Introduction
Xanthoria parietina is one of the most common and widespread species of lichen-forming ascomycetes in Europe. Its characteristic golden yellow thalli occur on a wide range of natural and anthropogenic substrata, from shaded to fully sunlit sites on nutrient-rich or eutrophic substrata. Its area of distribution ranges from the Caucasus to westernmost Europe and from North to South Africa; it is common on the east coast of northern North America, was found in Ontario and occurs on the west coast from Baja California to the southern part of British Columbia (Lindblom Reference Lindblom1997; Brodo et al. Reference Brodo, Sharnoff and Sharnoff2001, Reference Brodo, Lewis and Craig2007; McCune Reference McCune2004). Xanthoria parietina was introduced into Australia and New Zealand, where it grows side by side with morphologically similar native species (Galloway Reference Galloway1985; Rogers Reference Rogers1992; Eichenberger Reference Eichenberger2007). Honegger et al. (Reference Honegger, Zippler, Scherrer and Dyer2004b) concluded from fingerprint analyses of specimens from four continents, that the Australian X. parietina might have been introduced from southern Europe.
Xanthoria parietina forms no vegetative symbiotic propagules but is invariably fertile, its central thalline areas being covered by apothecia. Fingerprinting techniques (RAPD-PCR) applied to single spore isolates derivedfrom single asci (i.e., the progeny of meiosis) and to the sterile cultured vegetative mycelium of the ascocarp of X. parietina revealed identical fingerprints and, thus, it was concluded to be homothallic by Honegger et al. (Reference Honegger, Zippler, Gansner and Scherrer2004a). The genetic background was elucidated in molecular analyses of the mating type loci (MAT1-1-1 and MAT1-2-1) plus flanking regions of a rangeof Xanthoria species, where the homothallic X. parietina was shown to contain MAT 1-2-1 in all descendants of meiosis, MAT 1-1-1 having been lost (Scherrer et al. Reference Scherrer, Zippler and Honegger2005; nomenclature according to Turgeon & Yoder Reference Turgeon and Yoder2000); thus recombination seems unlikely to occur in X. parietina. Xanthoria elegans, another homothallic species, had MAT1-1-1 and MAT1-2-1 in all siblings (Scherrer et al. Reference Scherrer, Zippler and Honegger2005). Heterothallic species such as X. calcicola, X. polycarpa or X. capensis have either MAT1-1-1 or MAT1-2-1 in half of the siblings derived from one ascus and polymorphic fingerprints (2 × 4 genotypes among the 8 sporelings per ascus) resulting from successful recombination (Honegger et al. Reference Honegger, Zippler, Gansner and Scherrer2004a; Scherrer et al. Reference Scherrer, Zippler and Honegger2005).
As the homothallic X. parietina is unlikely to recombine we wondered whether natural populations have a clonal structure. Lindblom & Ekman (Reference Lindblom and Ekman2006, Reference Lindblom and Ekman2007) analysed the intraspecific genetic variation in populations of X. parietina samples in Norway and SW Sweden (Scania). Sampling was performed at irregular intervals over transects 30–150 m long, and epilithic and epiphytic samples were included. Part of the IGS and the complete ITS region were used as molecular markers. Genetic differences were documented within and between populations, but a high percentage (34% of IGS haplotypes, 24% of ITS haplotypes) of samples belonged to one haplotype. The authors concluded that X. parietina in these studies was characterized by intense gene flow within and between populations.
As shown in previous studies on Xanthoria elegans and X. parietina, a higher resolution of genetic variation among sample sets is achieved with fingerprinting techniques (e.g. RAPD-PCR markers; Murtagh et al. Reference Murtagh, Dyer, Furneaux and Crittenden2002; Scherrer & Honegger Reference Scherrer and Honegger2003; Honegger et al. Reference Honegger, Zippler, Scherrer and Dyer2004b) than with comparative sequence analysis. The present study aims to increase the resolution and expand the geographic range by analysing the genetic diversity within four populations of X. parietina in coastal, rural and urban areas in NW, SW and central France and NE Switzerland at the subspecific level with a fingerprinting technique (RAPD-PCR) applied to sterile cultured multispore isolates.
Materials and Methods
Collecting, isolation and sterile culturing
Collecting sites and collectors are listed in Table 1. Larger specimens were photographed and the outlines of smaller samples were painted on a transparent overlay prior to removal of single apothecia, whose site was noted on the photograph or overlay. Each multispore isolate was derived from one single apothecium per thallus. In three out of the four collecting sites, sampling was performed on two different substrata which were found at distances of a maximum of 30 m from each other. Up to eight microsites per collecting site were investigated. Within microsites (i.e. one sample set) the thalli had been growing side by side on the same stem or branch (i.e. on a small area).
Table 1. Collecting sites for Xanthoria parietina, collectors, numbers of microsites and isolates examined and percentage of polymorphisms detected

* thalli of vouchers nos 319 and 320 were documented photographically and left in situ, only small fragments carrying mature apothecia were removed.
Multispore isolates were obtained by placing a fully hydrated, single apothecium under an inverted Petri dish containing agarized Bolds Mineral Medium (BBM; Deason & Bold Reference Deason and Bold1960) with double the amount of nitrogen. Numerous packages of ascospores were usually ejected on the agar surface within a few minutes; they germinated within less than a week. After a few weeks the germlings were transferred to an agarized, later to liquid, nutrient medium as routinely used in our laboratory (a modified malt yeast extract medium, 1/10 strength, as described by Honegger & Kutasi Reference Honegger, Kutasi, Nardon, Gianinazzi-Pearson, Grenier, Margulis and Smith1990). Voucher specimens were air dried and stored at −20°C; they will be deposited in the mycological collection of the joint herbaria of the University and ETH Zürich (Z + ZT). Vouchers of the sterile cultured isolates are cryopreserved and stored under liquid nitrogen (LN2) in our laboratory.
DNA isolation and RAPD-PCR
Approximately 5 mg of mycelium from the liquid cultures was placed with c. six 1mm diameter glass beads in a 1·5 ml reaction tube, which was immersed in LN2 for a few minutes. It was then cryogenically disrupted with a MM 301 mixer mill (Retsch GmbH, Haan, Germany) at a rate of 30 vibrations/sec for 1 min. Total genomic DNA was isolated using the GenElute™ Gel Extraction Kit (Sigma-Aldrich, St. Louis) following the manufacturer's protocol.
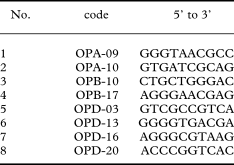
Eight decamer primers were used (Table 2), which had yielded good results in previous studies on Xanthoria species (Honegger et al. Reference Honegger, Zippler, Gansner and Scherrer2004a, Reference Honegger, Zippler, Scherrer and Dyerb). PCR amplifications were carried out with 1 µl of template DNA in 25 µl reaction volumes, consisting of 19·75 µl sterile ddH2O, ×1 reaction buffer for DyNAzyme, 0·2 mM dNTP, 0·2 µM RAPD primer and 0·5U of DyNAzyme DNA polymerase (Finnzymes, Espoo). The following cycles were run on a PTC-220 DNA Engine Dyad™ Peltier Thermal Cycler (MJ Research, Waltham, MA): initial denaturation for 3 min at 94°C, 40 cycles of 30 s at 93°C, 40 s at 37°C, 1 min 20 s at 72°C, 30 s at 93°C and a final extension of 5 min at 72°C. 15 µl of each amplification product were separated in 1·5% agarose gels in ×1 TBE buffer at 40 V for 5 h, stained with ethidium bromide and photographed under UV light. Constant reaction conditions and thermal profile during amplification were ensured (Meunier & Grimont Reference Meunier and Grimont1993). The reproducibility of this technique was tested in previous studies (Honegger et al. 2004; Honegger & Zippler 2007). High reproducibility is achieved provided that freshly isolated DNA is used and the PCR conditions are standardized. Only intense, unambiguous bands were visually scored and included in the presence-absence matrix.
Table 2. RAPD primers (Operon Decamer oligonucleotide primers) used in PCR in Xanthoria parietina

Data analysis
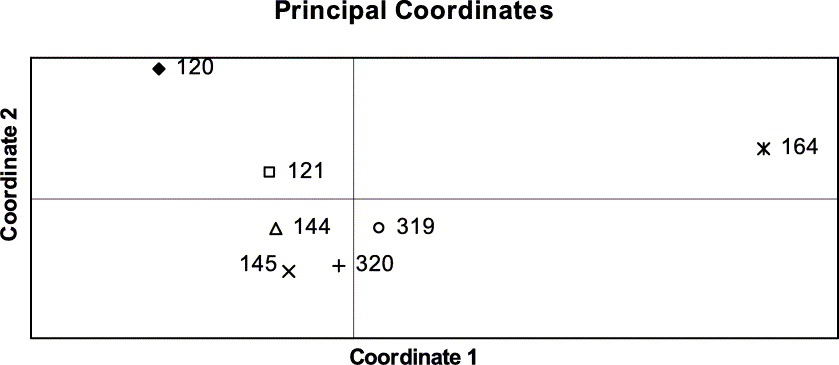
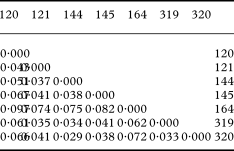
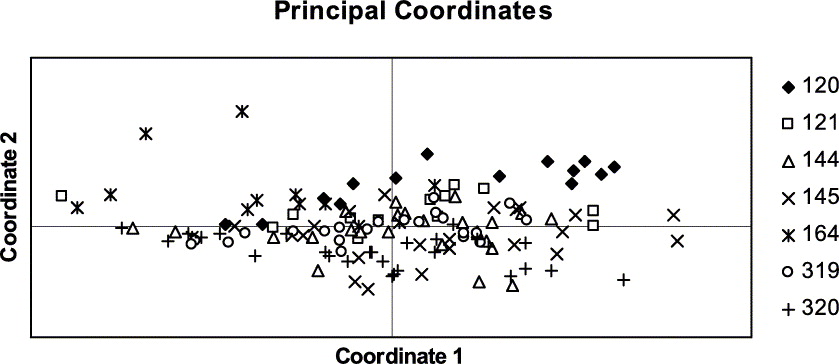
Presence (1) or absence (0) of 150 polymorphic RAPD bands (as achieved with eight primers) were visually scored and recorded in a binary data matrix. Bands of equal fragment size were assumed to be homologous. The computer program GenAlEx 6 (Peakall & Smouse Reference Peakall and Smouse2006) was used to calculate the statistics of genetic variation based on individual haplotypes for each population. To assess the distribution of the total genetic variation per population we calculated the percentage of polymorphic bands (P) and Nei's standard genetic distances between pairs of populations (Nei Reference Nei1972, Reference Nei1978). Genetic distances between pairs of isolates were calculated with the Nei coefficient (Nei Reference Nei1972). Genetic distances were spatially visualized with the principal coordinates analysis (PCO) and plotted in a chart. This analysis shows the relationships between isolates and between populations, based on the first two principal coordinates of each distance matrix.
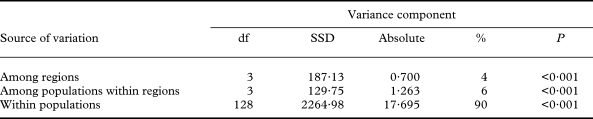
The partitioning of genetic variance within and among populations and groups of populations was assessed by an analysis of molecular variance AMOVA (Excoffier et al. Reference Excoffier, Smouse and Quattro1992). The significance test was performed with 999 permutations. This analysis also calculates fixation indices (Φ statistics), which are indices of population subdivision analogous to F-statistics (Weir Reference Weir1996).

Fig. 1. Phenotypic plasticity in Xanthoria parietina in the field and laboratory (symbiotic vs. aposymbiotic phenotypes). A, phenotypically homogenous population with confluent thalli on a branch of poplar in SW France (voucher No. 120, microsite III); all thalli contain large amounts of parietin (see fingerprints of these four samples in Fig. 3); B, phenotypically diverse thalli on Tamarix shrubs in coastal Bretagne (NW France; voucher No 164) with varying amounts of parietin; this is not due to shading effects (see C) [samples with asterisks all have chemosyndrome A (according to Søchting (Reference Søchting1997) in TLC (H. Gansner, unpublished data)], see fingerprints of some of these samples in Fig. 4; C, population of juvenile thalli developing on a fully sunlit bench 1 m from the Tamarix shrubs whence samples shown in B were collected, high parietin producers (intensely yellow to orange) grow next to pale, greyish thalli with very low parietin contents, the same situation is seen in voucher nos 164/5, 164/11 and 164/12 (see B), where only the apothecial discs are intensely yellow; D, sterile cultured isolates from voucher nos 120 and 121, all kept in the same culturing medium for the same period of time and revealing different coloration and secreting different amounts of secondary compounds into the medium.
Results
Phenotypic variation among Xanthoria parietina samples in the field and laboratory
In three of the four collecting sites the populations of X. parietina appeared phenotypically homogenous. The thalli revealed the characteristic coloration: intensely yellow on fully sunlit parts (Fig. 1A) and greenish grey on shaded parts, best visible in single thalli which grow around branches. Apothecial discs (i.e. the hymenial layer) and the ostioles of pycnidia always revealed an intense yellow coloration due to the deposition of crystals of the anthraquinone parietin (Søchting Reference Søchting1997). However, in one exceptional population from coastal Bretagne a whole spectrum of colours from greyish to orange-yellow was seen in thalli which had the same exposure and illumination, low and high anthraquinone producers growing next to each other (Fig. 1B & C). Some thalli had greyish lobes, but intensely yellow margins (Fig. 1B4, 1B11, 1C). In seven out of 13 samples of voucher 164 the lichen products were studied with thin-layer chromatography methods according to Culberson & Ammann (Reference Culberson and Ammann1979; H. Gansner, unpublished data); all revealed chemosyndrome A (parietin as the main secondary product) according to Søchting (Reference Søchting1997), as typically found in X. parietina.
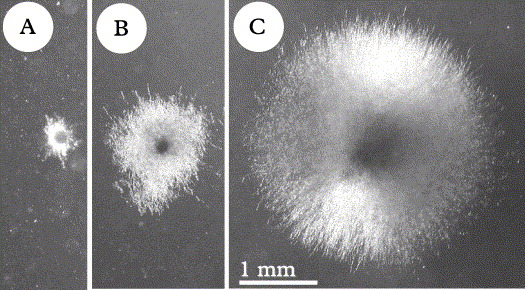
Even when populations were homogenous in the field, phenotypic differences were observed in sterile cultured multispore isolates (Fig. 1A & D1). Germlings of different samples from the same collecting site revealed distinctly different growth rates on non-nutrient mineral medium (Fig. 2A–C). Differences in growth rates were less prominent on nutrient media, but differences in pigmentation (probably carotenoids; these contribute to the coloration of the thallus of X. parietina and other Xanthoria spp. in nature, as shown by Czeczuga Reference Czeczuga1983) of the thallus-like fungal colonies and in the release of coloured (unidentified) secondary compounds into the culturing medium were evident (Fig. 1D).
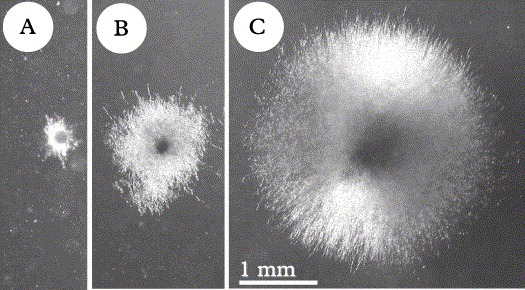
Fig. 2. Growth rates of Xanthoria parietina germlings from the same collecting site (University of Zurich, Irchel Park) after 5 months culturing on agarized mineral medium, same culturing conditions [date of isolation (ascospore ejection): 26 viii 2002; photographs were taken on 30 i 2003]. Each colony is a multispore isolate, as grown out of the eight ascospores contained in one ascus. All colonies from the same apothecium (multispore isolates) revealed the same growth rate and the same phenotype (see also Honegger et al. 2004a); A shows minimum, C maximum, B average growth rate as seen in most of the isolates. A, isolate 320 I bd; B, isolate 319 I f2d; C, isolate 319 IV ad. Same magnification in A–C.
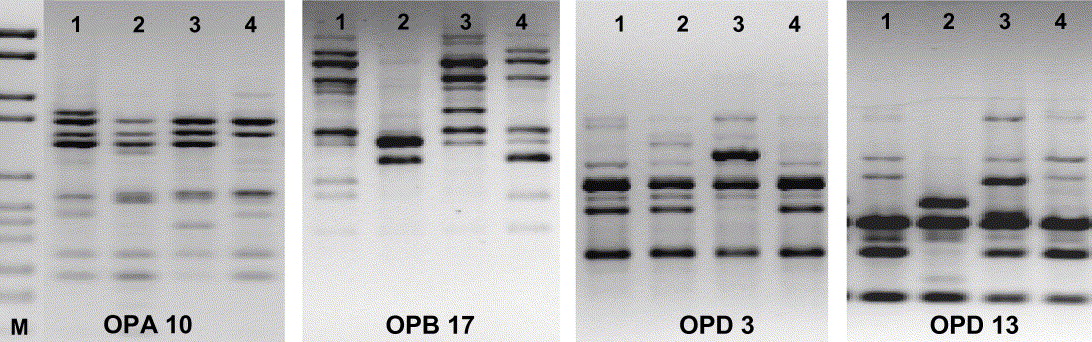
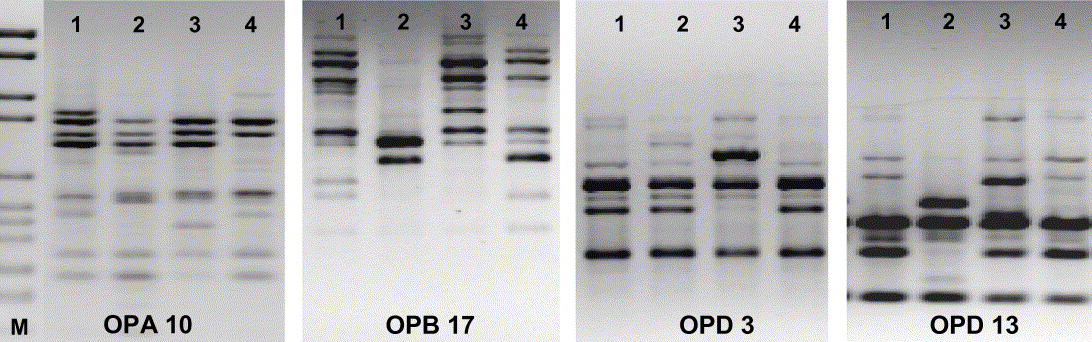
Fig. 3. RAPD-PCR (four primers) of four Xanthoria parietina isolates from thalli, which had been growing side by side in a phenotypically homogenous population in SW France (voucher no. 120, microsite III, see Fig. 1A). Each isolate represents a different haplotype.

Fig. 4. RAPD-PCR (three primers) of sterile cultured multispore isolates of samples 1–11 of the phenotypically diverse population of Xanthoria parietina from coastal Bretagne, NW France (voucher no. 164; see Fig. 1B). Each isolate represents a different haplotype.
Genetic diversity of Xanthoria parietina within and among populations
In our fingerprint analyses of epiphytic X. parietina, a high level of polymorphism was found in all populations, ranging from 57·3–80·7 % (13–22 isolates per site investigated; Table 1), whereas the only population from rock revealed 38% polymorphism (3 isolates tested; Table 1). The average (P) in the total sample was 73·5%. It is not surprising that the phenotypically heterogenous population from coastal Bretagne (164; Fig. 1B) was genetically highly diverse, but the phenotypically homogenous population from SW France (120; Fig. 1A) reached even slightly higher values (Table 1). No genetic variation was found in isolates which derived from different apothecia on the same thallus (3 samples tested; results not shown).
The genetic distances between pairs of populations ranged between 0·029 and 0·098 (Table 3). The PCO grouped similar populations together, the phenotypically diverse population from coastal Bretagne being the most distant one (Fig. 5). The population structure was assessed by analysing the molecular variance (AMOVA). Here the populations were grouped in four regions (SW, NW and central France and Switzerland).
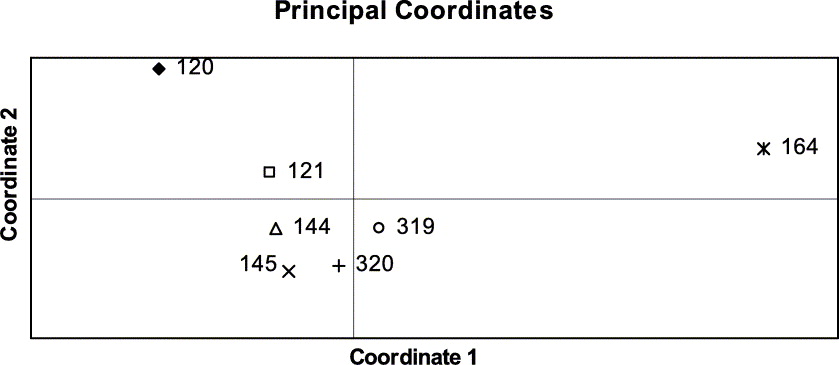
Fig. 5. Grouping of Xanthoria parietina populations with Principal Co-ordinates Analysis.
Table 3. Pairwise Population Matrix of Nei Genetic Distance between pairs of populations of Xanthoria parietina
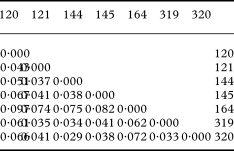
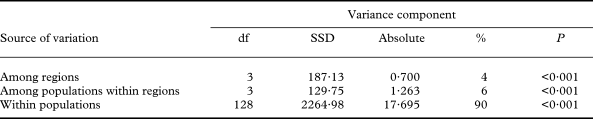
When the RAPD dataset was partitioned within and between populations, most of the genetic variation resided within populations (90%; Fig. 6), relative to the amount of variation among regions (3·9%) or among populations within regions (6·4%; Table 4). AMOVA produced significant (P < 0·001) fixation indices. The global differentiation of populations (ΦST) amounted to 0·103, the differentiation among populations within regions (ΦSC) to 0·067, and the proportion of differentiation between regions (ΦCT) to 0·039.

Fig. 6. Principal Coordinates Analysis of all isolates of Xanthoria parietina.
Table 4. AMOVA, analysis of molecular variance in Xanthoria parietina
Discussion
The high genetic diversity within populations of X. parietina, as observed in the present study, is surprising. As a homothallic species (with MAT1-2-1 in all progeny of meiosis, MAT1-1-1 having been lost; Scherrer et al. Reference Scherrer, Zippler and Honegger2005), recombination seems unlikely to occur. However, the ultimate proof for obligate homothallism, that is ascocarp and ascospore formation by a haploid mycelium derived from one single ascospore, is not yet available due to culturing problems, no sexual reproductive stages being formed in sterile cultures. Moreover a nonsense mutation, that is a stop codon at the beginning of the HMG box, was found in MAT1-2-1 of epiphytic inland X. parietina (Scherrer et al. Reference Scherrer, Zippler and Honegger2005; S. Scherrer, unpublished data); its impact on transcription and thus on gene expression remains to be explored. As an ascomycete X. parietina has to form a new dikaryon on the haploid mycelium prior to the formation of every fruiting body, but it is not known how this proceeds. Pycnidia as potential donors of spermatia are present and ascogonia are easy to find within and slightly below the algal layer of submarginal thallus areas (Janex-Favre & Ghaleb Reference Janex-Favre and Ghaleb1986), but no trichogynes have so far been detected with light or electron microscopy techniques (R. Honegger, unpublished data). Therefore we conclude that spermatization, a common and widespread mode of dikaryon formation in lichen-forming ascomycetes (Honegger Reference Honegger1984; Honegger & Scherrer Reference Honegger, Scherrer and Nash2008), is not the mode of dikaryotization in X. parietina.
Where does the high genetic diversity in populations of the homothallic X. parietina originate? In a homothallic species facultative gene flow might occur via parasexual events, for example via DNA transfer through hyphal anastomosis between different haplotypes. Such phenomena have not yet been detected among lichen-forming fungi, but cannot be excluded. In their pioneering work on mating systems in lichen-forming ascomycetes Murtagh et al. (Reference Murtagh, Dyer and Crittenden2000) found uniform fingerprints among sporelings from the same apothecium, but polymorphisms among sporelings from different apothecia on the same thallus of Ochrolechia parella or from different thalli of Graphis scripta; thus they concluded the patterns were the result of facultative homothallism or opportunistic outbreeding, respectively. In O. parella and G. scripta the molecular basis of homothallism is unknown.
Accumulation of mutations and intense genotype rather than gene (allele) flow might be the main causes of the high genetic diversity in X. parietina. High genotype flow is typically found in fungal species that disperse primarily by means of vegetative propagules. Thousands of conidia are asexually produced on a single infection of powdery mildew, and hundreds of uredospores detach from a rust pustule. Both can be transported by wind over hundreds of kilometres and germinate immediately upon landing on a suitable host (for a review see McDonald & Linde Reference McDonald and Linde2002). The heterothallic rice blast fungus Magnaporthe grisea, a main fungal pathogen on rice worldwide, lacks fruiting bodies in many regions, for example because only one mating type is present, as observed in North American and Korean populations (Viji & Uddin Reference Viji and Uddin2002; Tredway et al. Reference Tredway, Stevenson and Burpee2003; Park et al. Reference Park, Milgroom, Han, Kang and Lee2008); thus allele flow via outcrossing occurs rather infrequently, yet populations are genetically diverse. This is caused by the accumulation of mutations (Park et al. Reference Park, Milgroom, Han, Kang and Lee2008), efficient dispersal via asexually produced propagules (conidia) and partly also due to parasexual exchange of DNA via hyphal anastomoses, as observed in sterile co-cultures in the laboratory (Zeigler et al. Reference Zeigler, Scott, Leung, Bordeos, Kumar and Nelson1997).
Xanthoria parietina forms no vegetative symbiotic propagules but is invariably fertile, its central thalline areas being covered by apothecia. It was therefore assumed to disperse exclusively via ascospores, which have to re-lichenize (Ott Reference Ott1987). As shown in field experiments free-living photobiont cells are more common than previously assumed (Sanders Reference Sanders2005). However, vegetative dispersal via thallus fragments was also documented in the field (Honegger Reference Honegger1996). The high regenerative capacity of X. parietina facilitates the establishment of new thalli from larger or smaller fragments under natural or experimental conditions (Honegger et al. Reference Honegger, Conconi and Kutasi1996). Upon being glued to adequate substrata, thallus fragments of X. parietina grow very well within short periods of time, that is 3 mm in 10 months (Armstrong Reference Armstrong1993; Honegger Reference Honegger1993, Reference Honegger1995, Reference Honegger1996, Reference Honegger1998). Short distance dispersal of minute fragments by grazing invertebrates might be more common than previously assumed. The fecal pellets of the ever present lichenivorous mites (acari) were shown to contain viable ascospores of X. parietina and viable cells of its unicellular green algal photobiont, Trebouxia arboricola (Meier et al. Reference Meier, Scherrer and Honegger2002). The frequency of vegetative dispersal in X. parietina is not comparable to dispersal via asexually produced propagules of non-lichenized fungi (e.g. conidia, uredospores, etc.), but precise data are missing. Wind dispersal over long distances is documented for vegetative symbiotic propagules (soredia) of lichens (Marshall Reference Marshall1996; Tormo et al. Reference Tormo, Recio, Silva and Muñoz2001; Muñoz et al. Reference Muñoz, Felicísmo, Cabezas, Burgaz and Martínez2004; Seaward Reference Seaward and Nash2008). Vertebrates, especially birds, are active and passive vectors of lichen fragments and propagules (Seaward Reference Seaward and Nash2008). The importance of resident and migratory birds as passive transporters of either ascospores, tiny thallus fragments of X. parietina, of lichenivorous mites on X. parietina and/or their fecal pellets over short or long distances remains to be explored, but has certainly been underestimated. Xanthoria parietina grows abundantly at sites where birds land and rest (Honegger et al. Reference Honegger, Conconi and Kutasi1996) and nutrient input via droppings facilitate the growth of this nitrophilous species at the resting places. However, passive genotype transfer from adjacent populations might provide the starter material for establishing new populations and later for increasing the genetic diversity of existing ones. Theoretically only one viable photobiont cell and one or a few cells of the fungal partner (cells of the haustorial complex or germ tubes of ascospores), as found on bark substrata (Bubrick et al. Reference Bubrick, Galun and Frensdorff1984; Sanders Reference Sanders2005), might be sufficient to start a new thallus.
Taking homothallism and the different options for vegetative (symbiotic or aposymbiotic) dispersal into account, a clonal structure was expected at microsites, that is among vicinal thalli, which might have derived from one ‘founder thallus’ in phenotypically homogenous populations of X. parietina, as investigated in the present study. The Swiss population in the urban collecting site was expected to reveal a lower level of genetic variation because Irchel Park around the University campus was built and young trees planted 16 years before we did our sampling. In contrast, the populations in SW and central France included old trees at rural sites. However, no major differences were evident, probably because all sites are embedded within the main area of distribution of X. parietina and genotype flow proceeded in a similar manner throughout. A lower level of genetic variation might be found outside the main area of distribution, for example at sites where X. parietina was anthropogenically introduced (Australia, New Zealand).
Although the very common and widespread Xanthoria parietina is one of the best investigated lichens worldwide, many aspects of its fascinating biology remain unexplored.
Our sincere thanks to our colleagues Undine Zippler for performing preliminary RAPD-PCR tests and Heidi Gansner for allowing us to refer to her unpublished TLC data and to the Swiss National Science Foundation for generous financial support (grant No. 3100A0-116597 to R.H.).