INTRODUCTION
Mistletoes comprise a diverse group of hemiparasitic flowering plants that have become specialized to access nutrients and water from host trees via a haustorium (Stewart & Press Reference STEWART and PRESS1990). Mistletoes vary widely in their degree of host specificity, ranging from extreme specialists that parasitize a single host species to generalists that use many different host species with no apparent infection difference among host species (Dean et al. Reference DEAN, MIDGLEY and STOCK1994, Norton & Carpenter Reference NORTON and CARPENTER1998, Norton & de Lange Reference NORTON and DE LANGE1999). In some mistletoe species, host infection varies geographically such that at a given location a mistletoe species may infect only part of its potential host set (Okubamichael et al. Reference OKUBAMICHAEL, GRIFFITHS and WARD2011a, Rödl & Ward Reference RÖDL and WARD2002, Thorogood et al. Reference THOROGOOD, RUMSEY and HISCOCK2009). It could be argued that mistletoes use the most abundant trees in the community simply because there are few alternatives (neutral interactions governed by species abundance) but a mechanism of host adaptation is still required. The factors that explain why mistletoe species only infect a subset of the available host species in a given locality are not clear.
Host specificity in mistletoes is a composite measure of relative abundance of mistletoes on the parasitized host species (Mathiasen et al. Reference MATHIASEN, NICKRENT, SHOW and WATSON2008). Differential parasitism of mistletoes of the available host species is often expressed as host preference (i.e. only a subset of host trees are infected). Host compatibility at the genetic, mechanical, physiological and biochemical level are most likely to affect the growth and survival of mistletoes on host trees, subsequently determining host preference in mistletoes (Fadini Reference FADINI2011, Okubamichael et al. Reference OKUBAMICHAEL, GRIFFITHS and WARD2011a, Yan Reference YAN1993a, Yan & Reid Reference YAN and REID1995). Host preference may not be necessarily driven by traits of host trees that directly affect the growth and survival of mistletoes. For example, birds may influence seed dispersal patterns and thus affect host preference in mistletoes. It is therefore crucial to quantify the differential parasitism of mistletoes in the field and to test host compatibility.
Genotype performance across environments can be reflected in reaction norms tested by an interaction effect in analysis of variance (Lynch & Walsh Reference LYNCH and WALSH1998). If the genotypic responses to environmental changes of two or more reaction norms are non-parallel, it indicates a genotype by environment (G × E) interaction (Japhet et al. Reference JAPHET, ZHOU and ZHANG2009). In this particular study, we tested the contribution of G × E interactions to local adaptation of mistletoes on host species at a specific site by means of reciprocal transplant experiments in two combinations of mistletoe–host populations, using hypocotyl length and survival as indices of growth performance. The growth of the hypocotyl – the structure that eventually forms the haustorium – is hypothesized to be an essential trait determining the establishment success of parasitic plants (Yoder Reference YODER1999).
The aims of this study were to quantify the degree of host specificity and to evaluate the factors that determine the local distribution and specialization of the mistletoe Agelanthus natalitius (Loranthaceae) in two populations in South Africa. We predicted that host recognition and preference occurs during the early developmental stages of germination and haustorium formation in mistletoes and would be reflected in differential survival of mistletoes.
METHODS
Study sites and species
We surveyed the tree community and the population distribution of the mistletoe Agelanthus natalitius in two sites approximately 110 km apart in KwaZulu-Natal, South Africa: Highover (29°54′S, 30°05′E) and Mtontwane (28°48′S, 29°56′E). Highover is a privately owned nature reserve 25 km south-west of Richmond and Mtontwane is a game ranch adjacent to Weenen Game Reserve. The vegetation of Mtontwane is characterized by Acacia caffra, A. karroo, A. tortilis and A. nilotica woodlands and thickets. The vegetation of Highover is characterized by A. karroo, A. caffra and A. ataxacantha woodlands and thickets, with a denser vegetation and steeper terrain than at Mtontwane.
Agelanthus natalitius is widely distributed throughout southern Africa (Polhill & Wiens Reference POLHILL and WIENS1998, Visser Reference VISSER1981). This mistletoe species parasitizes at least 11 tree genera, including Acacia, Carya, Citrus, Combretum, Dichrostachys, Dombeya, Grewia, Pterocarpus, Punica, Sclerocarya and Terminalia (Visser Reference VISSER1981, Wiens & Tölken Reference WIENS and TÖLKEN1979). However, geographic variation in the infection patterns over the parasite's range suggests that A. natalitius may be locally specialized on particular host species.
Field survey
Host composition was assessed by quantifying the tree community in the two study sites (Highover and Mtontwane). We randomly selected and surveyed 64 plots (32 plots in each site) (20 × 50 m). We identified all tree species in each plot; any species that had at least one infected tree in the study site was recorded as a host species. We counted the number of mistletoes on each tree and measured tree size (height and diameter) because it may be an important parameter in determining the infection pattern of mistletoes among host trees (Overton Reference OVERTON1994, Roxburgh & Nicolson Reference ROXBURGH and NICOLSON2007, Ward et al. Reference WARD, SHRESTHA and MUSLI2006). Tree height was measured with a measuring pole; if the tree was inclined or growing on a slope, trigonometric calculations were applied to determine tree height from the ground. Diameter at breast height (dbh) of the trunk was measured approximately 1.5 m above the base of the stem; in the case of multi-stemmed trees the following calculation was used: (dbh = SQRT [sum (stem diameter2)]). Trees below 2 m in height and < 3 cm in diameter were excluded, because these were never parasitized by a mistletoe (pers. obs.).
Reciprocal transplant germination experiment
We carried out field reciprocal transplant germination experiments using seeds of mistletoes parasitizing the two main host species, A. karroo and A. caffra, in our two field sites. First, we bagged unripe mature fruits using nylon mesh bags to protect fruits from bird consumption 1 mo prior to collection. To avoid pseudoreplication, we randomly selected 20 individual mistletoes in different host trees from each of the two main host species in both sites. We collected fully ripe and undamaged fruits by hand picking. For each seed used for the experiment, we manually removed the exocarp (pulp cover) and endocarp (the skin covering the seed). This is essential because the layers covering the seed can act as barriers to germination in mistletoes (Ladley & Kelly Reference LADLEY and KELLY1996, Okubamichael et al. Reference OKUBAMICHAEL, RASHEED, GRIFFITHS and WARD2011b). Furthermore, this enables the sticky viscin surrounding the seed to be exposed, which facilitates the temporary attachment of mistletoe seeds to host branches. We worked the viscin by hand to increase its stickiness (for a similar method, see Ladley & Kelly Reference LADLEY and KELLY1996, Sargent Reference SARGENT1995).
We used non-parasitized individual trees in our experiment to avoid any effects of previous infection and susceptibility. Trees ranged in height from 2–6 m and were all located in open areas to avoid shade effects (Okubamichael et al. Reference OKUBAMICHAEL, RASHEED, GRIFFITHS and WARD2011b). We monitored a total of 64 individual trees that were marked at Mtontwane and Highover. For each host species in each site we had two groups: one group received seeds of mistletoes obtained from A. caffra and the other group from A. karroo. Each group consisted of eight trees. For each experimental tree, we selected two healthy branches at the same position within the canopy and of similar size (8–12 cm girth, although mistletoes are capable of growing on much smaller twigs). Twig size was based on the optimal size for seedling establishment based on previous studies in this particular mistletoe species. In addition, this host twig size enabled us to inoculate many seeds on a single twig. Most importantly, if many seeds had established during the experiment, this branch size would maintain many seeds better than smaller twigs that would not withstand high infection of mistletoes (Sargent Reference SARGENT1995). Each branch received 10 seeds linearly orientated and placed 3 cm apart; of these, five seeds were from Highover and five seeds from Mtontwane. Each seed was marked with a distinctly coloured pin. We applied a paired design (one local and one non-local seed) for each pair to experience identical environmental conditions including the current host species, bark surface and branch diameter (Rödl & Ward Reference RÖDL and WARD2002).
We monitored the seed germination, hypocotyl growth and survival after 1 wk, after 1 mo and after 6 mo at both sites. At each time period we recorded the condition of each seed as germinated (indicated by protrusion of the fresh green seed embryo), dead (where colour had changed to black and the seed had become dried and shrivelled) or lost in situ. Where germination occurred we measured hypocotyl length from the base of the viscin layer to the distal end of the protruded hypocotyl. Hypocotyls that curved towards the substrate and attached to the host substrate were considered to have successfully established because the haustorium will form in this position. However, if the hypocotyls grow away from the host bark they do not attach to the host and do not become successfully established. The experiment was designed to test the G × E interactions in response to site, source and current substrate while other abiotic factors were equivalently experienced by mistletoe seeds.
Statistical analysis
Host species and germination (expressed as the percentage of seeds that germinated) were analysed for differences in frequency using χ2 tests (SPSS 18.0 for Windows). The difference in prevalence (i.e. the percentage of individual trees carrying at least a single mistletoe infection) between host species and correlations of prevalence with tree height and dbh were tested using binary logistic regression. We also tested prevalence of grouped host trees with height (1-m class width) and with dbh (10-cm class width) after prevalence was arcsine-square root-transformed. The relationship between intensity of infection and tree height and trunk dbh was further analysed with GLIM, as the frequency distribution of parasitism among the two host species followed a negative binomial distribution (Krebs Reference KREBS1989) (variance/mean = 6.30/0.79 and k = 0.16, N = 1464, χ2 for goodness of fit = 9.17, df = 3, P = 0.027). We used ANOVA to test the differences among host species in mean height and dbh, as well as among infected and uninfected trees in mean height and dbh. We used ANOVA to test the effect of site, source, current substrate and time on hypocotyl length in the mistletoe seedlings (SPSS 18.0 for Windows). Significant interactions in an ANOVA indicate that the reaction norms are not parallel (i.e. that there is a G × E interaction). We performed Kaplan–Meier survival analysis (Kaplan & Meier Reference KAPLAN and MEIER1958, SPSS 18.0 for Windows) for the 6-mo period. When overall significance was confirmed we did a pairwise comparison and a new alpha value was computed to account for the Bonferroni correction. We fitted a sigmoid curve to determine the effect of hypocotyl length within 6 mo on survival of the mistletoe seedlings.
RESULTS
Field survey
At the two study sites, five host species were recorded as being parasitized by the mistletoe Agelanthus natalitius, namely Acacia caffra, Acacia karroo, Acacia tortilis, Acacia nilotica and Leucaena leucocephala (all Fabaceae; nomenclature after Van Wyk & Van Wyk Reference VAN WYK and VAN WYK1997). Acacia tortilis, A. nilotica and L. leucocephala were excluded from further analyses because these species were either rare in the study sites or had few infected individuals. Acacia tortilis was absent in Highover and only one A. nilotica was recorded in the survey plots at that site. Similarly, only two individuals of L. leucocephala were recorded in a single plot at Highover, both of which were infected. At Mtontwane, only a few individuals of A. nilotica (N = 3) and A. tortilis (N = 9) were infected, each supporting a single Agelanthus natalitius individual. Thus, all statistical analyses were applied to the two most common host species, A. karroo and A. caffra, which grow abundantly at both sites and were recorded with many trees infected by Agelanthus natalitius.
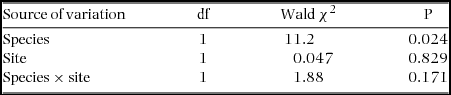
We recorded a total of 1464 trees (Acacia karroo and A. caffra) hosting 1202 Agelanthus natalitius mistletoes in the 64 surveyed plots at the two sites (Highover and Mtontwane). Acacia karroo was significantly more abundant than A. caffra at both sites; there were almost four times as many A. karroo as A. caffra at Highover and three times as many A. karroo as A. caffra at Mtontwane (Table 1). There was no significant difference in prevalence of mistletoe infection on the two host species at Highover (prevalence of A. karroo = 22% and A. caffra = 26%), but a significantly greater percentage of A. caffra trees were parasitized at Mtontwane (A. karroo = 25% and A. caffra = 34%) (Table 2). Infection intensity (number of mistletoes per tree) was higher for A. karroo (0.73 ± 0.04 and 1.03 ± 0.64 mistletoes per tree) than for A. caffra (0.66 ± 0.01 and 0.89 ± 0.035 mistletoes per tree) at Highover and Mtontwane, respectively (Table 3). At Highover, infected trees of A. karroo had an average of 3 mistletoes per infected tree as compared with 2.5 per infected tree for A. caffra. At Mtontwane, infected trees of A. karroo had an average of 4 mistletoes per infected tree as compared with 2.5 per infected tree of A. caffra. If we had excluded the two highly infected trees at Highover and one at Mtontwane that many mistletoe-dispersing birds used for nesting (pers. obs.), the number of mistletoes per infected tree of A. caffra would drop to 1.
Table 1. Chi-square test on the frequency of two tree species (Acacia karroo and A. caffra) that are hosts of the mistletoe Agelanthus natalitius at two sites (Highover and Mtontwane). Acacia karroo was significantly higher in abundance than A. caffra in both sites (N = 1464).
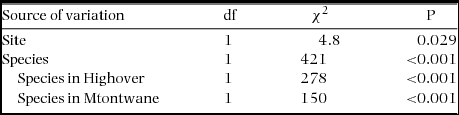
Table 2. Generalized linear model test results for the prevalence of infection (% of trees with a mistletoe infection) of two host species (Acacia karroo and A. caffra) in two sites (Highover and Mtontwane) (N = 1464).
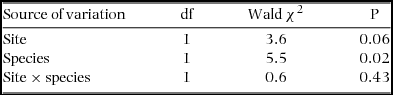
Table 3. Generalized linear model test results for the intensity of mistletoe infection (mean number of parasites per tree) for two host species at two sites. Intensity of infection was significantly higher on A. karroo than on A. caffra (N = 1464 trees parasitized by 1202 mistletoes).
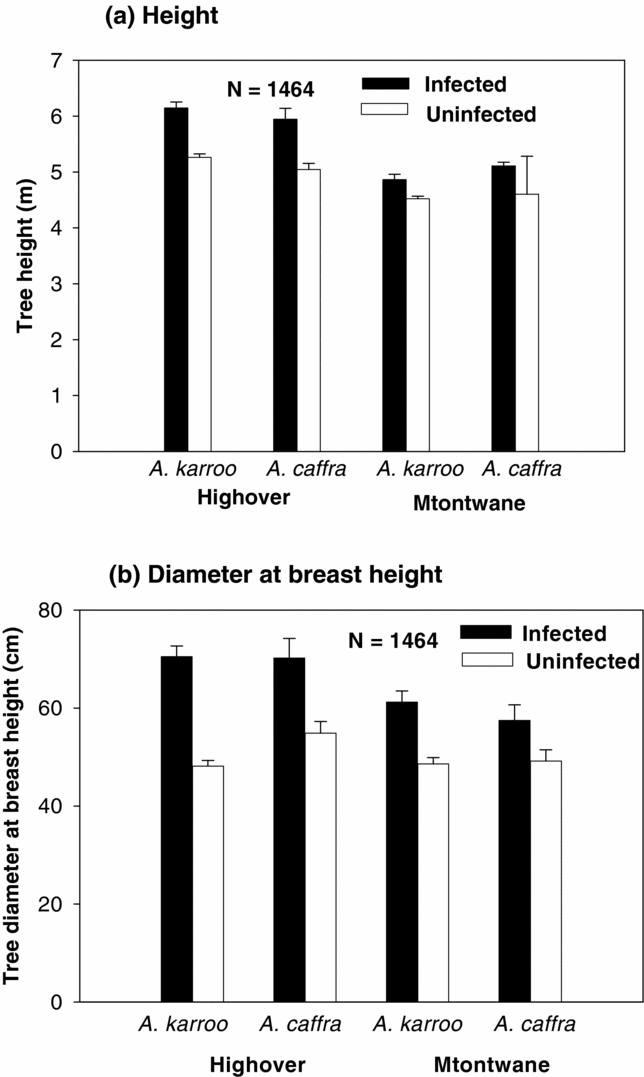
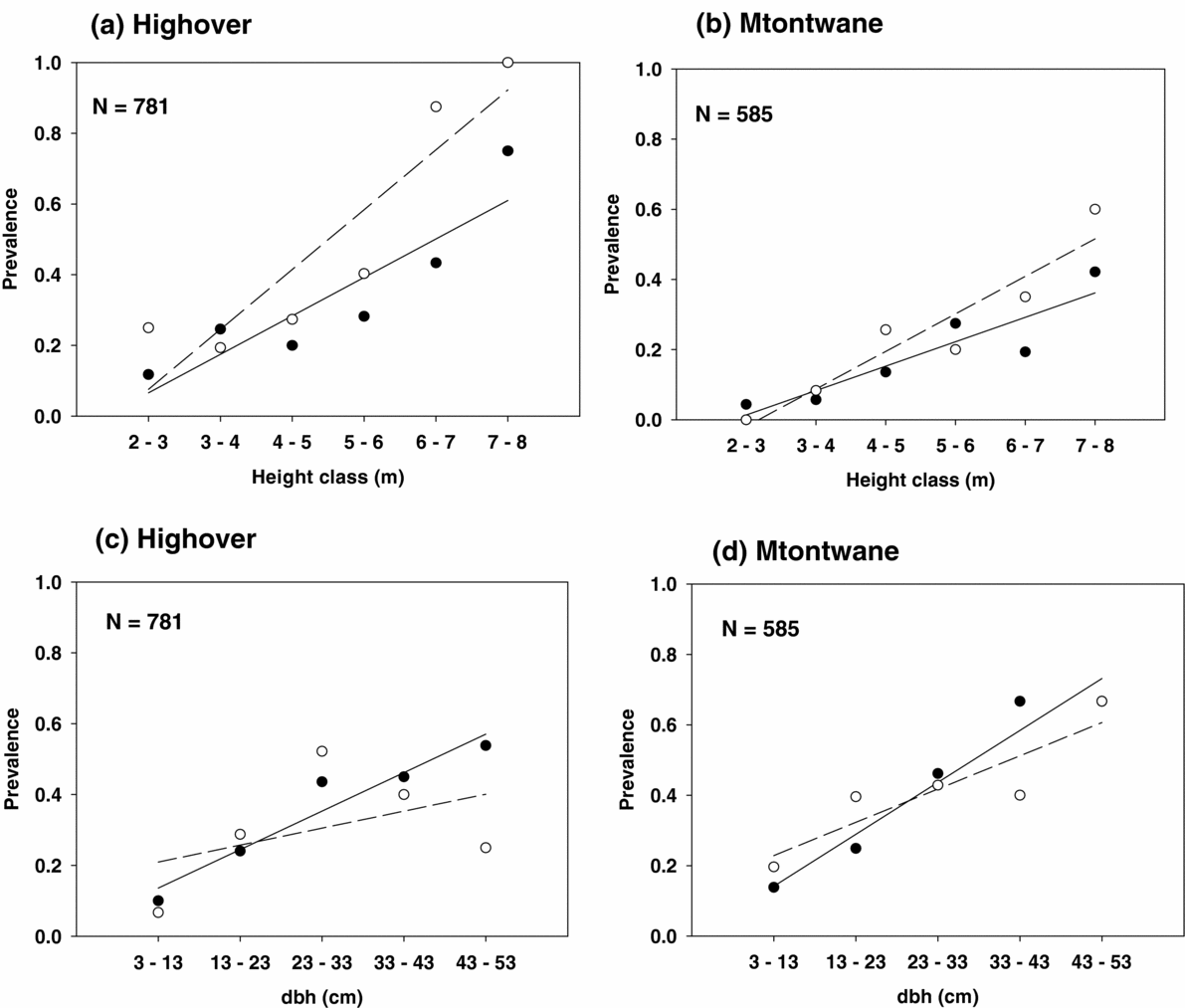
There was no significant difference (species, site and species × site) in tree height (range in Wald χ2 = 0.001–0.074, range in P = 0.786–0.927, N = 1464) and trunk dbh (range in Wald χ2 = 2.8–6.3, range in P = 0.227–0.435, N = 1464) between A. karroo and A. caffra trees at either site. However, the mean height and trunk dbh of infected trees were significantly greater than for uninfected trees for both species in both sites (Table 4, Figure 1). The logistic regression analysis indicated that both height and dbh had a significant positive effect on the probability of infection (slopes for prevalence and height ranged from 0.38–0.85, range in Wald χ2 = 12–40, P < 0.001, range in N = 157–622). A similar result was obtained for dbh, although there was a lower slope (slopes for prevalence and dbh ranged from 0.050–0.096, range in Wald χ2 = 5–41, P < 0.001, range in N = 157–622). Prevalence of grouped trees was the proportion of infected trees in the given class. Prevalence was positively correlated with height (1-m class width) and with dbh (10-cm class width) (results after prevalence was arcsine-square root-transformed, height, range in r = 0.90–0.95, range in F = 17–40, P < 0.05, range in N = 157–622; and dbh, range in r = 0.90–0.97, range in F = 12–50, P < 0.05, N = 157–622). However, the dbh class of A. caffra at Highover was not significantly positively correlated with prevalence (r = 0.50, F = 1.00, P = 0.39, N = 157) (Figure 2).
Table 4. GLIM of test for the height and diameter at breast height (dbh) of infected and uninfected trees (status) of two host species (Acacia karroo and A. caffra) at two sites (Highover or Mtontwane).
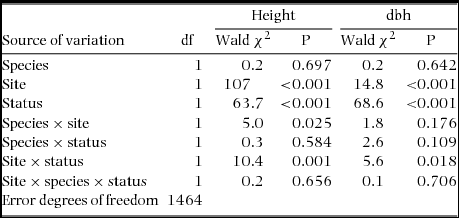

Figure 1. Height (a) and diameter at breast height (b) (mean ± SE) of host trees Acacia karroo and Acacia caffra infected and uninfected by the mistletoe Agelanthus natalitius at Highover and Mtontwane.

Figure 2. Prevalence of the mistletoe Agelanthus natalitius against tree height and dbh of the two host species, Acacia karroo (solid circles with solid regression line) and Acacia caffra (hollow circles and dashed regression line). (a) Prevalence against tree height at Highover, (b) prevalence against tree height at Mtontwane, (c) prevalence against dbh at Highover and (d) prevalence against dbh at Mtontwane. All were significantly positively correlated with the exception of prevalence of A. caffra, which was not significantly correlated with tree dbh at Highover.
The distribution of Agelanthus natalitius among host trees was strongly aggregated, meaning that most potential hosts were not infected, while a few individual host trees were highly infected and supported most of the parasites (e.g. we observed a single host tree with 56 mistletoes in our study). The GLIM analysis showed that the number of mistletoes per host tree (infection intensity) had a positive significant relationship with tree height (range in slopes = 0.30–0.70, P < 0.001, range in N = 157–622). A similar result was obtained for dbh, although with a lower slope (range in slopes = 0.024–0.032, P < 0.001, range in N = 157–622).
Reciprocal transplant germination experiment
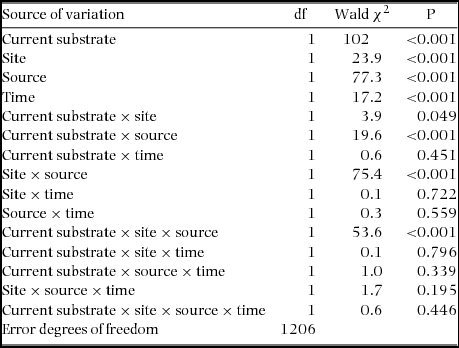
Germination of Agelanthus natalitius seeds started within 1 d in both sites and reached 100% after 1 wk, independent of host substrate and site. Within the first mo, 7% of the germinated seeds of A. natalitius were unsuccessful (either they died or were lost in situ) and there was no significant difference in germination success whether they were placed on a source or non-source host species and whether they had been translocated to a different site or were germinated within their locality (χ2 = 5, P = 0.78, N = 1280). In this case, we compared eight combinations at each site. In contrast to germination success, hypocotyl length was significantly influenced by the three-way interactions (site × source × current substrate). This is important to note because it reflects the presence of genotype × environment (G × E) interactions (Table 5).
Table 5. GLIM of the reciprocal transplant experiment of Agelanthus natalitius. Site = Highover or Mtontwane; Source = source (original) host species; Current substrate = host that the mistletoe was transferred to manually.

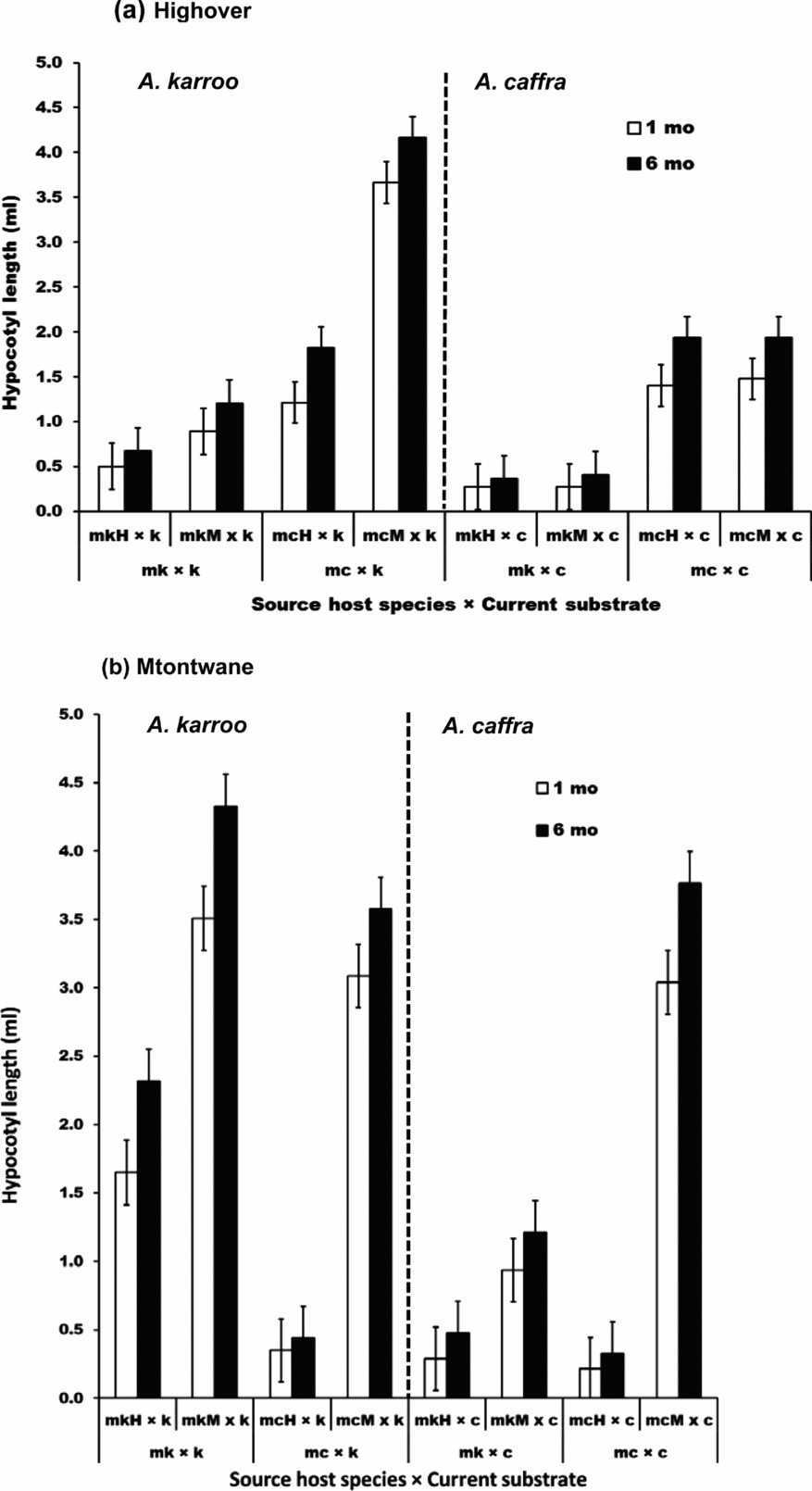
At Highover, mistletoe seeds placed on the same current substrate as their source host within their site grew significantly longer hypocotyls than those transferred to non-source hosts, except for mistletoes on Acacia karroo (Figure 3a). There was an unidentified pathogen on mistletoe seeds placed on A. karroo in Highover and we suspect that this resulted in very short hypocotyl growth of these mistletoes (Figure 3a). As a result, mistletoes from A. karroo at Highover that were placed on A. karroo at Highover grew much shorter hypocotyls than mistletoes from A. karroo at Mtontwane that were placed on A. karroo at Highover (Figure 3a).

Figure 3. The hypocotyl length after 1 mo (white bar) and 6 mo (black bar) (mean ± SE) of the germinated mistletoe seedlings of Agelanthus natalitius in the reciprocal transplant experiments of all combinations of source host species × current substrate at both sites, Highover (a) and Mtontwane (b). Abbreviations: source host species, mk = mistletoe seedlings obtained from mistletoes that grew originally (source) on Acacia karroo and mc = mistletoe seedlings obtained from mistletoes that grew on Acacia caffra and current substrate; k = A. karroo and c = A. caffra; source host species with their respective site; mkH = mistletoes from A. karroo at Highover, mkM = mistletoes from A. karroo at Mtontwane, mcH = mistletoes from A. caffra at Highover, mcM = mistletoes from A. caffra at Mtontwane.
In Mtontwane, mistletoe seeds placed on the same current substrate as their source host within the same site grew the longest hypocotyls (Figure 3b). Mistletoe seeds obtained from Acacia caffra and placed on A. karroo within the same site had longer hypocotyls than those obtained from A. karroo at Highover (Figure 3b). Similarly, mistletoes obtained from A. karroo hosts but placed on A. caffra within the same site grew longer hypocotyls than those obtained from Highover.
Overall, if substrate only was compared, mistletoe seeds placed on A. karroo had longer hypocotyls than mistletoe seeds placed on A. caffra at Highover and Mtontwane (Figures 3). For mistletoes that were transferred within a site, mistletoes from A. karroo grew less on A. caffra while mistletoes from A. caffra placed on A. karroo grew much longer hypocotyls in both sites.
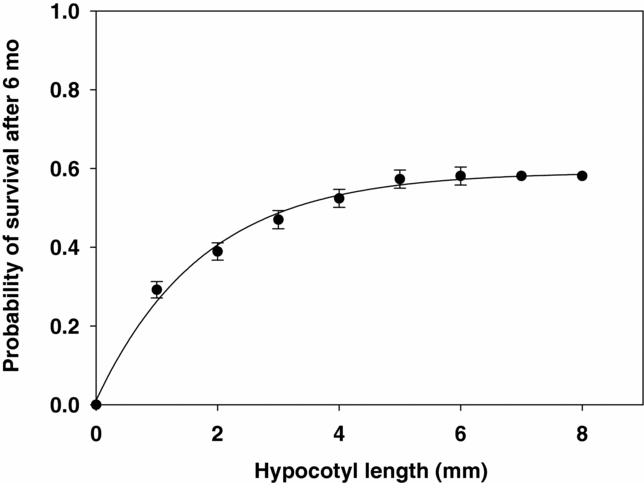
The number of hypocotyls that curved towards and contacted the host substrate was also higher when mistletoe seeds were placed on the source host species (χ2 = 97, P < 0.01, N = 309). In this case, we also compared the eight combinations at each site. Even when we excluded source and site effects by considering current host substrate only we found that the hypocotyls of mistletoe seedlings attached to the host substrate more frequently on A. karroo (χ2 = 28, P < 0.01, N = 309). Hypocotyl length over 6 mo was positively correlated with the probability of survival of mistletoe seedlings (r = 0.95, F1, 8 = 57.5, P < 0.001) (Figure 4).

Figure 4. Relationship between hypocotyl length of mistletoe seedlings of Agelanthus natalitius and their survival.
Survival
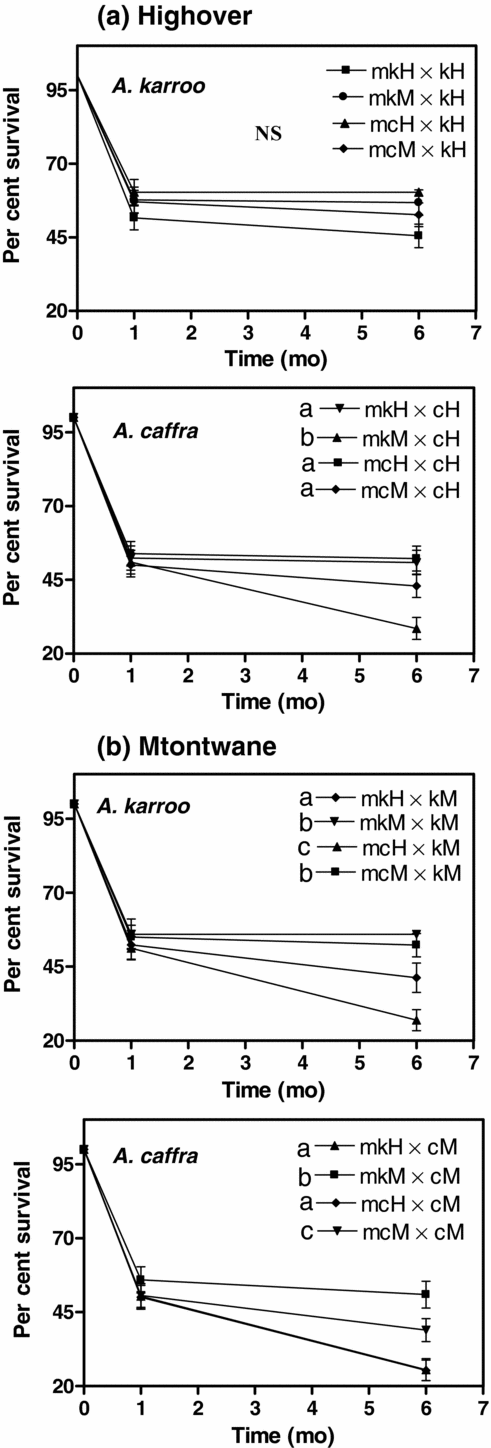
A log-rank test showed that overall survival curves showed significant differences across all 16 combinations over 6 mo (χ2 = 51.3, P < 0.001, df = 15). Three groups (mistletoes on A. karroo at Highover and mistletoes on both host species at Mtontwane) showed a significant difference in survival (range in χ2 = 8.61–10.60, range in P < 0.001–0.035, df = 3), but mistletoes on A. caffra at Highover did not show any significant difference in survival (χ2 = 3.57, P = 0.31, df = 3) (Figure 5). Overall, survival was higher for mistletoe seeds on Acacia karroo than on A. caffra (Figure 5).

Figure 5. Percentage survival over 6 mo of Agelanthus natalitius mistletoe seedlings. Two groups of mistletoes received from two different host species placed on two host species at two sites (= 16 combinations in total). (a) Highover survival curves of mistletoes placed on Acacia karroo and Acacia caffra, (b) Mtontwane survival curves of mistletoes placed on Acacia karroo and Acacia caffra. Abbreviations for mistletoe sources: mkH = mistletoes from A. karroo at Highover, mkM = mistletoes from A. karroo at Mtontwane, mcH = mistletoes from A. caffra at Highover, mcM = mistletoes from A. caffra at Mtontwane. Abbreviations for hosts: kH = A. karroo from Highover, kM = A. karroo from Mtontwane, cH = A. caffra from Highover and cM = A. caffra from Mtontwane. NS = not significant. The lowercase letters denote significant differences between groups of mistletoe seeds.
At Highover, mistletoes placed on Acacia karroo did not show any significant differences in survival regardless of the site or host species from which they were obtained (Figure 5a). However, mistletoes placed on A. caffra at Highover showed significant differences in survival (Figure 5a). Survival of mistletoes from A. karroo at Mtontwane placed on A. caffra at Highover (mkM × kH) was significantly lower than for the other three combinations at Highover.
At Mtontwane, mistletoes obtained from Acacia karroo at the same site had the highest survival on A. karroo (mkM × kM), which was followed by mistletoes obtained from A. caffra and placed on A. karroo at Mtontwane (mcM × kM) (Figure 5b). Mistletoes from A. karroo at Highover placed on A. karroo at Mtontwane had intermediate survival, while mistletoes obtained from A. caffra at Highover placed on A. karroo at Mtontwane had the lowest survival of all seeds inoculated on A. karroo. Mistletoes from A. karroo at Mtontwane placed on A. caffra at Mtontwane (mkM × cM) had the highest survival, followed by mistletoes of A. caffra from Mtontwane placed on A. caffra in Mtontwane (mcM × cM) (Figure 5b). Mistletoes of A. karroo from Highover placed on A. caffra at Mtontwane (mkH × cM) and mistletoes of A. caffra from Highover placed on A. caffra at Mtontwane (mcH x cM) had the lowest survival of seeds inoculated on A. caffra (Figure 5b). This demonstrates that mistletoe seedlings perform better on the same host species as the parent plant and in the same site from which they were obtained.
DISCUSSION
Host abundance
Agelanthus natalitius parasitizes several host species and its local distribution can be patchy. Our study found Acacia karroo to be the most compatible host species for Agelanthus natalitius in two field sites in KwaZulu-Natal, South Africa, based on the fact that higher infection intensity (number of mistletoes per tree) was recorded on A. karroo. The reciprocal transplant germination experiment in the field also showed that overall the hypocotyls of Agelanthus natalitius seedlings grew better on A. karroo than A. caffra. This demonstrates a general preference by the mistletoe for the most abundant host species, A. karroo. These results were consistent with those of other studies demonstrating that host specificity can be influenced by host abundance, given that abundant host species are encountered most frequently and are more reliable hosts through space and time (López de Buen & Ornelas Reference LÓPEZ DE BUEN and ORNELAS2002, Norton & de Lange Reference NORTON and DE LANGE1999, Zuber & Widmer Reference ZUBER and WIDMER2000). In contrast, Roxburgh & Nicolson (Reference ROXBURGH and NICOLSON2005) found no relationship between observed prevalence among host species and compatibility of the mistletoe Plicosepalus kalachariensis in Zambia.
Many bird species disperse Agelanthus natalitius seeds to the same tree as the maternal plant or to nearby trees (Green et al. Reference GREEN, WARD and GRIFFITHS2009, Okubamichael et al. Reference OKUBAMICHAEL, GRIFFITHS and WARD2011a, Roxburgh Reference ROXBURGH2007). This may reduce colonization of new sites, but might improve chances of landing in a safe site (Norton & Carpenter Reference NORTON and CARPENTER1998, Norton & de Lange Reference NORTON and DE LANGE1999, Okubamichael et al. Reference OKUBAMICHAEL, GRIFFITHS and WARD2011a). Thus adaptation of a mistletoe to the most abundant host species would facilitate dispersal efficiency. Rare host species are less likely to receive mistletoe seeds by chance, except when birds differentially perch on those host species due to the presence of fleshy fruits or some other trait that would make them attractive to birds.
Host tree traits
Birds differentially disperse mistletoe seeds to tall trees (Aukema & Martínez del Rio Reference AUKEMA and MARTÍNEZ DEL RIO2002, Ward et al. Reference WARD, SHRESTHA and MUSLI2006), so any difference in size among potential host species could result in differential distribution of mistletoes among host trees. However, A. karroo and A. caffra trees did not differ in size (height and dbh) in either site, so differences in mistletoe infection cannot be attributed to size differences in the host species. We found that infected trees were taller and had a greater trunk diameter than uninfected host trees for both host species in both sites. This may be a consequence of the behaviour of dispersers, as birds differentially perch on tall trees and may deposit mistletoe seeds in the process. Moreover, tall trees are generally older and have had more time to become infected by mistletoes (Aukema & Martínez del Rio Reference AUKEMA and MARTÍNEZ DEL RIO2002, Donohue Reference DONOHUE1995, Overton Reference OVERTON1994). Thus, large trees are frequently observed to have a greater number of mistletoe infections than smaller trees (Aukema Reference AUKEMA2004, Donohue Reference DONOHUE1995, Roxburgh & Nicolson Reference ROXBURGH and NICOLSON2007, Ward et al. Reference WARD, SHRESTHA and MUSLI2006).
Overton (Reference OVERTON1994) explained the frequency of mistletoe infection as an accumulation function of infection with time as the tree gets older. As in previous studies (Aukema Reference AUKEMA2004, Donohue Reference DONOHUE1995, Roxburgh & Nicolson Reference ROXBURGH and NICOLSON2007), linear regression analysis in this study produced a weak correlation between height of tree and infection intensity. This analysis may be inappropriate, however, because the data in these other studies followed a negative binomial distribution. An alternative (GLIM) analysis explained the observed patterns better because the frequency distribution of the number of mistletoes per tree (infection intensity) is a good fit to the negative binomial distribution (i.e. most individual trees are free of mistletoes and a few individuals are highly infected). Previous infection increases the likelihood of further infection and it causes a clumped or aggregated distribution which is likely due to the limited dispersal distance of mistletoe seeds from their source (Aukema Reference AUKEMA2004, Overton Reference OVERTON1994, Ward & Paton Reference WARD and PATON2007).
There was a steep slope for the regression of tree height to mistletoe prevalence because birds differentially visit tall trees. Trees with big trunks are often tall but this is not always the case; this may explain why there is a weaker relationship between prevalence and dbh as compared with tree height. For example, at Highover, A. caffra trees were tall but they did not have big trunks. In addition, tall trees are usually more branched and provide more twigs with a suitable diameter than short trees (Sargent Reference SARGENT1995). Mistletoes deposited on tall trees also have greater success because tall trees are less likely to be shaded by neighbours, thereby providing adequate light for mistletoes (Lamont Reference LAMONT1982, Okubamichael et al. Reference OKUBAMICHAEL, GRIFFITHS and WARD2011a, Ward & Paton Reference WARD and PATON2007, Ward et al. Reference WARD, SHRESTHA and MUSLI2006). Tall trees may supply more nutrients and water to the mistletoes due to deeper and broader root systems (Ward et al. Reference WARD, SHRESTHA and MUSLI2006). Tall trees may also protect mistletoes from browsing by large herbivores (Roxburgh & Nicolson Reference ROXBURGH and NICOLSON2005, Reference ROXBURGH and NICOLSON2007). Mistletoes are often selected by herbivores over their host trees because the mistletoes have higher mineral and nitrogen concentrations and have few physical and chemical defence mechanisms. Thus, herbivores can limit mistletoes in natural communities (Midgley & Joubert Reference MIDGLEY and JOUBERT1991). Associated with this, Agelanthus natalitius growing on A. karroo may be better protected against foragers because A. karroo has longer spines than A. caffra and is better defended against herbivores (see also Martínez del Rio et al. Reference MARTÍNEZ DEL RIO, SILVA, MEDEL and HOURDEQUIN1996).
Reciprocal transplant experiment
Our study supports previous findings that germination of mistletoes is independent of site and substrate provided that the pericarp is removed (Ladley & Kelly Reference LADLEY and KELLY1996, Lamont Reference LAMONT, Calder and Bernhardt1983, Rödl & Ward Reference RÖDL and WARD2002, Roxburgh & Nicolson Reference ROXBURGH and NICOLSON2005). Hypocotyl length and growth form, however, showed crossed reaction norms, indicating that there were significant differences due to G × E interactions in this mistletoe species. The growth patterns of the hypocotyl varied based on site, source and the substrate on which they were placed. This implies that morphologically identical mistletoes may be genetically different, such that early seedling development is greatest when there is correspondence between maternal and seedling host species. Several studies also demonstrated that the development of the haustorium is more successful when mistletoe seeds are placed on their source host species (Arruda et al. Reference ARRUDA, CARVALHO and DEL-CLARO2006, Clay et al. Reference CLAY, DEMENT and REJMANEK1985, Rödl & Ward Reference RÖDL and WARD2002, Yan Reference YAN1993b). Unfortunately, measuring lifetime fitness differences in reciprocal transplant experiments in long-lived perennial plants requires more time than was available for this study. For this reason quantitative genetic analysis should be considered in future studies of the relationship between this mistletoe species and its hosts.
Host compatibility, host selection, host recognition, localization of gene flow and spatial segregation of host species or populations promote host race formation in mistletoe populations (Glazner et al. Reference GLAZNER, DEVLIN and ELLSTRAND1988). Our findings show that differences in host utilization of source and non-source host species may have a genetic basis, leading to differential success of individuals from one mistletoe population when grown on a different host species or in different areas. However, it is still not clear what mechanisms the mistletoes use to differentiate between hosts of different species and from different localities.
The haustorium is a distinct and unifying structure of parasitic plants. Thus, investigating haustorium formation and adaptation provides a holistic approach to study both infection patterns and host specificity in parasitic plants (Calvin & Wilson Reference CALVIN and WILSON2006, Thorogood et al. Reference THOROGOOD, RUMSEY and HISCOCK2009). Although the chemical interactions between aerial parasites and their hosts are as yet unstudied, it has been found in root parasites that host-derived chemicals stimulate germination and haustorium formation (Bouwmeester et al. Reference BOUWMEESTER, MATUSOVA, ZHONGKUI and BEALE2003, Chang & Lynn Reference CHANG and LYNN1986, Matvienko et al. Reference MATVIENKO, TORRES and YODER2001, Tomilov et al. Reference TOMILOV, TOMILOVA and YODER2004, Yoder Reference YODER1999). The results of this study suggest that chemical interactions between mistletoes and their host trees may occur during post-germination development and this may be the basis for the G × E interactions we observed at the early stage of the mistletoe growth (see also Runyon et al. Reference RUNYON, MESCHER and DE MORAES2006, Thorogood & Hiscock Reference THOROGOOD and HISCOCK2010).
Survival over 6 mo clearly showed that mistletoes transferred to non-source host species and to non-local sites had lower survival. This demonstrates that mistletoes have mechanisms that facilitate compatibility to their parental hosts. In addition, the fact that mistletoes on A. caffra did not show any difference in survival at Highover may indicate that this population of mistletoes uses A. caffra opportunistically and the mistletoes that can grow on A. caffra may not be as specific as those that grow on A. karroo at this site. We found that mistletoe seedlings had longer hypocotyls when grown on A. karroo, regardless of the host their parent plant occurred on, which could reflect an adaptation to the most frequently encountered host tree. Similar findings were reported by Norton & Carpenter (Reference NORTON and CARPENTER1998) and by Rödl & Ward (Reference RÖDL and WARD2002). Further studies on mistletoe performance (in terms of hypocotyl growth, survival and reproduction) should focus on combinations of reciprocal transplant experiments. For example, differences in mistletoe performance should be tested on parental versus non-parental hosts and preferred versus non-preferred hosts to determine the underlying mechanisms that determine host compatibility.
ACKNOWLEDGEMENTS
We thank Leeza Musthafa for help in the field and Vanessa Stuart for technical assistance. Many thanks for the hospitality and assistance of the managers at Highover and Mtontwane. We thank J.R. Kambatuku for comments on an earlier draft. We are grateful for the Claude Leon Foundation for postdoctoral research funding to M.E.G. and the National Research Foundation for funding to D.W.