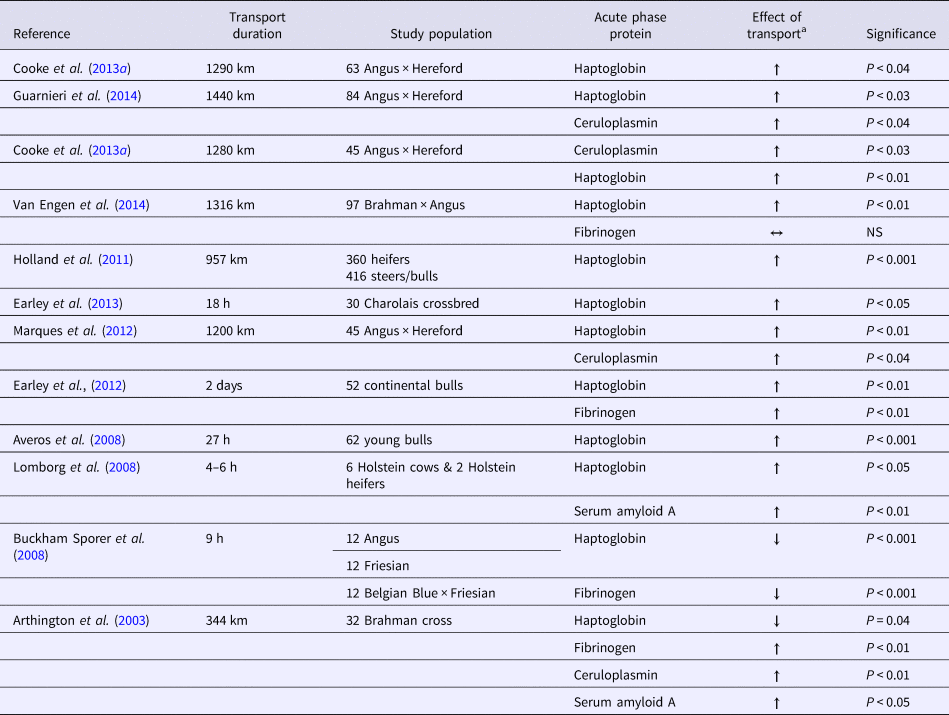
a All arrows are in reference of transported animal's acute phase protein response in comparison with either baseline value or a non-transported control group.
Serum amyloid A has also been implicated as part of acute inflammatory disease processes and has potential as a marker for respiratory disease (Alsemgeest et al., Reference Alsemgeest, Kalsbeek, Wensing, Koeman, Vanederen and Gruys1994). The production of serum amyloid A is directly related to inflammatory cytokine production, and is usually a response to viral infection (Yamada, Reference Yamada1999). Large increases in circulating serum amyloid A concentrations are associated with decreased antibody production (Benson and Aldobenson, Reference Benson and Aldobenson1982), enhanced monocyte chemotaxis (Badolato et al., Reference Badolato, Wang, Murphy, Lloyd, Michiel, Bausserman, Oppenheim and Kelvin1994), and decreased neutrophil oxidative burst capacity (Linke et al., Reference Linke, Bock, Valet and Rothe1991). The serum amyloid A concentration can increase 10-fold by 48 h after transportation (Lomborg et al., Reference Lomborg, Nielsen, Heegaard and Jacobsen2008). Even when cattle were exposed to only a three-h transportation event, there was a 46% increase in serum amyloid A concentration over the 7 days following transportation (Arthington et al., Reference Arthington, Eicher, Kunkle and Martin2003). Serum amyloid A concentration is a sensitive marker for a respiratory infection or, through interpretation, the inflammation of disease (Orro et al., Reference Orro, Pohjanvirta, Rikula, Huovilainen, Alasuutari, Sihvonen, Pelkonen and Soveri2011).
Ceruloplasmin is a tissue protectant produced during iron-mediated free radical injury. It has anti-inflammatory properties and has potential as an indicator of infection (Murata et al., Reference Murata, Shimada and Yoshioka2004) The metalloenzyme concentration is correlated with the serum copper concentration and has been suggested as a useful indicator of nutritional copper status for cattle (Pourjafar and Dehkordi, Reference Pourjafar and Dehkordi2008). Investigations of events with infectious etiologies have found significant increases in young calves (<30 days of age) clinically infected with rotavirus (Rocha et al., Reference Rocha, Silva, Bortoletto, Silva, Buzinaro, Zafalon and Fagliari2016). One study found that there was a 28% increase in ceruloplasmin concentration at 7 days after shipment in one experiment; a 48% increase occurred during the 21-day post-transport period in another experiment (Arthington et al., Reference Arthington, Eicher, Kunkle and Martin2003). The results of most investigations quantifying ceruloplasmin during transportation are consistent with the increasing trend found in the serum. The increase in ceruloplasmin has been associated with decreases in growth rates in cattle (Cooke et al., Reference Cooke, Arthington, Austin and Yelich2009). Other studies have found that ceruloplasmin concentration is negatively correlated with average daily gain and positively correlated with circulating cortisol level (Araujo et al., Reference Araujo, Cooke, Hansen, Staples and Arthington2010).
Fibrinogen is a substrate that allows for fibrin formation; it is key in tissue repair by providing support and a binding matrix for the extravasation of cells associated with inflammation (Murata et al., Reference Murata, Shimada and Yoshioka2004). Fibrinogen has been found to increase and decrease after transportation. After a long-distance transport by sea and road, calf fibrinogen levels were increased by 29% compared with the pre-transport values and were 22% higher than the control values (Earley et al., Reference Earley, Murray, Prendiville, Pintado, Borque and Canali2012). These increases occur within circulating serum. The results for fibrinogen measured within the lung during times of stress indicate that an opposite response occurs. Analysis of lung fluid extracted using bronchial alveolar lavage in stressed and non-stressed treatment groups revealed a marked decrease in fibrinogen 12 h after exposure to the stressor was terminated; this result suggests there was a loss of permeability due to the stress-associated release of glucocorticoids and catecholamines (Mitchell et al., Reference Mitchell, Clark, Siwicky and Caswell2008). There is a stronger correlation between circulating fibrinogen levels and bacterial respiratory pathogens (e.g., M. haemolytica and Pasteurella multocida) than to viral pathogens under field conditions (Nikunen et al., Reference Nikunen, Hartel, Orro, Neuvonen, Tanskanen, Kivela, Sankari, Aho, Pyorala, Saloniemi and Soveri2007). The critical role of fibrinogen in the bovine is a major focus of disease and clinical pathology interpretation.
Acute phase proteins are a potential hematological identifier of transport stress and predisposition to BRD. However, even though general increases occur, concentrations can vary between breeds and values be interpreted with caution (Qiu et al., Reference Qiu, Arthington, Riley, Chase, Phillips, Coleman and Olson2007). Rather than using the change in a singular biomarker, reporting the magnitude of changes in multiple circulating markers could be used for decision making. The current understanding of acute phase proteins and other proteomics concentrates heavily on a small number that is found in high concentrations. The transition to liquid chromatography-mass spectrometry screening to identify novel proteins at lower concentrations may increase the number of potentially useful proteins.
Mitigation strategies
Investigation of methods to alleviate the negative effects of transport, thus to prevent morbidity and mortality and to enhance receiving performance in the feed yard, continues. Pre-transportation and on-arrival treatments and methods vary. Most methods are based on a nutritional, behavioral, and/or non-antimicrobial pharmaceutical approach. These give insight into other potential things to consider in a model.
Vitamin, mineral, and caloric supplementation
Pre-transportation treatment using vitamin A, vitamin D, and vitamin E had no effect on shrink associated with transport (Jubb et al., Reference Jubb, Pinch and Petty1993). Investigation of the use of vitamin E supplementation on arrival in a population of high-risk calves experiencing 64.5% morbidity revealed a decrease in the treatment costs associated with the cattle that were fed 2000 IU of vitamin E (Carter et al., Reference Carter, Gill, Confer, Smith and Ball2000). This paper did not report any results indicating increased weight gain or feed efficiency with treatment. However, a study of heifers stressed through transport were treated with vitamin E after shipment, and it was found that at days 14–28 there was an increased feed efficiency and average daily gain, but other performances and health variables were unaffected (Choat et al., Reference Choat, Krehbiel, Gill, Ball, Stovall, Shriver and Carter2000). Trace minerals such as chromium have also been administered on arrival as an additive feed supplement in the range of parts per million. Chromium supplementation results in increases in average daily gain, presumably through increased dry matter intake at differing levels of Cr supplementation. The decreases in morbidity that occurred from day 2 to 30 were greatest in the group supplemented with 0.2 ppm of Cr (Moonsieshageer and Mowat, Reference Moonsieshageer and Mowat1993).
Nutrition supplements in the form of energy, specific amino acids, and electrolytes that are given prior to transportation result in gains in hot carcass weight as cattle are transported to harvest (Grumpelt et al., Reference Grumpelt, Hoffer, Curie, Jones, Jones, Kimmel, Mcdonald, Paterson and Schaefer2015). Supplementation for caloric gain in arrival diets from rumen-protected poly unsaturated fatty acids exhibited negative effects on weight performance and intake. However, a decrease in circulating haptoglobin concentration was a beneficial effect (Araujo et al., Reference Araujo, Cooke, Hansen, Staples and Arthington2010). The differences in supplementation with a linolenic or a linoleic acid can promote differences in specific inflammatory prostaglandin production (Yaqoob and Calder, Reference Yaqoob and Calder2007). Ultimately, the differences in the inflammatory mediators could change the immune response to an anti-inflammatory or inflammatory T-cell response.
Electrolyte supplementation in cattle before transportation helps to mitigate changes in osmolality on arrival compared with control animals (Schaefer et al., Reference Schaefer, Jones, Tong, Young, Murray and Lepage1992). Compiled evidence was insufficient to substantiate a reduction in stress; however, increased performance at harvest was noted through increased hot carcass weights and decreased live weight loss for treated animals (Schaefer et al., Reference Schaefer, Jones and Stanley1997). Multiple-day transport durations on a boat are associated with electrolyte benefits in cattle when compared with controls (Beatty et al., Reference Beatty, Barnes, Taplin, Mccarthy and Maloney2007). Overall, the literature does not support a production or health advantage for live cattle entering a feed yard with electrolyte supplementation prior to transportation. Most electrolyte research results indicate that there is an added benefit at harvest (Schaefer et al., Reference Schaefer, Jones and Stanley1997).
Behavior influence
Behavior methods aimed at decreasing morbidity have also been investigated. The use of trainer cows (cattle acclimated and used to the pen, bunks, and waterers) for incoming steers upon arrival after transport resulted in an increase in performance and health for some of the treatment groups, but overall the results between groups were not consistent enough to be conclusive (Loerch and Fluharty, Reference Loerch and Fluharty2000). Remote early disease identification was used to monitor behavior in calves within the first 45 days of arrival at the feedlot (White et al., Reference White, Goehl, Amrine, Booker, Wildman and Perrett2016). Behavior such as location in the pen, social interactions, time at feed bunks, and waterers. This method shows promise for detection of calves that are ill or will become ill before the traditional method of visual identification can be used. It also identified animals that had lower rectal temperatures. It might be useful for identification of animals during the early stages of the disease process (i.e. before cytokines are released in response to illness) (White et al., Reference White, Amrine and Goehl2015).
Other pharmacologic practices
Strategies based on the use of pharmacological techniques have also been investigated as options to mitigate negative effects (e.g., loss of performance, stress, and pain) and promote animal welfare. Some approaches used in older studies have aggressively targeted the stress response. Therapeutics involving the use of an adrenolytic agent (metyrapone) to suppress cortisol production prior to transportation have been studied (Agnes et al., Reference Agnes, Sartorelli, Picotti, Arrigoni and Locatelli1990). Metyrapone treatment does not reduce the increase in non-esterified fatty acid levels after transportation; the long-term effects were not described. Improved veterinary practices for transported animals include the use of nonsteroidal anti-inflammatory drugs (NSAIDs). Intravenous administration of flunixin meglumine (an FDA-approved NSAID for the treatment of pyrexia in cattle) did not restore performance on arrival at the feed yard, but the results suggested that it contributed to a reduction in cortisol, haptoglobin, and ceruloplasmin until day 4 after transport (Cooke, Reference Cooke, Cappellozza, Guarnieri and Bohnert2013a, Reference Cooke, Guarnieri, Cappellozza and Bohnert2013b). Flunixin treatment did result in a significant increase in non-esterified fatty acid concentrations that persisted through the fourth day after transport, compared with the non-treated transport group. The NSAID meloxicam may reduce effects of stress and inflammation. Inverse relationships between circulating plasma drug concentration and cortisol, neutrophil, and basophil counts have been reported (Van Engen et al., Reference Van Engen, Stock, Engelken, Vann, Wulf, Karriker, Busby, Lakritz, Carpenter, Bradford, Hsu, Wang and Coetzee2014). Beyond the suggestion of a decrease in stress and inflammation, there may also be an added benefit in terms of increased performance on arrival. Meloxicam-treated calves had greater and more efficient weight gain upon arrival. Meloxicam also reduced haptoglobin production, compared with an untreated transport group (Guarnieri et al., Reference Guarnieri, Cooke, Cappellozza, Reis, Marques and Bohnert2014). The translation of these added benefits has not been proven to carry through into the post-transport period or to result in decreased morbidity. Additional investigation of pharmacological practices that are not associated with metaphylaxis antibiotic treatment is warranted.
Conclusion
Transport effects vary and so do the subsequent physiological responses. Other variation is attributed to differences in age, sex, temperament, and recent environmental exposure. Physiologic responses are influenced by multifaceted effects that ultimately begin with dehydration, feed deprivation, tissue damage, and diesel fumes. These connections to stress-related glucocorticoids and inflammatory cytokines require emphasis during research investigation and veterinary communication with producers. A potential investigation needs to encompass these biomarkers in outcomes of BRD cattle rather than focusing solely on a singular entity of predicting BRD. Large gaps in data indicate a need for underlying normalcy of biomarkers prior to interpretation during a transportation event investigated. Other gaps, such as the effects of fumes and truck design, require further research. Using all of the potential biomarkers provided in this review, we could use these in a model to predict risk levels. Drastic and prolonged changes in stress, acute phase proteins, and cytokines leads to inflammation and immunosuppression. This cascade of factors is likely critical in understanding the pathogenesis of BRD in feedlot health. There is a clear need to develop a model to predict BRD and to develop therapeutic measures that concentrate on decreasing the aforementioned markers to promote active immune function without the use of antibiotics. This goal is attainable through the continued understanding of cattle physiologic responses and focusing research on addressing the variables discussed in this review. Moving forward, research projects conducted on cattle transportation should monitor for BRD and report findings. There is a need for connection of clinical BRD morbidity to these markers.