INTRODUCTION
Reef-building corals live in a symbiotic association with unicellular dinoflagellate algae, referred to as zooxanthellae (genus Symbiodinium). Translocated algal photosynthates satisfy most host energetic requirements and are essential for coral survival in oligotrophic tropical shallow waters (Muscatine & Porter, Reference Muscatine and Porter1977). Algal symbionts supply the coral host with sugars, glycerol and amino acids, while algae benefit from host metabolic products, such as CO2, phosphates and nitrogenous compounds (Hallock, Reference Hallock and Stanley2001).
Symbiodinium density in coral colonies varies at several spatial and temporal scales, both under normal conditions (i.e. in the absence of perturbation) and after particular disturbances. Algal endosymbiont density can vary among coral species (Drew, Reference Drew1972), as well as among colonies of the same species, at both local and regional scales (Fitt et al., Reference Fitt, Brown, Warner and Dunne2001). Light (D'Croz et al., Reference D'Croz, Maté and Oke2001; Bhagooli & Yakovleva, Reference Bhagooli and Yakovleva2004), sedimentation and eutrophication (Brodie et al., Reference Brodie, De'ath, Devlin, Furnas and Wright2007; Sawall et al., Reference Sawall, Teichberg, Seemann, Litaay, Jompa and Richter2011), water motion (Finelli et al., Reference Finelli, Helmuth, Pentcheff and Wethey2006), water temperature (Steen & Muscatine, Reference Steen and Muscatine1987; Sunagawa et al., Reference Sunagawa, Cortes, Jimenez and Lara2008) and salinity (Hoegh-Guldberg & Smith, Reference Hoegh-Guldberg and Smith1989; Sunagawa et al., Reference Sunagawa, Cortes, Jimenez and Lara2008) are known to influence the density of Symbiodinium. Despite these advances, the amount and causes of variability in coral algal endosymbionts density at a small spatial scale, i.e. from the colony scale (1–10 cm) to the reef scale (1–10 km), remain poorly documented in natural populations. The symbiotic relationship between Symbiodinium and its coral host is vulnerable and highly sensitive to environmental or anthropogenic disturbances, and may be disrupted (Bhagooli & Yakovleva, Reference Bhagooli and Yakovleva2004; Weis, Reference Weis2008). The disruption of this symbiosis is commonly referred to as coral bleaching, which is broadly defined as the drastic loss of endosymbiotic dinoflagellates or their associated pigments from the coral host cells (Douglas, Reference Douglas2003). Mass-bleaching events, which occur over large spatial scale, are typically associated with higher than average seawater temperature periods (Goreau & Hayes, Reference Goreau and Hayes1994; Baker et al., Reference Baker, Glynn and Riegl2008), often in conjunction with increased light (Lesser et al., Reference Lesser, Stochaj, Tapley and Shick1990). These events can cause mass mortality within coral populations and subsequent cascading effects on coral-associated fauna (McClanahan et al., Reference McClanahan, Weil, Cortés, Baird, Ateweberhan, van Oppen and Lough2009; Leal et al., Reference Leal, Nunes, Alexandre, Silva, Reis, Dinis and Calado2012), and may induce a long-term shift in the composition of reef assemblages (Adjeroud et al., Reference Adjeroud, Michonneau, Edmunds, Chancerelle, Lison de Loma, Penin, Thibaut, Vidal-Dupiol, Salvat and Galzin2009). Surviving coral colonies often show decreased growth and fecundity, reduction in competitive abilities and increased susceptibility to diseases (McClanahan et al., Reference McClanahan, Weil, Cortés, Baird, Ateweberhan, van Oppen and Lough2009). In recent decades, mass bleaching events have raised increasing concern, especially in the present situation of climate change (Baker et al., Reference Baker, Glynn and Riegl2008). In this context, studies have been set up to estimate coral reef health and document the consequences and extent of catastrophic disturbances such as mass bleaching events. Most of these programmes only document the abundance of coral colonies, and do not take into account physiological processes underlying coral health, such as the coral–Symbiodinium relationship (Fitt et al., Reference Fitt, Brown, Warner and Dunne2001). However, estimating coral–Symbiodinium symbiosis health through measures of parameters such as Symbiodinium density has been demonstrated to be relevant in studies investigating bleaching events (see, for example, Fagoonee et al., Reference Fagoonee, Wilson, Hassell and Turner1999; Stimson et al., Reference Stimson, Sakai and Sembali2002; Shenkar et al., Reference Shenkar, Fine, Kramarsky-Winter and Loya2006; Li et al., Reference Li, Yu, Shi, Chen, Zhao and Zhao2008). In this context, documenting Symbiodinium density variation under natural, non-bleaching conditions is critical to provide a baseline allowing comparisons when a bleaching event occurs. This is especially true in the present context of climate change, in reef systems under the influence of recurrent mass bleaching events, such as islands of the Central Pacific (Salvat, Reference Salvat1992; Adjeroud et al., Reference Adjeroud, Chancerelle, Schrimm, Perez, Lecchini, Galzin and Salvat2005, Reference Adjeroud, Michonneau, Edmunds, Chancerelle, Lison de Loma, Penin, Thibaut, Vidal-Dupiol, Salvat and Galzin2009; Penin et al., Reference Penin, Adjeroud, Schrimm and Lenihan2007, Reference Penin, Vidal-Dupiol and Adjeroud2013).
In this context, the present study aims at documenting intra-colony and small scale natural spatial variation in Symbiodinium density in a sentinel coral species under non-bleaching conditions during the summer season (warm period). Symbiodinium density was chosen because it is an inexpensive and easy-to-measure variable that is a good proxy for the health of the coral–Symbiodinium relationship (Moothien-Pillay et al., Reference Moothien-Pillay, Willis and Terashima2005). The method used can be implemented in many locations with very basic laboratory equipment (Bürker type haemocytometer and dissecting microscope).
The present study documents intra-colony variation (colony scale: 1–10 cm) as well as small spatial scale variation in the field thanks to a hierarchical sampling design encompassing the station scale (1–10 m), the location scale (50–100 m), and the reef scale (4–7 km). Additional measurements of light intensity, sedimentation rate, and water motion allowed the spatial patterns of variation of these key environmental factors to be compared with Symbiodinium density, thus providing a better understanding of the implications of these factors for the coral–Symbiodinium relationship in the field.
MATERIALS AND METHODS
Sampling strategy
The present study focused on the coral Acropora globiceps (Dana, 1846), a major reef-building species in Moorea. Acropora globiceps is a ubiquitous species in the Society Archipelago, abundant both in the lagoon and on the whole depth range of the outer slope, and is easy to identify in the field. It is widespread in the Indo-Pacific, from the central Indian Ocean (Andaman Sea) to south central Pacific (Pitcairn) via the Great Barrier Reef, Micronesia and Polynesia (Wallace, Reference Wallace1999). It is also highly sensitive to changes of environmental conditions, and particularly to temperature variations, like most species of this genus (Marshall & Baird, Reference Marshall and Baird2000; Penin et al., Reference Penin, Adjeroud, Schrimm and Lenihan2007, Reference Penin, Vidal-Dupiol and Adjeroud2013; Kayal et al., Reference Kayal, Lenihan, Pau, Penin and Adjeroud2011). As a consequence, A. globiceps can be considered as a sentinel species and an adequate candidate for surveys documenting coral health in the Society Archipelago.
Moorea Island (17°30′S, 149°50′W, Society Archipelago, French Polynesia) exhibits a narrow coral reef belt surrounding the island, which compresses the spatial organization along highly marked environmental gradients (Adjeroud, Reference Adjeroud1997); therefore, it is a unique system to study spatial variability of Symbiodinium density and the role environmental factors may play in causing these patterns.
First, colony scale variability of Symbiodinium density was studied at three different depths (6, 12 and 18 m) at one site (Vaipahu). At each of these three depths, eight colonies were randomly chosen. For each colony, the extremity of four branches (2 cm long apex), two internal, and two external, were collected for comparison of Symbiodinium density (Oliver, Reference Oliver1984).
A hierarchical sampling design (Figure 1), which includes various depths and locations was used to determine small spatial scale variation of Symbiodinium density: (1) at the station scale (1–10 m), among colonies within a sampling station; (2) at the location scale (50–100 m), among stations implemented at different depths (6, 12 and 18 m) within a location; and (3) at the reef scale (4–7 km), among three locations within the outer reef slope of Moorea Island (Figure 1: Haapiti on the west coast, Tiahura and Vaipahu on the north coast; see Penin et al. (Reference Penin, Adjeroud, Schrimm and Lenihan2007) for habitat description). Since no significant differences were observed at the colony scale, small spatial scale variability was assessed through sampling three branches of each of eight colonies randomly chosen within a 100 m2 area at the nine sampling stations.

Fig. 1. Map of Moorea indicating the position of the nine sampling stations encompassing three locations (Haapiti, Tiahura and Vaipahu) and three depths (6, 12 and 18 m) on the outer reef slope. Variation of Symbiodinium density has been characterized at the colony scale as well as at three different hierarchical small spatial scales represented by different line patterns. Distances among stations within a location are not to scale.
Because Symbiodinium density is known to vary seasonally (Fagoonee et al., Reference Fagoonee, Wilson, Hassell and Turner1999; Moothien-Pillay et al., Reference Moothien-Pillay, Willis and Terashima2005), coral samples were collected in March 2007, which is the warmest month of the year in Moorea (CRIOBE temperature data), in order to provide a baseline corresponding to warm period, i.e. when mass bleaching events are most likely to happen (Adjeroud et al., Reference Adjeroud, Michonneau, Edmunds, Chancerelle, Lison de Loma, Penin, Thibaut, Vidal-Dupiol, Salvat and Galzin2009). Indeed, about one month after the sampling, the first signs of bleaching were observed around Moorea (mid-April; Penin et al., Reference Penin, Vidal-Dupiol and Adjeroud2013).
Sample analysis
Coral samples were initially preserved at −20°C. Tissues of the frozen fragments were then separated from the coral skeleton with a high-pressure water jet (Water-PickTM; Fitt et al., Reference Fitt, McFarland, Warner and Chilcoat2000) using 0.22 µm-filtered seawater (50 ml per sample) and allowed to settle. The slurry was then ground in a glass tissue homogenizer and fixed with 4% formalin for further counts and observations (Lasker, Reference Lasker2003). Density was determined from counts of three replicate aliquots, using a haemocytometer (Bürker type), under an optical microscope. The Symbiodinium cell counts were normalized to total coral surface using a paraffin method adapted from Chancerelle (Reference Chancerelle2000), based on the weight difference between the clean and dry skeleton and the same skeleton coated with paraffin (i.e. sealing fragments of coral skeletons with a varnish and single dipping in paraffin wax at 65°C).
Other methods exist to document coral–Symbiodinium symbiosis performances (see for example Frade et al., Reference Frade, De Jongh, Vermeulen, Van Bleijswijk and Bak2008a, Reference Frade, Englebert, Faria, Visser and Bakb). However, they imply using expensive equipment and laboratory facilities (such as aquarium systems, pulse-amplitude modulation fluorometers, etc.), that are not always available, especially in remote locations like the French Polynesian islands. Symbiodinium counting method used in the present study presents the advantage of being easy to implement with basic laboratory equipment.
Environmental factors
To identify major factors potentially associated with spatial variations of Symbiodinium density, light intensity (relative photosynthetic photon flux, rPPF, in μmol.m−2.s−1), sedimentation (total sedimentation rate, SR, in mg.cm−2.d−1), and water motion (diffusion factor, DF) were measured at each station. Variability in light intensity was assessed through a relative photosynthetic photon flux (rPPF), which is calculated as the ratio between underwater and surface photosynthetic photon flux (PPF, µmol.m−2.s−1), within the range of the photosynthetically active radiations (400–700 nm). Measures were made using a MQ-200 quantum meter (Apogee Instruments Inc., Logan UT, USA) at zenith and on cloud-free days. For each replicate, underwater and surface PPF were measured five times within 60 s at each of five random replicate plots. Three replicates were performed at each station, on three different days. Variability in water motion was characterized through comparison of diffusion factor (DF), calculated as the ratio between weight loss of clod cards deployed in the field for 24 h and weight loss of identical cards kept in a motionless seawater tank (Thompson & Glenn, Reference Thompson and Glenn1994). At each station, five replicate racks, each encompassing four clod cards, were used on each of five randomly chosen days. Variability in sedimentation was quantified through a comparison of dry sediment weight deposited per cm2 and per day. At each station, five sediment collectors were deployed for ten days in three replicate periods, following Stewart et al. (Reference Stewart, Caldwell, Cloutier and Flight2006). Temperature was not measured, because it does not significantly vary within the studied depth range at these sites during the warm season (Penin et al., Reference Penin, Adjeroud, Schrimm and Lenihan2007).
Statistical analysis
Due to lack of normality and homoscedasticity of the distributions of Symbiodinium densities, even after appropriate transformations, parametric statistics like ANOVA could not be used. As a consequence, non-parametric statistical analyses were used. Intra-colony comparisons (between internal and external branches) were performed using Mann–Whitney rank tests (MW). For comparisons among stations, locations and depths, Kruskal–Wallis rank tests (KW) were conducted, completed by MW rank tests for post-hoc pairwise comparisons. Spatial variability of light intensity, water motion, and sedimentation rate were explored through the use of KW rank tests and complementary MW rank tests for pairwise comparisons. Non-parametric Spearman correlations were used to detect significant relationships between variability of Symbiodinium density and variability of light intensity, sedimentation, and water motion among the nine sampling stations. Results are presented with two values of α, the first one being the classical 0.05, and the second one being the α obtained after standard Bonferroni corrections, which is a method aiming at adjusting the α risk to the number of tests run, thus limiting the risk of Type I errors (i.e. rejecting H0 when H0 is true). Results were virtually identical with the two methods, but we choose to present both due to controversy raised by the use of Bonferroni corrections (Cabin & Mitchell, Reference Cabin and Mitchell2000; Moran, Reference Moran2003).
RESULTS
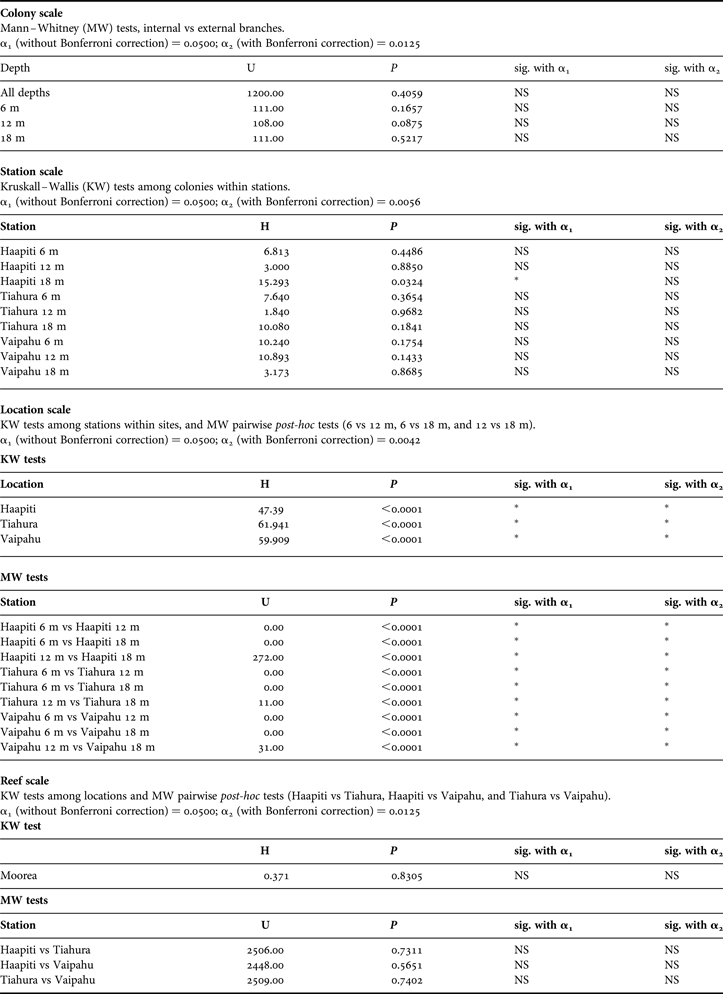
Symbiodinium density ranged from 0.77 to 2.32 × 106cm−2. No difference was observed between internal and external branches at the Vaipahu site, whatever the depth being considered (Figure 2; Table 1). Similarly, Symbiodinium density did not significantly vary at the station scale (i.e. among colonies within a sampling station, Table 1). In contrast, significantly higher densities were observed at deep stations than at shallow stations at all three locations (Figure 3; Table 1). Marked gradients were also observed among the nine sampling stations for the measured environmental variables. The rPPF decreased with increasing depth, but no significant variation was observed among locations (Figure 4; Table 2). Total dry sediment weight did not vary among depths, but was significantly lower at Haapiti than at Vaipahu or Tiahura (Figure 4; Table 2). Diffusion factor decreased with depth, and significant differences were detected among locations, Haapiti presenting the highest values and Vaipahu the lowest (Figure 4; Table 2).
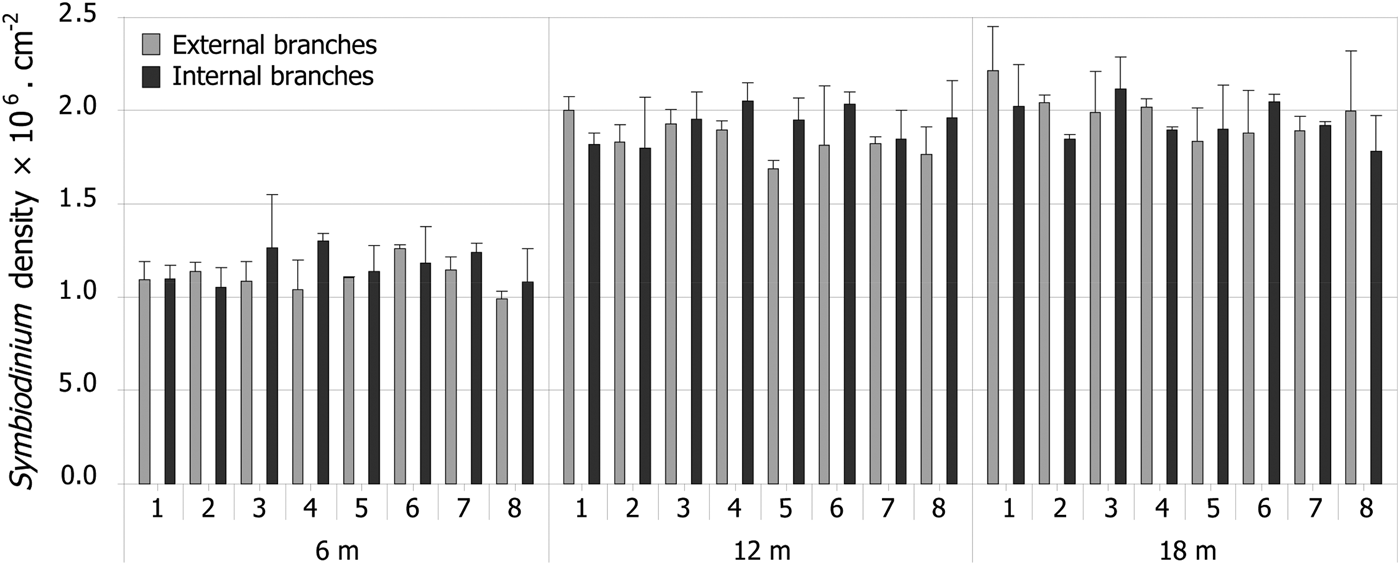
Fig. 2. Colony-scale variation of Symbiodinium densities: mean Symbiodinium density (zoox.cm−2) in external vs internal branches for each of the eight colonies (1–8) sampled at each of the three depths (6, 12 and 18 m) at Vaipahu site. Error bars represent standard deviations.

Fig. 3. Multi-scale variation of Symbiodinium densities: mean Symbiodinium density (zoox.cm−2) for each of the eight colonies sampled at each of the nine study stations around Moorea. Error bars represent standard deviations.

Fig. 4. Spatial variation of: (A) light intensity (relative photosynthetic photon flux rPPF); (B) sedimentation (dry sediment weight, mg.cm−2.d−1); (C) water motion (diffusion factor) over the nine stations. Error bars represent standard deviations.
Table 1. Non-parametric statistical analysis of spatial variation of Symbiodinium density at the colony, station, location and reef scales.

Table 2. Kruskal–Wallis tests among stations (N = 9 stations), among locations (N = 3 locations) and among depths (N = 3 depths) on light (photosynthetic photon flux), sedimentation (dry sediment weight), and water motion (diffusion factor). α1 (without Bonferroni correction) = 0.0500; α2 (with Bonferroni correction) = 0.0167.

Spatial variability in Symbiodinium density was strongly and negatively correlated with light intensity, but not with sedimentation rates or water motion (Figure 5; Table 3).

Fig. 5. Relationships between variation of Symbiodinium density (zoox.cm−2) and environmental factors among the nine stations: (A) light intensity (relative photosynthetic photon flux); (B) sedimentation (dry sediment weight, mg.cm−2.d−1); (C) water motion (diffusion factor). rho is the Spearman's rank correlation coefficient.
Table 3. Spearman non-parametric correlations between Symbiodinium density and environmental factors (light, sedimentation, and water motion). α1 (without Bonferroni correction) = 0.0500; α2 (with Bonferroni correction) = 0.0167.

DISCUSSION
Symbiodinium density observed in Acropora globiceps tissues around Moorea was of the same order of magnitude as values previously measured in A. palmata and A. cervicornis in the Caribbean (Fitt et al., Reference Fitt, McFarland, Warner and Chilcoat2000), in A. formosa in the Indian Ocean (Fagoonee et al., Reference Fagoonee, Wilson, Hassell and Turner1999), in different Acropora species in the South China Sea (Li et al., Reference Li, Yu, Shi, Chen, Zhao and Zhao2008), and also in A. millepora at the Palm Island Group, Great Barrier Reef, Australia (Moothien-Pillay et al., Reference Moothien-Pillay, Willis and Terashima2005). This suggests that the range of Symbiodinium density is relatively consistent within the Acropora genus, even for highly divergent host species and symbiont clades, and from different biogeographic regions or environmental conditions.
At the colony scale, results showed homogeneity of Symbiodinium densities between inner and outer branches in A. globiceps at the Vaipahu site, regardless of the depth considered. This outcome seems in contradiction with results of previous surveys on other Acropora species (Oliver, Reference Oliver1984; Moothien-Pillay et al., Reference Moothien-Pillay, Willis and Terashima2005) and suggests that intra-colony variation in Symbiodinium density in reef-building corals could be species-specific. At Moorea, this absence of intra-colony differences in Symbiodinium density could also be due to high light intensity (>200 µmol.m−2.s−1) and water motion (>3.5; Figure 4) observed at all study sites and/or to the presence of only small differences in these parameters between internal and external branches, especially when considering the upper part of the branches, which usually contain less Symbiodinium (Allemand et al., Reference Allemand, Tambutté, Zoccola, Tambutté, Dubinsky and Stambler2011). These hypotheses could be addressed through intra-colony measurements of light intensity and water motion on different species of the Acropora genus.
At the station scale, no differences were observed among colonies in the density of Symbiodinium. This homogeneity within a particular habitat indicates the preponderance of extrinsic vs intrinsic factors, and suggests environmental factors are probably homogeneous enough at this scale not to induce significant variability in Symbiodinium density. At the location scale, a marked and consistent increase in Symbiodinium density with increasing depth was observed at all three locations. At the reef scale, significant differences in Symbiodinium density have been observed among the nine stations, but not among the three locations (Figure 3). This shows that variability in Symbiodinium density is mostly driven by depth and associated parameters such as light, rather than by location. This depth pattern is probably related to the strong negative correlation observed between light intensity and Symbiodinium density, and underlines the importance played by light in the coral–algal symbiosis (Falkowski et al., Reference Falkowski, Dubinsky, Muscatine and Porter1984). A similar depth/light pattern in Symbiodinium density was demonstrated in other cnidarians, such as other scleractinian corals (Drew, Reference Drew1972; Dustan, Reference Dustan1979) or the sea anemone Aiptasia tagetes (Steele, Reference Steele1976). Reduced light intensity is known to induce an increase of Symbiodinium density and photosynthetic pigments concentration under experimental conditions (Titlyanov et al., Reference Titlyanov, Titlyanova, Yamazato and van Woesik2001) or in the field, in relation with depth (Li et al., Reference Li, Yu, Shi, Chen, Zhao and Zhao2008) or cloud cover (Titlyanov et al., Reference Titlyanov, Titlyanova, Yamazato and van Woesik2001; Sunagawa et al., Reference Sunagawa, Cortes, Jimenez and Lara2008). Titlyanov et al. (Reference Titlyanov, Shaposhnikova and Zvalinskii1980) have also established a relationship between the increase of Symbiodinium density and the decrease of light. These patterns are linked with acclimatization to low light, which involves maximization of the light harvesting capacity by increasing photosynthetic pigment concentration in Symbiodinium, and Symbiodinium population density in coral branches. Another mechanism for corals to acclimatize to low light may be to change their Symbiodinium clades (Rowan & Knowlton, Reference Rowan and Knowlton1995; Toller et al., Reference Toller, Rowan and Knowlton2001; Bongaerts et al., Reference Bongaerts, Riginos, Ridgway, Sampayo, van Oppen, Englebert, Vermeulen and Hoegh-Guldberg2010), in a similar way to that sometimes observed with acclimatization to high temperature (Stat et al., Reference Stat, Carter and Hoegh-Guldberg2006). The different clades present variable volume and circumference (Wilkerson et al., Reference Wilkerson, Kobayashi and Muscatine1988), and deeper corals generally harbour smaller Symbiodinium (Wilkerson et al., Reference Wilkerson, Kobayashi and Muscatine1988). Moreover, there is a relation between the size and density of symbionts and the host tissue volume (space availability for symbionts; Jones & Yellowlees, Reference Jones and Yellowlees1997). In the present study, we did not detect any visible difference in Symbiodinium size, and we can thus assume that variability in size of Symbiodinium, in order to counterbalance higher density, is probably limited. However, it would be of particular interest to examine this hypothesis in further studies aiming to precisely quantify and qualify the size and the clade of Symbiodinium extracted from Acropora globiceps sampled at different depth.
The observed higher Symbiodinium density at deep stations can also be one of the causes of spatial variability in corals response to high temperature observed during the bleaching event that occurred at Moorea a few weeks after this study. During this bleaching event, corals at deeper stations displayed a higher bleaching response than the shallower ones (Penin et al., Reference Penin, Vidal-Dupiol and Adjeroud2013). Coral bleaching is clearly linked to photodamages faced by Symbiodinium under thermal stress (Venn et al., Reference Venn, Loram and Douglas2008). These damages cause overproduction of reactive oxygen species (ROS) leading to coral bleaching via a complex cellular cascade (Weis, Reference Weis2008). As a consequence, corals from deeper stations, with high Symbiodinium density and high concentration of photosynthetic pigments might suffer from higher oxidative stress during temperature anomalies than corals at shallower stations, characterized by lower Symbiodinium and pigment densities (Stat et al., Reference Stat, Carter and Hoegh-Guldberg2006).
Results of this study demonstrate that Symbiodinium density in Acropora globiceps is strongly influenced by light intensity, as it is the case for photophysiological and symbiotic mechanisms in reef-building coral species (Venn et al., Reference Venn, Loram and Douglas2008; Mass et al., Reference Mass, Kline, Roopin, Veal, Cohen, Iluz and Levy2010). Homogeneity in Symbiodinium density at the colony (i.e. between branches of the same colony) and station scales (i.e. between colonies of the same habitat) allows considering Symbiodinium density in A. globiceps as a potential biomarker of coral health in monitoring surveys, since Symbiodinium density seems typical of a particular habitat. In the present study, Symbiodinium density was measured in non-disturbed conditions (i.e. in the absence of major perturbations), just before the season when bleaching events generally occur (Penin et al., Reference Penin, Adjeroud, Schrimm and Lenihan2007, Reference Penin, Vidal-Dupiol and Adjeroud2013), and at various depths and locations. Therefore, it provides a valuable baseline that could be used in the future as a reference, to be compared with measures realized in disturbed conditions, such as during a bleaching event. In this perspective, Symbiodinium density can represent an inexpensive and easy to implement biomarker of coral–Symbiodinium symbiosis health, and complement other tools used in studies investigating the effects of bleaching events on coral reef health.
ACKNOWLEDGEMENTS
We would like to thank CRIOBE staff for field logistics and support, and particularly Yannick Chancerelle, Pascal Ung, Pauline Bosserelle, and volunteers of Planète Urgence for logistical help in data collection.
FINANCIAL SUPPORT
We would like to acknowledge the following financial support: FNRS (Fonds National de la Recherche Scientifique, Belgium)—O.L. and S.R.; European Marie Curie Outgoing Fellowship (PIOF-GA-2008-220798)—L.P.; La Polynésienne des Eaux (French Polynesia) and Planète Urgence, France0 – M.K.