Introduction
Bovine mastitis places a major economic burden on the dairy industry globally (Bradley, Reference Bradley2002; Wellenberg et al., Reference Williamson and di Menna2002; Petrovski et al., Reference Petrovski, Trajcev and Buneski2006; Carrillo-Casas and Miranda-Morales, Reference Carrillo-Casas, Miranda-Morales and Chaiyabutr2012; Man'ombe, Reference Man'ombe2014), and is a major concern for the dairy cattle community in Africa. This disease results due to prokaryotic (bacterial) and eukaryotic (mycotic and algal) species invading the udder tissue and subsequent inflammation of the mammary glands (Bradley, Reference Bradley2002). Economic costs associated with bovine mastitis are the result of poor quality and reduced yield of milk (Petrovski et al., Reference Petrovski, Trajcev and Buneski2006; Halasa et al., Reference Halasa, Nielen, Roos, Van Hoorne, de Jong, Lam, van Werven and Hogeveen2009), that seems to vary with each causative pathogen (Gröhn et al., Reference Gröhn, Wilson, González, Hertl, Schulte, Bennett and Schukken2004). Direct and indirect losses contribute massively to economic impacts of the disease. Direct losses result from milk wastage due to pathogenic contamination, antimicrobials used for treatment or adulteration in appearance, and treatment expenditure. Oftentimes, indirect losses are not realized by the farmer as they are concealed. Many sources of indirect losses include pre-mature culling, decreased quality and quality of the harvested milk, expenditure on prevention and health problems associated with the disease and the zoonotic potential (du Preez, Reference du Preez2000; Gruet et al., Reference Gruet, Maincent, Berthelot and Kaltsatos2001; Bradley, Reference Bradley2002; Petrovski et al., Reference Petrovski, Trajcev and Buneski2006).
To date, more than 140 potentially pathogenic species of bacteria (including Mycoplasma), fungi, algae, and viruses cause bovine mastitis (Watts, Reference Watts1988; Petrovski et al., Reference Petrovski, Williamson, Lopez-Villalobos, Parkinson and Tucker2011). This is a big number of species for a single disease, and could potentially alter the veterinarians’ interpretation and determination of the epidemiology of the disease and resulting economic losses. As such, the infectious pathways of these causative pathogens should be addressed meticulously as some may overlap. Mastitis develops as one of two major types, namely contagious or environmental (Bradley, Reference Bradley2002), both of which severely damage the udder tissue of affected cows. Although routes and types of infection are universally accepted, etiologies of different pathogens, incidences, prevalence and management of mastitis are yet to be comprehensively documented for most African countries. As a result, costs remain underestimated and difficult to calculate, while existing treatment regimens are supported by limited evidence-based veterinary medicine. Consequently, these pitfalls hinder prevention, detection and treatment of the disease per cow, client and herd, and will ultimately impact negatively on dairy farming profitability.
Dairy production in Africa pales in comparison with the dairy industry in developed countries, such as the USA and those in the European Union (EU), in terms of licensed herds (USAD, 2010; Lacto data, 2015). However, the average herd size is larger in African countries, including South Africa (USAD, 2010; Lacto data, 2015). Consequently, milk has become an important food commodity in African farming enterprises while its production is a source of income for commercial farmers. For many smaller farms, the dairy industry mainly feeds households because of milk production-related labor requirement inputs, and generates income in poor communities. Several factors further influence the vitality of the dairy industry in many African communities. These include milking practices, processing, distribution, skilled human resources and indigenous beliefs, attitudes and values attached to the consumption of dairy products including milk. Combined, these factors could influence mastitis management practices and associated policies presently held in Africa. Given this background, this review provides the current understanding of the occurrence and importance of bovine mastitis for the African continent.
The review process
A review of the scientific literature was conducted following standard practices published by O'Connor et al. (Reference O'Connor, Anderson, Goodell and Sargeant2014) and Sargeant and O'Connor (Reference Sargeant and O'Connor2014). Databases and scientific search engines (e.g. African Journals Online, Web of Science, CAB Abstracts, PubMed, Google Scholars, ScieELO and Scopus) were searched for English language peer-reviewed articles, theses and reviews. General key terms, such as ‘mastitis’, ‘mastitis pathogen’, ‘mastitis organism’, ‘mastitis cost’, ‘mastitis economics’, ‘dairy’, ‘cattle’, ‘cow’ and ‘bovine’, were used. Terms describing specific forms of mastitis were also used, including ‘mastitis algae’, ‘mastitis bacteria’, ‘mastitis fungus’, ‘mastitis virus’, ‘clinical mastitis’ and ‘sub-clinical mastitis’. All search terms were used as major descriptors and combined with search terms ‘Africa’ or ‘African continent’ or specific country names to specify prevalence locations. The search was enriched by doing a manual search in various journals, and was finalized on 15 September 2016. Papers retrieved were screened manually for relevance, focusing on full length articles reporting on mastitis from the African continent and excluding papers with no relevance to Africa. Additionally, reports published before the year 2000 were excluded, and this has limited the review to roughly the past decade and a half.
Statistical analyses were conducted as follows. In terms of more than one paper being retrieved, provided that each was presenting data from different localities of a country, data were pooled. When multiple papers retrieved were presenting data from the same region in a country, an average of the data sets was calculated and used in the final analysis. Data from different countries were imported and saved into the StatPlanet data editor (StatSilk, 2012) using indicators ‘cows sampled’, ‘disease prevalence’, and ‘pathogen diversity’. Integrative maps were constructed using StatPlanet version 3.0 (StatSilk, 2012) and an online version of Plotly (https://plot.ly/plot/). Statistical data used to measure whole fresh milk production relative to animal trend in Africa were retrieved from the statistics division of the Food and Agriculture Organization of the United Nations (FAOSTAT, 2015).
Milk production in Africa
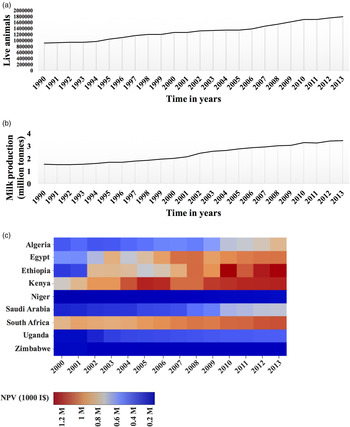
Milk is an important food and income source in developing countries. In sub-Saharan Africa and Asia, cattle account for about 75 and 50% of total milk produced, respectively. As shown in Fig. 1(a), there has been an observable increase in milk producing animals and a concomitant increase in the production of whole fresh milk across Africa (FAO, 2015). However, despite an increase in these two entities, analysis based on net milk production yield portrays a disturbing picture for certain African countries (Fig. 1b). For instance, although there has been an increase in the net yield between 2000 and 2013 in Egypt, Kenya and South Africa, other countries including Niger and Zimbabwe have encountered little, if any, gains during this period. Other countries, such as Algeria and Uganda, have experienced a sluggish increase in net milk production yield. A similar trend was observed in Asia, where buffaloes are major producers of milk, between 1999 and 2009 where only India, China and Pakistan experienced gains in milk production (Sharma et al., Reference Sharma, Rho, Hong, Kang, Lee, Hur and Jeong2012). Countries including Bangladesh, Nepal and South Korea experienced sluggish gains, while Thailand, Vietnam and Sri Lanka experienced little to no gains (Sharma et al., Reference Sharma, Rho, Hong, Kang, Lee, Hur and Jeong2012). These intercontinental milk variations can be attributed to numerous challenges including poor hygiene and/or servicing of malfunctioning milking equipment or milk storage bulk tanks. Lack of awareness among farmers regarding mastitis may also be a common cause. However, subclinical mastitis is an important contributor that is mutual between Asia and Africa, accounting for substantial milk losses. This prompts study of this disease on a continental scale in order to comprehend its prevalence and end results on milk production systems and the economy. Such analysis will identify severely affected regions in the developing world, such as Africa, and will enable a combined effort to deal with the disease.

Fig. 1. Whole fresh cow milk production in Africa. (a) Animal trend determined from number of cows in selected countries (Top) and milk production shared by region (Bottom). (b) Milk production determined as Net Production Value (NVP) and expressed as international prices or US dollars (I$) derived using a Geary–Khamis formula for the agricultural sector. Source: FAOSTAT (2015).
Etiologies of bovine mastitis
Clinical and subclinical mastitis is caused by more than 140 different pathogenic bacterial species (including Mycoplasma), in addition to fungi, algae, and viruses (Watts, Reference Watts1988; Petrovski et al., Reference Petrovski, Williamson, Lopez-Villalobos, Parkinson and Tucker2011). Understanding the diversity of causative pathogens may help explain how they interact to produce intricate clinical patterns displayed by the disease. Because each country on each continent may have different cattle breeds, follow particular feeding mechanisms and be influenced, to some extent, by a particular cultural or indigenous beliefs, pathogen diversity may also vary on the different continents such as Africa, at herd, cow and quarter level. Below, a discussion on the etiologies of bovine mastitis and its relevance to the situation in Africa is provided.
Bacterial mastitis
The most common bacterial mastitis pathogens have been identified and classified into two distinct groups – contagious (or host-adapted) and environmental. Contagious mastitis involves introduction of pathogens during milking processes via milking equipment (e.g. malfunctioning pulsation and vacuum controllers) or milkers’ hands. Some of the more commonly listed species, which exploit this mode of transmission include Staphylococcus aureus, Streptococcus agalactiae, Corynebacterium bovis and Mycoplasma spp.
Mycoplasma spp. show some differences in transmission, thus a brief discussion is warranted. Due to lack of a cell wall, Mycoplasma spp. can evade antimicrobial treatment that may target and disrupt the cell wall. Moreover, traditional tests rarely include Mycoplasma spp. in routine assessments, making cases of diagnosed Mycoplasma mastitis to be uncommon. Eventually, this hinders effective and rapid control measures that require early deployment. Such delays and a lack of effective detection system results in unforeseen and substantial economic consequences caused by Mycoplasma species. Affiliated species involved in Mycoplasma mastitis include, but are not limited to, Mycoplasma bovis, Mycoplasma bovigenitalium, Mycoplasma californicum, Mycoplasma canadense, and Mycoplasma alkalenscens. However, M. bovis is by far the most commonly isolated pathogen causing Mycoplasma mastitis on dairy farms globally. In addition to causing bovine mastitis, M. bovis is associated with a range of conditions including arthritis, reproductive (genital disorders) and respiratory diseases (pneumonia) (Pfützner and Sachse, Reference Pfützner and Sachse1996), which may exacerbate or be exacerbated by bovine mastitis. On the other hand, calves regarded as clinically healthy and young cattle tend to harbor M. bovis in the respiratory passages. Therefore, they can act as mutual reservoirs for this pathogen and will spread it across the herd, resulting in Mycoplasma bovine mastitis occurring at herd level. Transmission is thus facilitated through milk and respiratory mucus of infected cattle (Pfützner and Sachse, Reference Pfützner and Sachse1996).
Mycoplasma mastitis has been largely reported in parts of the USA (Fox et al., Reference Fox, Hancock, Mickelson and Britten2003, Reference Fox, Muller, Wedam, Schneider and Biddle2008; Olde Riekerink et al., Reference Olde Riekerink, Barkema, Veenstra, Poole, Dingwell and Keefe2006; Roy et al., Reference Roy, Francoz and Labrecque2008; Punyapornwithaya et al., Reference Punyapornwithaya, Fox, Hancock, Gay and Alldredge2012), and less frequently reported in developing countries in Asia and Africa (Ghazaei, Reference Ghazaei2006; Saidi et al., Reference Saidi, Khelef and Kaidi2013). Therefore, detection of Mycoplasma in bovine mastitis cases is deterred for the most part due to the requirement for specialized media and culturing techniques as well as an extended period of growth of more than 7 days. The emergence and spread of Mycoplasma mastitis in developed countries suggests that outbreaks in the African countries are possible. However, because an effective detection system is lacking in Africa, adverse economic effects will be hard to prevent. Therefore, research and laboratory diagnostics should focus on early detection.
Environmental mastitis is caused by pathogens found in the habitat of the cow, such as soil, plant material, manure, bedding, or a contaminated water source. Frequently, isolated causative pathogens that contribute to environmental bovine mastitis include members of streptococci and gram-negative bacteria, such as Escherichia coli and Klebsiella (Carrillo-Casas and Miranda-Morales, Reference Carrillo-Casas, Miranda-Morales and Chaiyabutr2012). Both contagious and environmental mastitis result in subclinical and clinical forms with serious economic implications for the dairy industry in developing countries.
Mycotic mastitis
Fungi such as Aspergillus fumigatus, Aspergillus nidulans, Candida spp., Pichia spp. and Trichosporon spp. are known to cause mycotic mastitis. These fungi have been isolated in various parts of the world affected by mastitis, including Brazil, Poland, New Zealand, and Tanzania (Williamson and di Menna, Reference Williamson and di Menna2007; Mdegela et al., Reference Mdegela, Ryoba, Karimuribo, Phiri, Løken, Reksen, Mtengeti and Urio2009; Wawron et al., Reference Wawron, Bochniarz and Piech2010; Dworecka-Kaszak et al., Reference Dworecka-Kaszak, Krutkiewicz, Szopa, Kleczkowski and Biegańska2012). However, mycotic mastitis is poorly characterized given the few studies conducted to characterize fungi in the context of this disease. In contrast, most studies have focused on prokaryotic etiologies, especially staphylococci. As a result, it remains unclear, which fungal species is predominantly causing mycotic mastitis. Nonetheless, there is no compelling evidence to suggest that mycotic mastitis may not develop into a costly disease, as is the case with bacterial mastitis, in any part of the world, but may certainly become a farm-specific problem. Since mycoses caused by fungi are common in mammals, such as humans and other warm blooded animals, the possibility of mycotic mastitis being costly should not be ignored.
Algal mastitis
Members of the genus order, Prototheca, cause incurable acute or chronic Protothecal mastitis in dairy cows. Protothecal species, such as Prototheca zopfii (genotype 2) and Prototheca wickerhamii, have been isolated in numerous clinical cases (Ranjan et al., Reference Ranjan, Swarup, Patra and Nandi2006; Pieper et al., Reference Pieper, Godkin, Roesler, Polleichtner, Slavic, Leslie and Kelton2012; Sobukawa et al., Reference Sobukawa, Yamaguchi, Kano, Ito, Suzuki, Onozaki, Hasegawa and Kamata2012; Krukowski et al., Reference Krukowski, Lisowski, Nowakowicz-Dębek and Wlazło2013). Mastitis outbreaks due to several predisposing factors linked to pßrotothecal bovine mastitis range from animal age, prolonged use of antimicrobials to quarters with a history of clinical mastitis (Ranjan et al., Reference Ranjan, Swarup, Patra and Nandi2006). Environments that are wet and humid tend to harbor Prototheca spp. These environments include muddy pastures and pens, and the infection can occur when an injured teat is exposed to large pathogen numbers. Bovine immune status also plays a major role in infection establishment (Ranjan et al., Reference Ranjan, Swarup, Patra and Nandi2006). Similar to Mycoplasma mastitis, the Protothecal form is rarely reported due to a lack of an effective detection system (Ranjan et al., Reference Ranjan, Swarup, Patra and Nandi2006). Molecular typing tools, such as 18S ribosomal DNA sequencing and restriction fragment length polymorphisms, have been applied in the detection of Prototheca cases of bovine mastitis in Brazil (Gonçalves et al., Reference Gonçalves, Lee, de Paula Arruda, Galles, Caetano, de Oliveira, Fernandes and dos Santos2015), Canada (Pieper et al., Reference Pieper, Godkin, Roesler, Polleichtner, Slavic, Leslie and Kelton2012), Italy (Ricchi et al., Reference Ricchi, Goretti, Branda, Cammi, Garbarino, Turchetti, Moroni, Arrigoni and Buzzini2010) and Japan (Osumi et al., Reference Osumi, Kishimoto, Kano, Maruyama, Onozaki, Makimura, Ito, Matsubara and Hasegawa2008). Because data are already available for the detection of Prototheca in bovine mastitis cases, a guide to identify such related cases in Africa can be formulated.
Viral mastitis
Viruses are isolated from cows affected with bovine mastitis, although they are not regarded as common etiological factors. Therefore, these infectious agents should not be dismissed, especially because algal, bacteriological and fungal agents do not account for 100% of mastitis cases. In addition, viruses, such as bovine herpesvirus (BHV), BHV4, foot-and-mouth disease virus and parainfluenza 3, have been associated with clinical bovine mastitis without isolation of bacterial pathogens (Wellenberg et al., Reference Wellenberg, Poelb and Oirschot2002), suggesting that viral mastitis may indeed occur. However, the evidence is not sufficient to argue, with great certainty, that viruses are causative agents of bovine mastitis.
Relevance to Africa
To address relevance of mastitis causing pathogens to certain African regions, papers reporting the different pathogenic species isolated from Botswana, Ethiopia, Nigeria, Sudan and Zambia were assessed. These regions show the highest variability in pathogens causing mastitis (>10; Fig. 2a). Kenya and Niger showed between six and seven pathogens, while South Africa, Tanzania, Uganda, and Zimbabwe showed fewer than six different pathogens (Fig. 2a). The most commonly reported pathogens were S. aureus, S. agalactiae, S treptococcus dysgalactiae, Streptococcus uberis, and E. coli., while S. aureus was the predominant pathogen identified in milk samples from across Africa (Fig. 2b). This mastitic pathogen had the highest prevalence in Kenya (>70%) and lowest prevalence compared with other pathogens in South Africa at <10% (Fig. 2b). An early survey examining distribution patterns of mastitis causing pathogens between 1996 and 2007 also reported that S. aureus isolates occurred in relatively low numbers (between 10 and 20%) compared with other pathogens in South Africa (Petzer et al., Reference Petzer, Karzis, Watermeyer, van der Schans and van Reenen2009). Several factors, such as climatic conditions, cattle nutrition in feed stocks or movement of cattle between herds, may influence the geographical distribution and dynamics of S. aureus and other pathogens. A larger sample size will be more informative in establishing continental prevalence of bovine mastitis and pathogen diversity.

Fig. 2. Continental outlook of causative pathogens in Africa. (a) Pathogen diversity. Abbreviations for countries are Algeria (ALG), Botswana (BOT), Egypt (EGY), Ethiopia (ETH), Jordan (JOR), Kenya (KEN), Morocco (MOR), Niger (NIG1), Nigeria (NIG2), Saudi Arabia (SAU), South Africa (SOU), Tanzania (TAN), Uganda (UGA), Zambia (ZAM) and Zimbabwe (ZIM). (b) Prevalence of Staphylococcus aureus in selected African regions.
The influence of bovine mastitis in Africa
Incidence and prevalence of clinical and sub-clinical mastitis has been reported for many regions of the world. Most reports come from developed countries, with some reported by Bradley (Reference Bradley2002) and Petrovski et al. (Reference Petrovski, Heuer, Parkinson and Williamson2009). Sharma et al. (Reference Sharma, Rho, Hong, Kang, Lee, Hur and Jeong2012) reviewed the impact of bovine mastitis on Asian cattle and buffaloes. In Africa, occurrence of this disease is well documented in 30% of countries. However, a unified presentation of findings from various studies that is continental for this disease in Africa is needed and will be attempted in this section.
Based on recent reports, released from 2011 to 2014 (Basdew and Laing, Reference Basdew, Laing and Méndez-Vilas2011; Saidi et al., Reference Saidi, Khelef and Kaidi2013; Gitau et al., Reference Gitau, Bundi, Vanleeuwen and Mulei2014; Kassa et al., Reference Kassa, Ayano, Abera and Kiros2014), negative effects of bovine mastitis on the African economy could be overwhelming. Therefore, evidence-based intervention is warranted. Data compiled from several reports shows that mastitis is generally of bacterial in nature, with the predominant species being S. aureus. A large number of reports were reviewed for this paper to demonstrate overall prevalence of bovine mastitis at the clinical and subclinical level in African bovine herds. Reports published from 2000 to 2014, based on surveys from small and large dairy farms in sub-Saharan Africa, with a few coming from the Middle-East and north Africa (Figs. 2–5), suggest that efforts to develop effective and economical methods for disease treatment may be fast-tracked in some regions. To address the extent to which bovine mastitis affected Africa, several aspects were analyzed, such as sample size, reported cases and resultant losses, as well as the economic aspects in selected regions based on published reports.

Fig. 3. Integrative map showing sample size used in bovine mastitis. Data compiled from reports published between 2000 and 2015. Abbreviations to country names are the same as in Fig. 2.

Fig. 4. Continental outlook of bovine mastitis. (a) Prevalence at cow or quarter level. (b) Relationship between quarter prevalence (QP), disease prevalence (DP), subclinical mastitis (SM), and clinical mastitis (CM). N/A, data not available. Abbreviations of countries are the same as in Fig. 2.

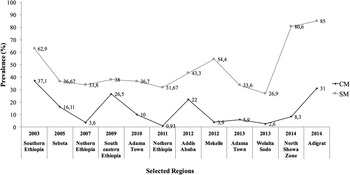
Fig. 5. Mastitis prevalence in selected Ethiopian regions. CM, clinical mastitis; SM, subclinical mastitis.
Sample size
In general, the Middle-Eastern and north African regions have a large number of sampled animals [Morocco (circa 1700), Jordan (circa 16,900) and Saudi Arabia (circa 11,200)] compared with other countries (Fadlelmula et al., Reference Fadlelmula, Dughaym, Mohamed, Deib and Zubaidy2009; Alekish et al., Reference Alekish, Al-Qudah and Al-Saleh2013; Boujenane et al., Reference Boujenane and El Aimani2015) (Fig. 3). Less than 1000 cows were surveyed for bovine mastitis in most reports, with Nigeria, Tanzania and Uganda having the lowest number of surveyed animals (≤200). The reasons for various sample sizes are most likely multifactorial and can range from poor accessibility to milk samples to extent of the disorder not being recognized. Lack of collaboration between practicing veterinarians holding mastitis data and scientists undertaking surveys may also be a cause of variability in sample size. Whatever the reason for observed variability in available sample size, only a margin of the African continent has been represented with some literature of prevalence and incidence of this disease, suggesting that Africa is progressing at a slower pace, compared with other continents, in surveying the disease.
Reported cases of clinical and subclinical mastitis
Udder health directly and indirectly imposes economic burden on dairy farms worldwide. Although initial impact is usually experienced at farm level, udder health issues may escalate to national and continental scale if not detected and addressed in a timely manner. This requires national monitoring system of mastitis occurrence to ensure treatment measures are sanctioned to reduce financial losses. As is the case for continents such as Asia, in Africa mastitis has been profiled in terms of clinical and subclinical forms, both of which contribute to disease prevalence (Fig. 3a). Notably, all reports evaluated in this review unanimously show that subclinical mastitis is steadily increasing, and this is likely the case in regions for which data is not yet published. Accordingly, countries in which disease prevalence is high, such as Ethiopia, Kenya, South Africa and Uganda, display high prevalence of subclinical mastitis of between 60 and 80% (Fig. 3b). Interestingly, more papers reporting on clinical and subclinical mastitis were published in Ethiopia than in any other sub-Saharan African country (Fig. 4). These reports show that >3400 cows have been tested for clinical and subclinical mastitis in dairy herds scattered in and around Ethiopia over the past decade or so (Dego and Tareke, Reference Dego and Tareke2003; Sori et al., Reference Sori, Zerihun and Abdicho2005; Lakew et al., Reference Lakew, Tolosa and Tigre2009; Abebe et al., Reference Abebe, Ike, Siegmund-Schultze, Mane-Bielfeldt and Zarate2010; Bitew et al., Reference Bitew, Tafere and Tolosa2010; Mekibib et al., Reference Mekibib, Furgasa, Abunna, Megersa and Regassa2010; Moges et al., Reference Moges, Asfaw and Belihu2011; Almaw et al., Reference Almaw, Molla and Melaku2012; Daka et al., Reference Daka, G/silassie and Yihdego2012; Girma et al., Reference Girma, Mammo, Bogele, Sori, Tadesse and Jibat2012; Haftu et al., Reference Haftu, Taddele, Gugsa and Kalayou2012; Tadesse and Chanie, Reference Tadesse and Chanie2012; Abera et al., Reference Abera, Lemma and Iticha2013; Belayneh et al., Reference Belayneh, Belihu and Wubete2013; Yohannis and Molla, Reference Yohannis and Molla2013; Zeryehun et al., Reference Zeryehun, Aya and Bayecha2013; Benti and Zewdie, Reference Benti and Zewdie2014; Hailemeskel et al., Reference Hailemeskel, Admasu and Alemu2014; Zenebe et al., Reference Zenebe, Habtamu and Endale2014). This suggests that some African countries have higher prevalence of bovine mastitis than others, and may be more involved in dealing with disease detection, treatment and impacts than other countries.
Although data from reviewed reports reflects information based on one visit per farm for most surveys, a general view of mastitis occurrence can be inferred. For instance, prevalence of subclinical mastitis between 2005 and 2012 was around 40%, while the clinical form was below this number until 2014 (Fig. 4); clinical signs of mastitis are easily detected, thus can be dealt with as soon as they appear. Whether this points to a common factor, such as the time of year (season) when samples were collected or common feeding schemes, remains a matter of speculation and demands further inquiry. Another generalization that can be made is that the occurrence of subclinical mastitis is predominantly higher than clinical mastitis in surveyed areas of Ethiopia, consistent with most parts of Africa (Fig. 3b). Therefore, prevalence of subclinical mastitis in Ethiopia and other African regions may impose substantial costs due to indirect losses (Petrovski et al., Reference Petrovski, Trajcev and Buneski2006; Halasa et al., Reference Halasa, Huijps, Østerås and Hogeveen2007). To this effect, cumulative data from Ethiopia can serve to develop a large-scale disease control program for other African countries.
Prevalence at cow or quarter level
Infections that occur at cows' quarters are good indicators of mastitis’ prevalence at animal level. This was the baseline to determine mastitis at this level in the African continent. Reports of cows with one or more udder quarters infected with clinical and/or subclinical mastitis for the African continent were gathered and examined. According to these reports, most countries display between 30 and 60% prevalence, with circa 30–40% infection in Morocco and Ethiopia and <13% infection in Niger and Sudan (Fig. 3a). Saudi Arabia, located north of Africa, showed the highest percentage of mastitis among cows (>70%) from a relatively large sample size (Fadlelmula et al., Reference Fadlelmula, Dughaym, Mohamed, Deib and Zubaidy2009). Evidence-based information of the prevalence of bovine mastitis in Africa could be maintained by sampling different localities at least two or three times a year.
Reported losses
Economic losses associated with bovine mastitis in developed countries are well documented. These include annual losses estimated per cow per year in the USA and EU countries (Blosser, Reference Blosser1979; Morin et al., Reference Morin, Petersen, Whitmore, Hungerford and Hinton1993; Yalcin et al., Reference Yalcin, Stott, Logue and Gunn1999; Costello, Reference Costello2004; Halasa et al., Reference Halasa, Nielen, Roos, Van Hoorne, de Jong, Lam, van Werven and Hogeveen2009; Viguier et al., Reference Viguier, Arora, Gilmartin, Welbeck and O'Kennedy2009). In contrast, the economic impact of bovine mastitis in Africa is not well documented due to a lack of published material. Therefore, production losses and expenditure associated with mastitis in Africa and other developing countries are generally underestimated and potentially miscalculated (FAO, 2014). In addition, different countries around the world apply distinct methodologies to calculate economic losses incurred due to mastitis (some examples are indicated in Table 1), and this makes comparison difficult (FAO, 2014). Therefore, to estimate economic impacts of bovine mastitis, a universal method is needed. As far as Africa is concerned, the research landscape for analysis of economic effects associated with bovine mastitis is wide open.
Table 1. Estimated costs incurred due to bovine mastitis in selected developing countries (FAO, 2014)

a ‘$’, cost estimates calculated in US dollars. CM, clinical mastitis; SM, subclinical mastitis; N/A, not applicable.
Reported interventions
A recent study conducted in South Africa to evaluate economic value of somatic cell count (SCC) in Holstein and Jersey cattle found that it is imperative for SCC to be incorporated into breeding objectives. A reduction in milk yields caused a concurrent profit reduction ranging from ZAR 491.48 to ZAR 1795.57 per cow per year, depending on the breed, production and payment system (Banga et al., Reference Banga, Neser and Garrick2014). To our knowledge, these estimated profit reductions were derived from milk of healthy cows, arguing that reductions are potentially severe in cows with subclinical mastitis. Therefore, information on milk SCC is vital in detecting subclinical mastitis as it may provide reliable estimates of milk production losses.
Mastitis diagnosis
Diagnosis of clinical mastitis is less complex, because clinically discernable signs, including swollen quarters/udder and poor milk quality, can be detected by farmers (Mahmmod, Reference Mahmmod2013). By contrast, subclinical mastitis cannot be visually diagnosed and requires application of diagnostic techniques. The wide range of mastitis causing pathogens can perpetuate the costs of developing treatment, and in some cases, may involve application of diagnostic methods tailored to specific pathogens.
Under field conditions, early detection is often assisted using traditional diagnostic tests, such as the California Mastitis Test (CMT) and/or SCC at herd level (Deb et al., Reference Deb, Kumar, Chakraborty, Verma, Tiwari, Dhama, Singh and Kumar2013; Duarte et al., Reference Duarte, Freitas and Bexiga2015). CMT is a simple cow-side indicator test commonly used to determine SCC for diagnosis of subclinical mastitis. Somatic cells mainly comprise macrophages, lymphocytes, erythrocytes and epithelial cells (Dohoo and Meek, Reference Dohoo and Meek1982; Pillai et al., Reference Pillai, Kunze, Sordillo and Jayarao2001; Sharma et al., Reference Sharma, Singh and Bhadwal2011) and the proportion of each cell type depends on infection status of the gland. In healthy udders, white blood cells constitute a third of cells, but during infection white blood cells may increase in proportions reaching 99%. Therefore, SCC can indicate the presence and extent of udder tissue damage caused by pathogenic species or malfunctioning milking equipment and, hence, safeguard milk quality. Consequently, regular examination of somatic cells in milk is recommended for dairy farms, despite the lack of a universal standard that exists for SCC in terms of poor or good milk quality. Additionally, an inverse linear relationship has been defined between low SCC (e.g. <100,000 cells ml−1) and high milk quality as well as high SCC (e.g. >200,000 cells ml−1) and declining milk quality (Bradley, Reference Bradley2002; Sharma et al., Reference Sharma, Singh and Bhadwal2011). Such measures should be considered for Africa to curb disease consequences.
Other common tests for detection of bovine mastitis include electrical conductivity, pH, NaOH (white side test), measurement of N-acetyl-b-D-glucosaminidase, lactate dehydrogenanse, bacterial culture of milk, and milk enzyme-linked immunosorbent assay as well as polymerase chain reaction (PCR) assay (Mahmmod, Reference Mahmmod2013). Several PCR assays, including multiplex conventional and real-time PCR, have been developed for detection of mastitic pathogens including Staphylococcus spp., E. coli, M. bovis, S. agalactiae and Enterococcus spp. (Koskinen et al., Reference Koskinen, Holopainen, Pyorala, Bredbacka, Pitkala, Barkema, Bexiga, Roberson, Solverod, Piccinini, Kelton, Lehmusto, Niskala and Salmikivi2009; Taponen et al., Reference Taponen, Salmikivi, Simojoki, Koskinen and Pyörälä2009; Shome et al., Reference Shome, Das Mitra, Bhuvana, Krithiga, Velu, Shome, Isloor, Barbuddhe and Rahman2011; Hiitiö et al., Reference Hiitiö, Riva, Autio, Pohjanvirta, Holopainen, Pyörälä and Pelkonen2015; Barbier et al., Reference Barbier, Boschiroli, Gueneau, Rochelet, Payne, de Cruz, Blieux, Fossot and Hartmann2016). A comprehensive background of the molecular epidemiology of mastitis pathogens, particularly at sub-species level, with relevance to public health is reported by Zadoks et al. (Reference Zadoks, Middleton, McDougall, Katholm and Schukken2011). The advent of the loop-mediated isothermal amplification method, which is another nucleic acid amplification technique (Tomita et al., Reference Tomita, Mori, Kanda and Notomi2008), has seen development of assays for detection of Staphylococcus spp., E. coli, M. bovis, S. agalactiae and Enterococcus spp. (Kato et al., Reference Kato, Yoshida, Ansai, Watari, Notomi and Takehara2007; Zhang et al., Reference Zhang, ZHnag, Yang, Huang, Zhang, Jia, Yuan and Li2011; Wang et al., Reference Wang, Wang, Xiao, Guo, Zhang, Wang and Liu2015; Bosward et al., Reference Bosward, House, Deveridge, Mathews and Sheehy2016).
Other molecular biological techniques developed for diagnosis of bovine mastitis include proteomics-based detection, biochips and biosensors (Deb et al., Reference Deb, Kumar, Chakraborty, Verma, Tiwari, Dhama, Singh and Kumar2013). Therefore, an inventory of diagnostic techniques exists, and can be applied as a first line of detection from milk samples in African countries. The challenge is to select a technique(s) with most of desirable qualities (e.g. most reliable, relevant, and rapid) to facilitate detection and downstream analyses.
Mastitis treatment
Developing an effective mastitis therapy remains a challenge for researchers due to high number of pathogens contributing to the disease. S. aureus is the most prevalent species in bovine mastitis due to resistance mechanisms, such as formation of abscesses within the udder (du Preez, Reference du Preez2000) or evasion of antibiotics by residing inside macrophages, thus avoiding antibiotics circulating in the bloodstream. Moreover, some strains of S. aureus can exist as latent bacteria within a capsule and can later reactivate growth when conditions normalize (du Preez, Reference du Preez2000). Furthermore, treatment is complicated by the presence of planktonic and biofilm growth. Evasion of, and resistance to, antibiotics as well as latency have obvious implications for treatment and costs. These factors are important to the success of S. aureus as a mastitic pathogen prevailing in Africa.
Currently, there is no universal procedure to treat mastitis. For most treatment campaigns recommended treatment depends on the extent of udder health deterioration (du Preez, Reference du Preez2000). In South Africa, an interesting development is the exploration of phages as an alternative therapy (Basdew and Laing, Reference Basdew, Laing and Méndez-Vilas2011; Basdew, Reference Basdew2012). Phages offer a number of benefits as natural therapeutic agents to control bacterial pathogens. These include host specificity, reduced toxicity, ease of isolation and propagation, prolonged shelf life and availability in the same environment as their bacterial hosts. Therefore, problems associated with antimicrobials, such as resistance, cost, and the need to continuously develop antimicrobials in response to their targets, can be eradicated as phages presumably evolve with the target host. However, it is possible that bacteria could develop mechanisms to avoid attack and killing by bacteriophages. Bacteria may secrete enzymes that target phage receptors on cell wall surfaces preventing recognition by, or altering specificity of, phages. As S. aureus is the dominant etiological agent in bovine mastitis in Africa, the great potential of bacteriophage therapy is currently being tailored for controlling strains of this species (Basdew and Laing, Reference Basdew, Laing and Méndez-Vilas2011; Basdew, Reference Basdew2012). Application of phage therapy should be expanded to accommodate other pathogens as antibiotic resistance is not limited to S. aureus alone.
Conclusions and future directions
More work is required for reliably diagnosing and treating bovine mastitis, as well as estimating the resulting economic impacts in Africa. While culture-based techniques allow strain isolation from field samples, molecular genetic techniques undoubtedly offer rapid, specific, and sensitive detection pipelines for mastitis. Nonetheless, African countries can largely benefit from developing a clear policy regarding diagnosis. Such a policy should emphasize application of rapid techniques as a first line of diagnosis for suspected infections, outbreaks, or be used during routine testing and confirmation at reference centers. As indicated in this paper, prevalence of subclinical mastitis is definitely on the rise in Africa, ranging from 50 to 80% in Ethiopia, Kenya, South Africa and Uganda. This is concerning, as this form of mastitis leads to increased antimicrobial resistance observed in most mastitis-causing pathogens and is by far the damaging and costly form of the disease as early detection is difficult. Thus, this review has highlighted some of the countries severely affected by subclinical mastitis, and should serve as a guide to strengthen subsequent analyses. As subclinical mastitis is common in affected cows and results in elevated SCC, it is recommended that there be clear communication regarding SCCs acceptable for a healthy udder in Africa. This will inform the initiation of treatment for potentially affected cattle. It is also important to address the issue of accuracy when using SCC to detect subclinical mastitis as farmers rely on this information to administer antimicrobials during therapy.
Only about 30% of African countries report cases of bovine mastitis, while approximately 70% lag behind. This is a small number compared with the developed world. Additionally, estimates of milk losses and costs of dealing with the disease are not well documented, suggesting that there is a delay in devising effective combat strategies. Measures such as increasing collaborations between the dairy industry, scientific community and economists, would result in the better use of limited resources and expertise. It is also advisable that farmers do regular cattle screening for subclinical and clinical signs of mastitis in order to iminently deal with direct and indirect losses.
Acknowledgments
Authors are grateful to L. Mwadzingeni for comments received during preparation of this manuscript. TEM is supported by the National Research Foundation (NRF) of South Africa (Grant no. SFP14070774252). The financial assistance of the NRF is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the author and not necessarily attributed to the NRF.