INTRODUCTION
Anaspideans are herbivorous, solely marine animals with worldwide distribution. They occur in intertidal and subtidal environments and are important members of marine ecosystems (Kandel, Reference Kandel1979). Anaspideans are extensively used as models in neuroscience research (e.g. genus Aplysia) aimed at understanding the cellular basis of behaviour, due to the simplicity and relatively large size of their underlying neural circuitry (Carew, Reference Carew2000; Kandel et al., Reference Kandel, Schwartz and Jessell2000; Narusuye et al., Reference Narusuye, Hamaguchi and Nagahama2013). They are also of further interest because of their peculiar defensive strategies, including secretions from ink and opaline glands, which are mixed in the mantle cavity and released against attackers (Nusnbaum & Derby, Reference Nusnbaum and Derby2010; Nusnbaum et al., Reference Nusnbaum, Aggio and Derby2012).
As euopisthobranch heterobranchs (Jörger et al., Reference Jörger, Stöger, Kano, Fukuda, Knebelsberger and Schrödl2010; Wägele et al., Reference Wägele, Klussmann-Kolb, Verbeek and Schrödl2014), anaspideans are usually recovered as a sister group for pteropods (Euthecosomata and Gymnosomata) in molecular phylogenies (Klussmann-Kolb & Dinapoli, Reference Klussmann-Kolb and Dinapoli2006; Malaquias et al., Reference Malaquias, Mackenzie-Dodds, Bouchet, Gosliner and Reid2009; Göbbeler & Klussmann-Kolb, Reference Göbbeler and Klussmann-Kolb2011). The monophyly of Anaspidea is well accepted and corroborated by several molecular studies (e.g. Medina & Walsh, Reference Medina and Walsh2000; Grande et al., Reference Grande, Templado, Cervera and Zardoya2004; Vonnemann et al., Reference Vonnemann, Schrödl, Klussmann-Kolb and Wägele2005; Klussmann-Kolb & Dinapoli, Reference Klussmann-Kolb and Dinapoli2006; Malaquias et al., Reference Malaquias, Mackenzie-Dodds, Bouchet, Gosliner and Reid2009; Göbbeler & Klussmann-Kolb, Reference Göbbeler and Klussmann-Kolb2011). Morphological evidence for a monophyletic Anaspidea is also consistent and supported by synapomorphic characters such as an opaline gland, an ink gland, an atrial gland, a filter chamber and rodlets in the oesophagus (Mikkelsen, Reference Mikkelsen1996; Klussmann-Kolb, Reference Klussmann-Kolb2004). On the other hand, Klussmann-Kolb & Dinapoli (Reference Klussmann-Kolb and Dinapoli2006) did not confirm its monophyly in their molecular systematic investigation of Thecosomata and Gymnosomata, which included representatives of eight anaspidean genera (Akera, Aplysia, Syphonota, Bursatella, Stylocheilus, Dolabrifera, Notarchus and Dolabella), rendering the relationships within Anaspidea still poorly resolved.
As it is currently understood, Anaspidea contains two traditional superfamilies: Akeroidea, which includes a single family Akeriidae and genus Akera with five species, and Aplysioidea which includes the family Aplysiidae, with 11 genera and 70 species (Bouchet, Reference Bouchet2010). The genus Aplysia is the most diverse within the Aplysioidea, comprising 34 species (Bouchet & Gofas, Reference Bouchet and Gofas2010) and five subgenera (Eales, Reference Eales1960): Aplysia Linnaeus, Reference Linnaeus1767, characterized by a low, bulky body, a broad head and foot, metapodium producing a rounded sucker, parapodia joined high up posteriorly and a broad and unarmed penis; Phycophila Adams, Reference Adams1861, a poorly known subgenus, having a slender body, broad cephalic tentacles and narrow foot with a long metapodium; Neaplysia Cooper, Reference Cooper1863, with a single species (A. californica) bearing a remarkable rectangular flattening in the shell, described as an accessory plate; Pruvotaplysia Engel & Hummelinck, Reference Engel and Hummelinck1936 with a narrow foot, strongly concave shell, and similarly coloured (black) border of the parapodia, foot, shell foramen, rhinophores and cephalic tentacles; Varria Eales, Reference Eales1960, with parapodia joined low down posteriorly, usually uniporous opaline gland and filiform, unarmed penis.
Aplysia depilans Gmelin, Reference Gmelin and Gmelin1791 is the type species of the genus and subgenus Aplysia. It is a large and common species, with occurrence restricted to Europe (Grigg, Reference Grigg1949; Thompson, Reference Thompson1976) and adjacent areas, namely Senegal (Poddubetskaia, Reference Poddubetskaia2003), West Africa and Madeira (Eales, Reference Eales1960). Despite the nearly 200 years elapsed after its description, only certain aspects of its anatomy have been addressed, e.g. external morphology and radula (Bebbington & Thompson, Reference Bebbington and Thompson1968; Bebbington, Reference Bebbington1970; Thompson, Reference Thompson1976); shell (Rang, Reference Rang1828; Pruvot-Fol, Reference Pruvot-Fol1933; Grigg, Reference Grigg1949; Bebbington, Reference Bebbington1970, Reference Bebbington1975; Cossignani et al., Reference Cossignani, Cossignani, Nisio and Passamonti1992); opaline gland (Pilsbry, Reference Pilsbry1895; Pruvot-Fol, Reference Pruvot-Fol1954); hermaphrodite reproductive system (Mazzarelli, Reference Mazzarelli1891; Thompson & Bebbington, Reference Thompson and Bebbington1969); and nervous system (Mazzarelli, Reference Mazzarelli1893; Wirz, Reference Wirz1952; Kandel & Tauc, Reference Kandel and Tauc1965). On the other hand, the digestive system of A. depilans has been investigated in relative detail, with several articles addressing its histochemical and ultrastructural properties (Lobo-da-Cunha, Reference Lobo-da-Cunha1999, Reference Lobo-da-Cunha2000, Reference Lobo-da-Cunha2001, Reference Lobo-da-Cunha2002; Lobo-da-Cunha & Batista-Pinto, Reference Lobo-da-Cunha and Batista-Pinto2003, Reference Lobo-da-Cunha and Batista-Pinto2005, Reference Lobo-da-Cunha and Batista-Pinto2007).
Aplysia depilans is commonly referred to as distinct from other European species (e.g. A. fasciata Poiret, Reference Poiret1789 and A. punctata Cuvier, Reference Cuvier1803) in having a broad head, broad and flat salivary glands, stout penis, penis sheath, parapodia joined rather high posteriorly, and a broad metapodium widely known as a ‘pedal sucker’ (Eales, Reference Eales1960; Bebbington, Reference Bebbington1970). However, these characters are diagnostic characters of the subgenus Aplysia as stated by Eales (Reference Eales1960), who provided the most recent and comprehensive revision of the genus, followed by several authors (e.g. Thompson & Brown, Reference Thompson and Brown1984; Rudman, Reference Rudman1999a). Adding to the overall confusion, other five allied species were further included in the subgenus Aplysia: Aplysia cedrosensis Bartsch & Rehder, Reference Bartsch and Rehder1939 (from Cedros Island in the Gulf of California, USA); Aplysia dura Eales, Reference Eales1960 (from Tristan da Cunha, South Atlantic Ocean); Aplysia juliana Quoy & Gaimard, Reference Quoy and Gaimard1832 (worldwide in warm seas); Aplysia nigra d'Orbigny, Reference d'Orbigny1837 (Australia, New Zealand and Peru); Aplysia vaccaria Winkler, Reference Winkler1955 (Southern California, USA) (Eales, Reference Eales1960).
Regardless of the relatively numerous studies, the external morphology and few other attributes so far invoked have been insufficient to accurately define generic and subgeneric taxa in Aplysiidae, which includes considerations on previously neglected anatomical aspects. New insights on the use of phenotypic characters for taxonomic and phylogenetic purposes are discussed herein, in order to facilitate identification and provide a better understanding of the evolutionary trends involving such an important group. To begin this analysis with the type species of a taxon that reaches order level (Anaspidea) seems like a wise and indispensable approach, and, as such, is the main objective of this paper.
MATERIALS AND METHODS
Studied specimens were not photographed alive and are deposited in the Museu de Zoologia da Univesidade de São Paulo, MZSP (São Paulo, Brazil), Museu Nacional do Rio de Janeiro, MNRJ (Rio de Janeiro, Brazil), University Museum of Bergen, ZMBN (Natural History, Norway) and Zoologische Staatssammlung München, ZSM (Munich, Germany). Dissections were performed by standard techniques, with specimens immersed in the preservative fluid under a stereomicroscope. Drawings were done with the aid of a camera lucida.
Note: While total body length is provided for all specimens, relative measurements are given for internal structures (absolute measurements were not taken and are not available here, but all specimens are available for examination in museums).
Material examined
United Kingdom, England, Cornwall, St Ives, 1 spec, ZMBN 102270 (preserved, ~120 mm long). France; Bretagne; Finistère, ZSM 20070855, 1 spec (preserved, ~140 mm long, Exp. Uni-Exkursion 2007, Haszprunar coll., 06/vi/2007). Portugal, Madeira Island, Porto Island, MZSP 84023, 5 m depth, 2 spec (under rocks, 20/viii/2006, P. Rosa coll.); Peniche; Abalo Beach, MZSP 914909, 2 spec (2008, J. Jardim coll.); Setubal; Tróia, MZSP 84022, 3 spec (under rocks, 15/x/2006, P. Rosa coll.); Viana do Castelo, MZSP 91472, 3 spec (2008, J. Jardim coll.). Spain; Ria Formosa; Golfo Ibero-Marroquino, MNHN, 1 spec (P. Bouchet coll., 8/vi/1988). Greece, Creta; Heraklion, 2 m depth, MZSP 100438, 1 spec, MZSP 100441, 1 spec (08/v/2003, D. Poursanidis coll.). Italy; Gulf of Naples, MNRJ 2422, 1 spec (preserved, ~200 mm long).
Abbreviations used in the figures
aa, anterior aorta; ab, abdominal ganglia; ag, gastric aorta; al, anterior parapodial lobe; an, anus; ap, posterior aorta; as, anal siphon; au, auricle; av, auriculoventricular valve; bc, bursa copulatrix; bg, buccal ganglion; bm, buccal mass; ca, caecum; cb, cerebrobuccal connective; cc, concentration of cells; cd, commissure of pedal ganglia; ce, cerebral ganglion; cf, crop folds; cg, pleuropedal connective; ci, aortic crest; cm, circular muscle of crop; co, cerebropedal connective; cp, cerebropleural connective; cr.a, crop anterior chamber; cr.p, crop posterior chamber; dd, duct digestive gland; df, dorsal fold; dg, digestive gland; dm, dorsal muscles; dv, dorsoventral muscles; es, oesophagus; ey, eye; fc, filter chamber; fe, oesophageal folds; fs, foot sole (mesopodium); gc, gizzard cluster; gf, gastric fold; gi, gill; gn, genital nerve; go, gonad; gp, gizzard plates; gs, single gizzard plates; gu, contents of digestive system; gz, gizzard; hd, hermaphrodite duct; he, hermaphrodite pore; ho, gastric hooks; in, intestine; jw, jaw; ll, left lateral columellar muscle; lm, foot longitudinal muscles; lr, right lateral columellar muscle; ki, kidney; mb, mantle border; mo, mouth; mp, penis protractor muscle; mr, penis retractor muscle; ms, muscle sheath; mt, metapodium; ne, nephrostome; np, pericardial nerve; nr, nerve ring; oa, aperture of opaline gland; od, odontophore; op, opaline gland; os, osphradium; os.n, osphradium nerve; ot, oral tentacle; pa, parapodium; pc, pericardium; pd, pedal ganglion; pe, penis; ph, penis sheath; pl, pleural ganglion; pn, penis aperture; po, propodium; pp, parapedal commissure; pv, pleurovisceral connective; ra, radula; rh, rhinophore; rn, radulae nerve; rs, radular sac; rt, rectum; rv, renal vein; sn, spines of penis sheath; sf, shell foramen; sg, salivary gland; si, circulatory sinus; sm, mantle skirt nerve; so, salivary gland opening; sr, seminal groove; ss, sheath sac; st, stomach; sv, semilunar valve; tc, cephalic tentacle; tf, tentacle fold; tm, foot transversal muscles; ty, typhlosole; ve, ventricle; vd, ventral edge of lateral columellar muscle; vn, blood vein.
RESULTS
External morphology
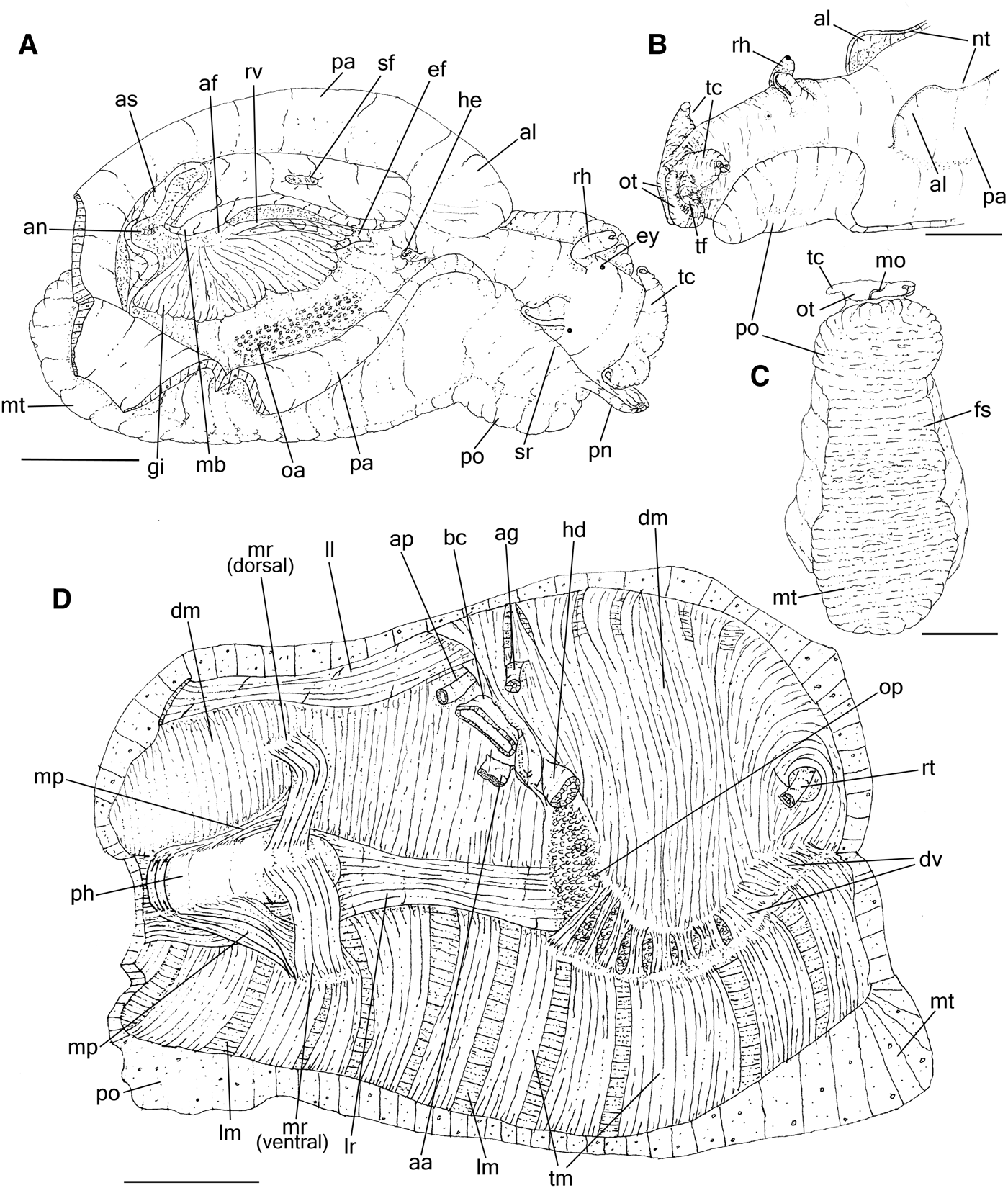
General features like rhinophores, cephalic tentacles, body shape and colour are described by Bebbington (Reference Bebbington1970) and Thompson (Reference Thompson1976); the body colour (Poddubetskaia, Reference Poddubetskaia2003) and shell (Cossignani et al., Reference Cossignani, Cossignani, Nisio and Passamonti1992; Cossignani & Ardovini, Reference Cossignani and Ardovini2011). Description complement: Size 120–140–200 mm. Parapodia (Figure 1A, B and Supporting Data S1A, B) about twice longer than wide, joined high posteriorly (jo); anterior edge slightly lobed, forming an anterior lobe (al) about a quarter of parapodia length, separated by a shallow notch more evident in live animals (Supporting Data S1B: nt). Foot (Figure 1C) dark grey, broad, muscular, with three well differentiated parts, each one about the same size in length, recognizable by muscular thickness: (1) propodium anteriorly rounded (Figure 1B, C and Supporting Data S1A, B: po), forming single lobe, muscular and thick; occupying same level as cephalic region, projecting laterally beyond the side of the body; (2) foot sole or mesopodium (Figure 1C: fs) as middle part of foot, about half of propodium thickness; (3) metapodium (Figure 1A, C: mt) slightly thicker than propodium, occupying posterior end of body, projecting posteriorly and laterally beyond the side of the body (Supporting Data S1A: mt).
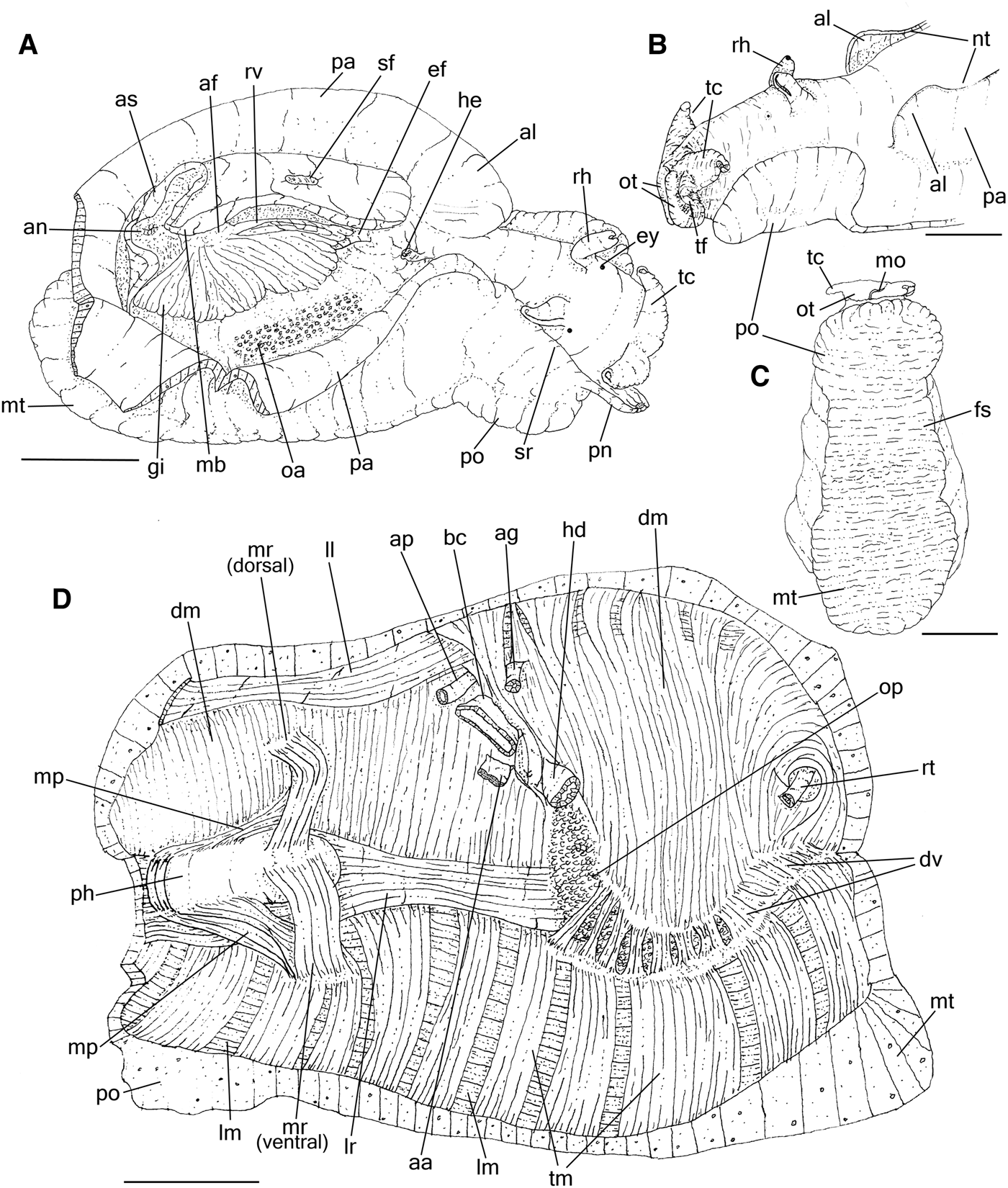
Fig. 1. Aplysia depilans, anatomical features; (A) right lateral whole view, some portions of right parapodium artificially sectioned, pallial roof deflected upwards; (B) detail of head, left view; (C) whole ventral view; (D) whole view of hemocoel, opened by longitudinal section along left side, left view, most inner structures removed. Scale bars: 20 mm. See Box for abbreviations used in figure.
Hemocoel muscles
Similar to that described by Brace (Reference Brace1977a) in A. punctata. Description complement: Pair of lateral columellar muscles (Figure 1D) right (lr) and left (ll), flattened, as large as buccal mass, about 4 times longer than wide, almost completely adhered to inner wall of hemocoel, except for free ventral edge corresponding about a quarter of muscles width (Figure 2A: vd); on right side crossing between hemocoel wall and penis sheath, skirting connection point of penis sheath with hemocoel wall.

Fig. 2. Aplysia depilans, reno-circulatory structures; (A) whole view of pericardial region, right-dorsal view, pallial roof and adjacent integument removed, most structures shown as in situ; (B) isolated heart, ventral view; (C) heart opened longitudinally, dorsal view. Scale bars: 10 mm. See Box for abbreviations used in figure.
Circulatory and excretory systems
General aspects as described by Eales (Reference Eales1921) in Aplysia punctata. Description complement: Gill (Figures 1A & 2A: gi) large, length about a third of body length, almost completely covered by the mantle, attached along the left wall of the mantle cavity; gill supported by a thick connection in the anterior edge and posterior edge of the mantle cavity, which are connected by the renal vein (rv) running attached in the left wall; afferent blood veins in posterior end of the gill, rise from two sinuses (si) coming from left and right sides of body wall respectively; the anterior edge receiving the efferent blood from the gill and renal veins from the kidney (ki). Pericardium (pc), large, about a third of gill length, rounded, longer than wide, located just antero-dorsally to pallial cavity, immediately anterior to shell. Heart (Figure 2A–C) occupying almost entire pericardial cavity. Auricle (au) wall thin, delicate, whitish; inner wall covered by interlacing strands of thin fibrous tissue; posterior to and slightly larger than ventricle. Ventricle (ve) thick, walls opaque and muscular. Connection between auricle and ventricle marked by constriction of pair of auriculo-ventricular valves (av), concentric flaps on each side of lumen, width about a third of ventricle width (av). Semilunar valve (sv) located in lumen of ventricle to aortic crest, composed of single semi-concentric flap, width less than a quarter of aortic crest width. Aortic crest (ci) arising antero-ventrally from ventricle, glandular, flattened, about three times longer than wide, ventral edges fixed on ventral wall of pericardium; its right end giving rise to anterior aorta (aa); left end giving rise to posterior (ap) and gastric (ag) aortas, both smaller, about a third of anterior aorta diameter. Kidney (ki) flattened, located dorso-laterally in the hemocoel wall, overlying posterior end of pericardium and visceral hump, covered by shell, colour pale-beige; reno-pericardial duct not seen. Nephrostome (ne) small, located on left and bottom of cavity, near base of gill.
Digestive system
As described by Eales (Reference Eales1921) and Howells (Reference Howells1942) in A. punctata. Radula characters observed here (Supporting Information S2) agree with previous knowledge on A. depilans (Pruvot-Fol, Reference Pruvot-Fol1954; Bebbington & Thompson, Reference Bebbington and Thompson1968; Bebbington, Reference Bebbington1970; Thompson, Reference Thompson1976). Description complement: Buccal mass cylindrical, length less than a quarter of digestive system length. Pair of jaw plates (Figure 3C: jw) large, thick, occupying about a quarter of dorsal anterior surface of oral cavity; composed of several rods (Figure 4D); rods slender, slightly laterally compressed, tip narrow and lobed. Pair of dorsal folds (df) of buccal cavity wide, running close to each other. Pair of long salivary gland (sg) ducts very narrow, running posteriorly, passing through nerve ring, attached to anterior edge gizzard; opening (so) in middle region of dorsal folds. Odontophore spherical (Figure 3C: od), occupying about half of oral cavity volume. Oesophagus (Figure 3A, C, D: es) walls thick, dark grey, longitudinally folded by narrow and tall folds; originating in dorsal region of buccal mass end, length about the same buccal mass length, presenting a constriction at posterior end delimitating junction with crop. Crop (Figure 3A, D: cr) small, reddish, about same volume as buccal mass, walls thick and muscular, externally covered by thin muscular fibres; internally chaotically folded (cf) by broad and low folds; thick circular muscle of crop (cm) present in middle region of crop, providing mild separation into anterior (cr.a) and posterior (cr.p) chambers of similar volume. Gizzard (Figures 3A, D & 4B: gz) spherical, walls thick, about same size as crop, presenting about 12 main gizzard plates (Figure 4A–C: gp); plates outline pyramidal, cream whitish to reddish, clustered into 2 lateral groups of 6 teeth each (Figure 4B: gc) and narrower and taller teeth located between them at posterior edge of gizzard (Figure 4B: gs); anterior edge covered by small gizzard plates, length less than a quarter of gizzards plate length; posterior end marked by muscular constriction; marking anterior edge of filter chamber (Figures 3A, D & 4B: fc), walls muscular, thinner and shorter than gizzard, occupying about a third of gizzard volume, anterior region slightly wider than posterior region, covered and crowded by small gastric hooks (Figure 4A, B: ho), which are smaller and slender than half gizzard plate length, base shape hexagonal and tip pointed, as tall as its base (Figure 4A). Stomach (Figure 3A, D: st) consisting of a small chamber, size equivalent to length of filter chamber; walls muscular, thin, located embedded in right anterior end of digestive gland; pair of gastric folds (Figure 3D: gf) running longitudinally on lateral wall, covered by irregular folds, wider than tall; anterior edge about twice as wide as posterior edge, ending in anterior edge of stomach close to hooks of filter chamber. Duct to digestive gland (Figure 3D: dg) opening dorso-posteriorly to each gastric valve. Caecum (Figure 3A, B, D: ca) walls thin, diameter about a third of stomach diameter and as long as digestive gland length; running ventrally almost completely embedded by digestive gland, tip coiled (1/2 whorls) and touching end on right side of digestive gland; aperture located in left ventroposterior end of stomach, close to origin of intestine; typhlosole (ty) dividing lumen by two longitudinal folds tall, extending along entire length of caecum, continuing in intestine, for about same length as stomach, distancing from one another and returning to join, forming arc. Intestine (Figure 3A, B, D: in) very thin walled, long, origin close to caecum aperture, inner surface mostly smooth. Rectum (Figures 1D & 3A, B: rt) short, passing through dorsal roof of hemocoel. Anus (Figure 1A: an) consisting of a very short papilla located at middle of anal siphon.
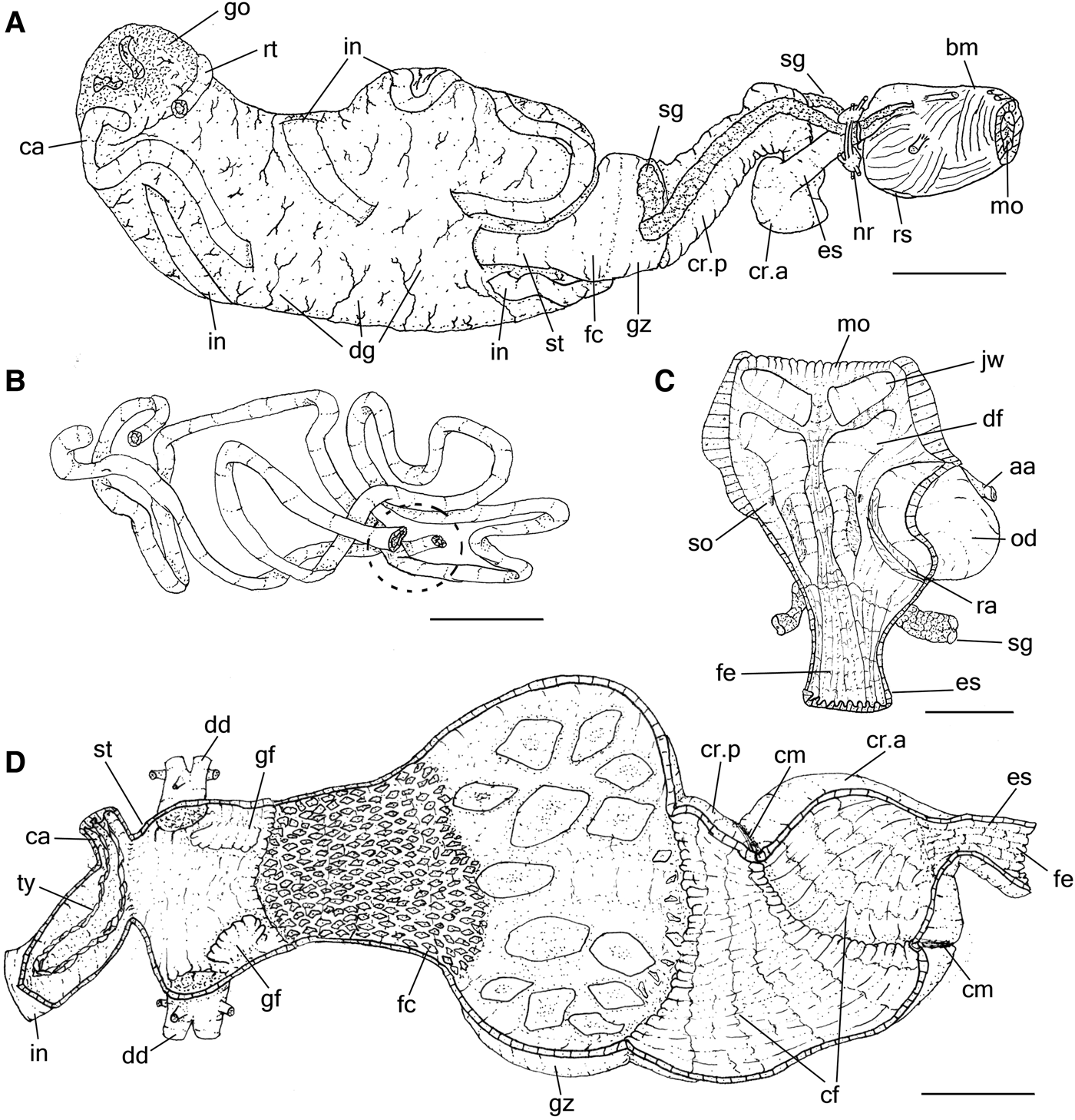
Fig. 3. Aplysia depilans, details of digestive system; (A) whole right view, most structures as in situ; (B) mid and hindgut loops as in situ, right view, some portions artificially sectioned, dotted region related to the stomach area; (C) buccal mass, ventral view, opened longitudinally; (D) fore and midgut, dorsal view, almost completely sectioned longitudinally to show inner surface. Scale bars: A, B and D, 10 mm; C, 5 mm. See Box for abbreviations used in figure.
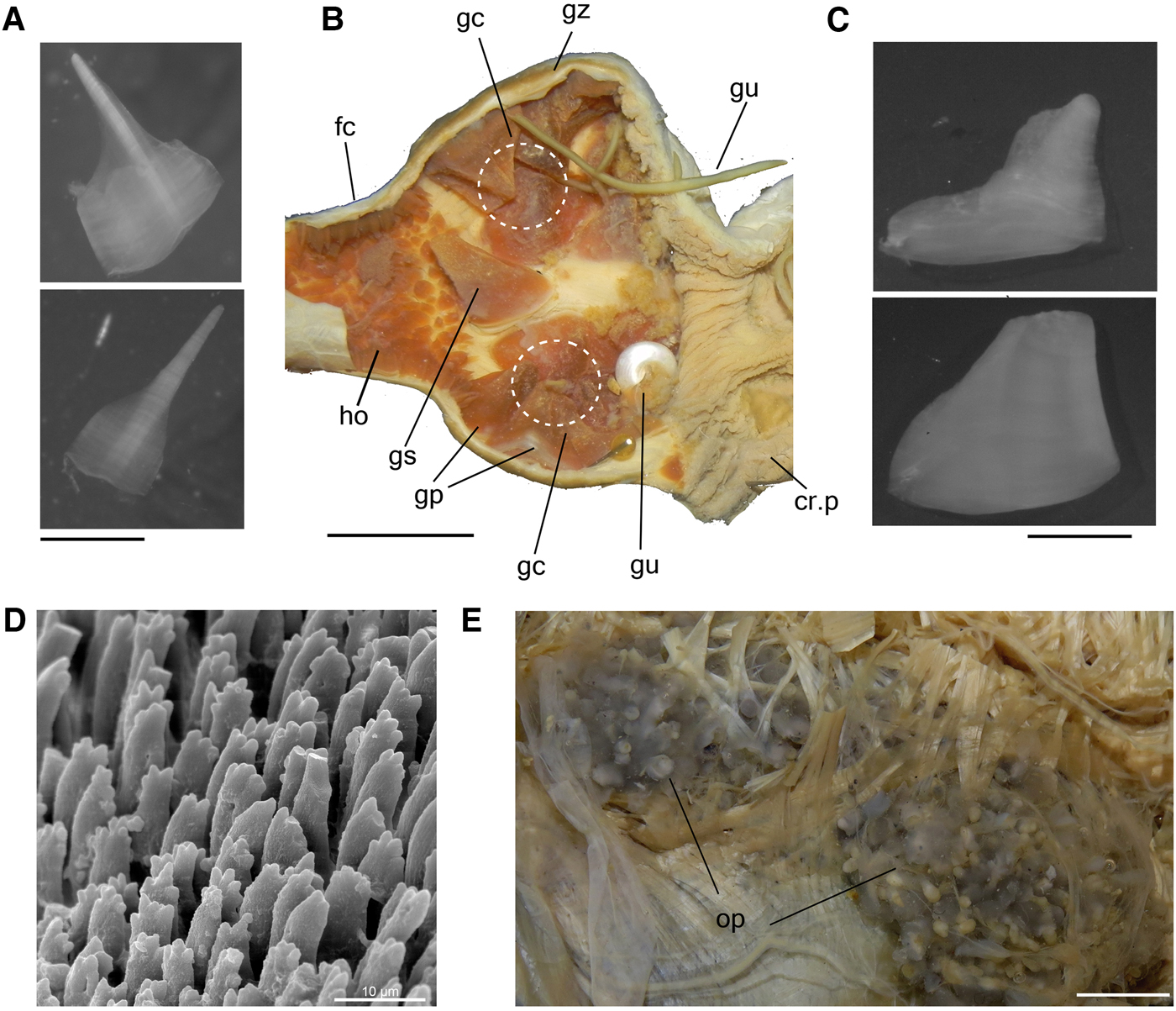
Fig. 4. Aplysia depilans, details of digestive system; (A) a pair of gastric hooks; (B) gizzard, dorsal view, opened longitudinally to show inner surface, circle indicating clusters; (C) gizzard plates; (D) rods of jaw in SEM, middle region; (E) opaline grand in situ. Scale bars: A, C, 2 mm; B, 10 mm; D, 10 µm; E, 5 mm. See Box for abbreviations used in figure.
Reproductive system
Hermaphrodite reproductive system similar to those described by Mazzarelli (Reference Mazzarelli1891) and Thompson & Bebbington (Reference Thompson and Bebbington1969) for A. depilans; the hermaphroditic duct open externally as the common genital aperture, seminal groove running anteriorly on the right side of the body to the penial aperture, located on the right dorsal side of the head, close to the cephalic tentacle. For the penis characters see Mazzarelli (Reference Mazzarelli1893), Bebbington & Thompson (Reference Bebbington and Thompson1968) and Thompson (Reference Thompson1976). Description complement: Penis sheath (Figures 1D & 5A: ph), length about a quarter of hemocoel length, located just between right anterior inner hemocoel wall and buccal mass, size equivalent to buccal mass length; divided into a muscle sheath, proximal to the hemocoel wall, and the sheath sac, distal edge; both limited by a point of thickening of seminal groove. Muscle sheath of penis sheath (Figure 5A: ms) limited anteriorly by sheath opening and posteriorly by thickening of seminal groove, wall thick, muscular, right inner wall highly folded, covered by small spines (Figure 5A, C, D: sn); opposite side smooth, limited by longitudinal fold housing continuation of seminal groove (sr); sheath sac (Figure 5A: ss) distal end of sheath, limited anteriorly by thickening of seminal groove; length about half of muscle sheath length, wall thin, having fewer and smaller spines (Figure 5E) organized radially on inner wall. Penis (Figure 5A, B: pe) black in colour, spatulate in shape, surface smooth, as long as sheath length. Penis retractor muscle (Figures 1D & 5A: mr) as a pair, short, about 4 times longer than wide, origin on lateral wall of sheath sac; dorsal branch inserting on dorsal inner surface of hemocoel, just at internal level between rhinophores; ventral branch inserting at lateroventral edge of hemocoel, just ventral to ventral edge of right lateral columellar muscle (Figure 1D). Penis protractor muscle (Figures 1D & 5A: mp) consisting of several fibres, origin at ventral and dorsal anterior edge of penis sheath, length about half of sheath length, longitudinally aligned, running posteriorly; dorsal branch inserting at anterior edge of dorsal branch of penis retractor muscle, and ventral branch inserting near insertion of ventral branch of penis retractor muscle.

Fig. 5. Aplysia depilans penis and sheath, the arrows showing the seminal groove; (A) penis opened longitudinally, some adjacent structures also shown; (B) penis, transverse section in middle level; (C) middle region of muscle sheath of young specimen, SEM (MZSP 91409); (D) same structure of adult; (E) SEM of sheath sac of same specimen. Scale bars: A, 5 mm; B, 2 mm; C, 100 µm; D, 300 µm; E, 60 µm. See Box for abbreviations used in figure.
Central nervous system
Like that described by Mazzarelli (Reference Mazzarelli1893) and Wirz (Reference Wirz1952). Description complement: Nerve ring (Figure 6A, B) located posteriorly to buccal mass and vertically to the visceral mass (Figure 3A: nr), surrounding oesophagus and salivary glands. Cerebral ganglia (ce), fused with each other, leaving connectives, width about the same pedal ganglion width, slightly rounded: cerebropleural (cp), short, length equivalent to pleural ganglion length; cerebropedal (co), about twice longer than cerebropleural connectives; cerebrobuccal (cb) surrounding anterior portion of oesophagus, long, about a third longer than the nervous ring length. Buccal ganglia (bg), about half of cerebral ganglia width, partially fused, with readily visible bundles; radulae nerve (rn) arising on posterior surface, thick, attaching to buccal mass ganglion, located below and close to connection of buccal mass and oesophagus. Pedal ganglia (pd) slightly rounded, largest ganglia in nerve ring, each ganglion about twice as large as cerebral ganglia; pedal commissure (cd) short, width about half of pedal ganglion width; parapedal commissure (pp) arising ventrally, surrounding anterior aorta, about twice as long and more slender than pedal commissure. Pleural ganglia (pl) asymmetric, slightly elongated in axis direction of cerebropleural connective; right ganglion about one third wider than cerebral ganglia and left ganglion about half of right ganglion length. Pleurovisceral connective (pv) arising from each pleural ganglion, about twice as long as nervous ring length, running towards posterior region and connecting to the abdominal ganglia (ab), which is bilobed; right hemisphere, emitting mainly osphradial (os.n) and pericardial (np) nerves; left hemisphere emitting genital (gn) and mantle skirt (sn) nerves. Presence of concentration of cells (cc) anterior to each hemisphere, producing a bulge. Pleurovisceral loop (streptoneurous condition) crossing in a point close in the middle of pleurovisceral connectives.
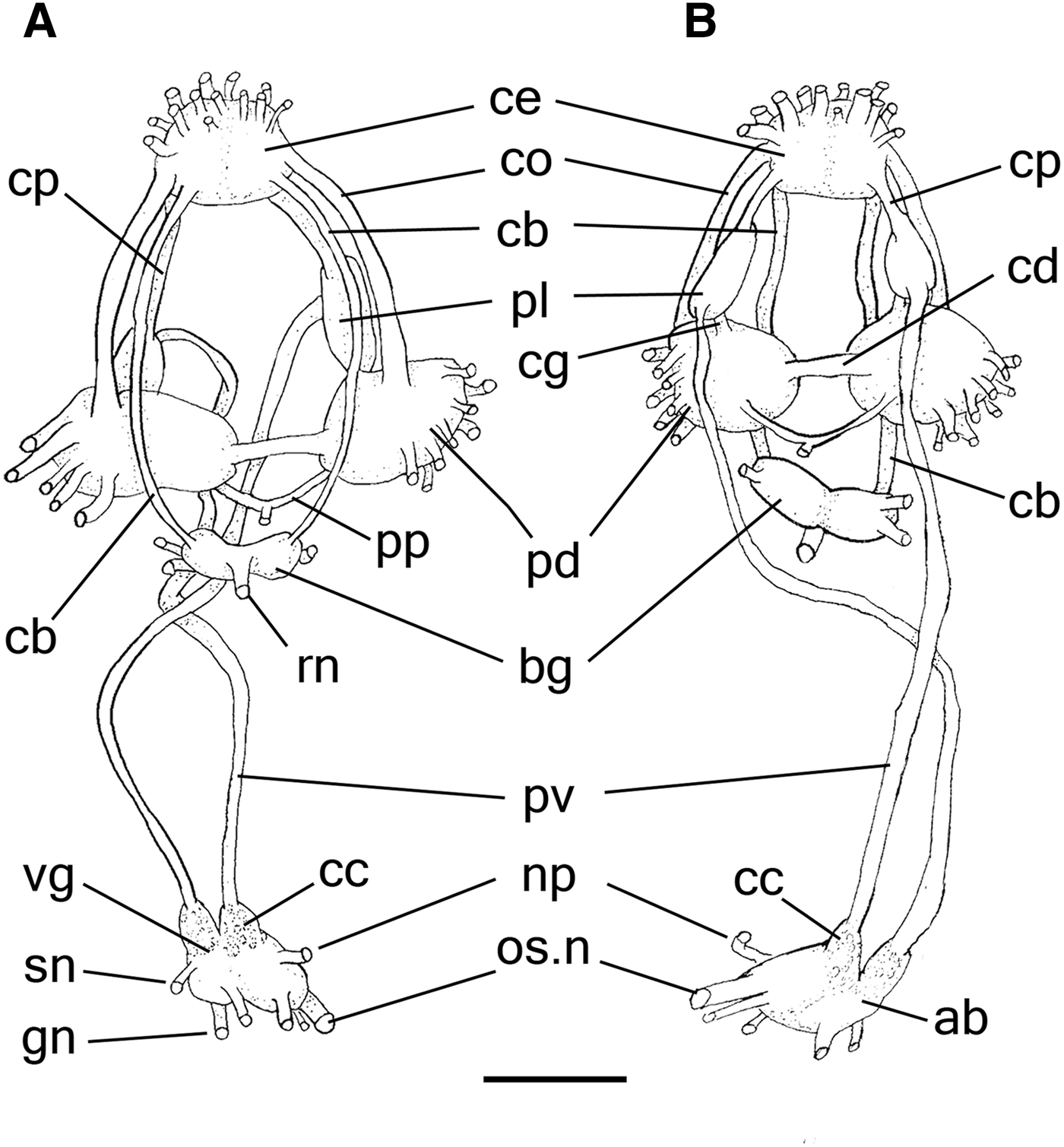
Fig. 6. Aplysia depilans, central nervous system (nerve ring); (A) anterior view. (B) posterior view. Scale bar: A, B, 2 mm. See Box for abbreviations used in figure.
DISCUSSION
Cephalic tentacles
Except for Akera, a pair of rolled cephalic tentacles is present in all other anaspideans. Klussmann-Kolb (Reference Klussmann-Kolb2004: character 5) reported to the genera Aplysia and Syphonota as having cephalic tentacle lobes joined in the median region, forming a large veil (see Hoffmann, Reference Hoffmann and Bronns1939). In the same paper, Klussmann-Kolb (Reference Klussmann-Kolb2004: character 8) stated that the genera Akera, Aplysia, Syphonota and Dolabella lack oral lobes. Hence, it, in fact, represents a fusion of cephalic and oral tentacles, once its base is located at the lateral edge of the mouth. Though it is relatively hard to distinguish, we observed a fold marking the delimitation between the fused tentacles (Figure 1B: tf).
Parapodia
Connected with the large metapodium (see below), the parapodia joined posteriorly in a point above half the height of the parapodia is a remarkable characteristic of the subgenus Aplysia (Eales, Reference Eales1960), as observed here and reported in the literature (Thompson, Reference Thompson1976; Thompson & Brown, Reference Thompson and Brown1984). Rather than favouring natation (Grigg, Reference Grigg1949; Eales, Reference Eales1960; Thompson, Reference Thompson1976), the shape of the parapodia in A. depilans would prevent it. This is different from most species of Aplysia, in which the parapodia are wide, also fused posteriorly with each other, but in a point beneath half the height of the parapodia, near the metapodium, giving the animal greater swimming capacity (e.g. A. dactylomela Rang, Reference Rang1828 and A. fasciata).
Although the parapodium is a conspicuous character virtually addressed in every taxonomic study on Aplysioidea, we observed a lobation of its anterior edge in A. depilans (Figure 1A: al). Rudman (Reference Rudman2003a) noticed a similar lobation in A. parvula Mörch, Reference Mörch1863, describing it as ‘almost semi-permanent lobes on the parapodial edge’, which he additionally remarked as ‘a feature’ of that species. Furthermore, the narrow foot of A. parvula could explain its ability to swim. This anterior lobation of the parapodia appears to be a recurrent character among anaspideans, e.g. Saad et al. (Reference Saad, Cunha, Colpo and Valdés2014) in A. brasiliana Rang, Reference Rang1828; Bebbington (Reference Bebbington1974) in A. juliana; Rudman (Reference Rudman1999c) in Syphonota geographica (Adams & Reeve, Reference Adams, Reeve and Adams1850); Zhang (Reference Zhang2009) in Bursatella leachii de Blainville, Reference de Blainville and Cuvier1817; Rudman (Reference Rudman2006) in Stylocheilus longicauda (Quoy & Gaimard, Reference Quoy and Gaimard1825); Revell (Reference Revell2002) in Stylocheilus striatus (Quoy & Gaimard, Reference Quoy and Gaimard1832); Rudman (Reference Rudman2003b) in Dolabrifera brazieri Sowerby II, Reference Sowerby1870. As was well demonstrated by Rudman (Reference Rudman1999b) in Dolabella auricularia (Lightfoot, Reference Lightfoot1786), the anterior edge of the parapodia could work like an inhalant siphon, drawing water into the enclosed mantle cavity and irrigating the gills. On the other hand, this is not clear in species of Dolabrifera, Phyllaplysia and Petalifera, which have the right parapodium much more developed than the left one (Bebbington, Reference Bebbington1974). Parapodium lobation is another step toward understanding the origin, evolution and function of the parapodia in Anaspidea, as well as character relationships within Euopisthobranchia. Although the presence of parapodia is also a feature uniting Cephalaspidea s.s., Anaspidea and Pteropoda, its homology within these groups has not yet been clarified (Klussmann-Kolb & Dinapoli, Reference Klussmann-Kolb and Dinapoli2006).
Propodium
An evident propodium is present in A. depilans (Figures 1A, B and Supporting Data S1A, B: po; Thompson, Reference Thompson1976: figure 78A–C), forming a single, anteriorly rounded, muscular and thick lobe, and extending laterally along the cephalic region (Figure 1A: po). Although a developed propodium is present in other aplysiids, e.g. Saad et al. (Reference Saad, Cunha, Colpo and Valdés2014; A. brasiliana), Bebbington & Brown (Reference Bebbington and Brown1975; A. parvula), Eales (Reference Eales1960; A. dura), it is more inconspicuous in A. depilans.
Metapodium
An analysis of the metapodium is relevant as it is a very variable structure among anaspideans. According to Klussmann-Kolb's character 16 (Reference Klussmann-Kolb2004), the size of the foot sole, or mesopodium, can be small or narrow when compared to the size of the lateral bulge of the parapodia (Akera bullata O.F. Müller, Reference Müller1776, Syphonota geographica, Aplysa parvula, A. punctata, A. extraordinaria (Allan, Reference Allan1932), A. dactylomela), or broad and oval (Dolabella auricularia, Dolabrifera. dolabrifera Rang, Reference Rang1828, Dolabrifera brazieri, Phyllaplysia taylori Dall, Reference Dall1900, Petalifera petalifera Rang, Reference Rang1828, Petalifera ramosa Baba, Reference Baba1959). Additionally, the same author stated that the posterior end (=metapodium) generally forms an elongate tail (except for Akera, where a metapodium is missing), whereas in the remaining Aplysiidae it is broadened, and the tail is reduced, producing an oval, slightly elongate shape.
A broad metapodium is known as a ‘pedal sucker’ because of its pressurizing function that keeps the animal attached to the substrate, especially rocks (Bebbington, Reference Bebbington1970). Aplysia depilans has a broad and thick muscular metapodium that does not agree with Klussmann-Kolb's (Reference Klussmann-Kolb2004) observations regarding Aplysia, since it is a character of species in the subgenus Aplysia and no such representatives were included in her study. We noticed in A. depilans that the lateral edge of the metapodium is continuous with the sole (mesopodium). It is thus different from A. juliana (Pruvot-Fol, Reference Pruvot-Fol1933; Engel & Eales, Reference Engel and Eales1957), which possesses a peculiar and well-defined posterior muscular disc that is completely separated from the sole. We were not able to compare it to other species within the same subgenus since the metapodium is usually not described in detail.
Opaline gland
Anaspideans possess a large gland near the mantle floor, known as the opaline gland or gland of Bohadsch (Mazzarelli, Reference Mazzarelli1890). According to Gosliner (Reference Gosliner, Harrison and Kohn1994) it is white in colour and produces a milky white secretion that blends itself with the ink gland's secretion when the animal is disturbed. The outline of the opaline gland in A. depilans is multiparous: the gland is splayed on the dorsal wall of the hemocoel, with multiple external openings on the pallial cavity's floor beneath the gill (Pilsbry, Reference Pilsbry1895; Pruvot-Fol, Reference Pruvot-Fol1954; Eales, Reference Eales1960). Eales (Reference Eales1960) referred to this gland in A. depilans as ‘pigmented’, secreting a white or ‘rarely a black’, copious and odoriferous fluid. We were not able to verify this, as we have only investigated preserved specimens. However, photos of A. depilans in natural environments show the presence of mixed purple (ink gland of mantle edge) and white fluids supposedly secreted by the opaline gland (Supporting Data S1B: ik), as described for other species of Aplysia (Eales, Reference Eales1960). Moreover, in all specimens analysed herein, we found a distinct opaline gland of dark grey to black colouration, a unique feature among aplysiids. Currently, A. depilans is the sole species of the subgenus Aplysia that occurs in Europe, and this character could be useful to distinguish it from other congeners. This is an important point since A. juliana is supposedly a cosmopolitan species (Eales, Reference Eales1960). It has been recorded in areas off the European coast, such as the Azores (Malaquias et al., Reference Malaquias, Mackenzie-Dodds, Bouchet, Gosliner and Reid2009), but it is not known from Europe. The same holds true for California (USA), where the related species of the subgenus Aplysia are A. cedrosensis, A. reticulopoda Beeman, Reference Beeman1960 and A. vaccaria (Bebbington, Reference Bebbington1977).
Columellar muscle
Important accounts concerning the columellar muscle of Akera bullata and Aplysia punctata were provided by Brace (respectively, Reference Brace1977a, Reference Brace1977b). At most, it is still a poorly investigated structure in Anaspidea. After analyses of other anaspideans (e.g. A. fasciata), the fact that A. depilans has the lateral columellar muscle running almost completely attached to the hemocoel wall, except for a free ventral edge (Figure 2A), caught our attention. This character deserves more investigation, as those muscles have different degrees of adherence to the hemocoel wall among anaspideans. Except for the origin and insertion, the muscles run completely free in species of Dolabrifera, Dolabella, Bursatella and Notarchus (personal observation).
Heart
The heart of Aplysia is an important model for scientific experiments (e.g. Souza & Scemes, Reference Souza and Scemes2000; Souza et al., Reference Souza, Stucchi-Zucchi, Cassola and Scemes2002; Jung et al., Reference Jung, Kavanaugh, Sun and Tsai2014). Nevertheless, no studies have addressed the morphology of this organ, especially from a taxonomic perspective. The heart of A. depilans is like that described for other monotocardian gastropods, with an auricle and ventricle (Figure 2B–C). Additionally, an aortic crest is present (Figure 2B: ci), arising anteroventrally from the ventricle; it is glandular and flattened, with its ventral edges adhering to the ventral wall of the pericardium (Eales, Reference Eales1921).
The aortic crest is located inside the pericardial cavity in A. depilans, whereas the other analogous blood glands are independent of it, as in Nudibranchia (Grande et al., Reference Grande, Templado, Cervera and Zardoya2004). Still, it is possible that this character has been poorly described in Euthyneura since the observation of blood glands is difficult in preserved specimens (Dayrat & Tillier, Reference Dayrat and Tillier2002). Focusing on other aplysiids, Cuvier (Reference Cuvier1803) described the aortic crest of A. fasciata as a chamber anterior to the ventricle. Mazzarelli (Reference Mazzarelli1893) and Eales (Reference Eales1921) adopted the name aortic crest henceforth, but Fujisawa et al. (Reference Fujisawa, Furukawa, Ohta, Ellis, Dembrow, Li, Floyd, Sweedler, Minakata, Nakamaru, Morishita, Matsushima, Weiss and Vilim1999) used the name to describe the heart innervation of A. kurodai Baba, Reference Baba1937. Guiart (Reference Guiart1901) schematically showed an aortic crest in Aplysia and Acera (=Akera), a structure apparently wanting in the non-anaspidean genera Acteon, Scaphander, Haminoea, Philine, Gastropteron, Doridium (=Aglaja) and Oscanius (=Pleurobranchus). Nonetheless, this issue needs to be further investigated, despite the hearts illustrated by Pruvot-Fol (Reference Pruvot-Fol1933) (Aclesia (=Stylocheilus) rufa Quoy & Gaimard, Reference Quoy and Gaimard1832) and Risbec (Reference Risbec1928) (Dolabella rumphii Cuvier, Reference Cuvier1817 and Aplysia odorata Risbec, Reference Risbec1928), not bringing any mention of the aortic crest.
A pair of auriculoventricular valves is found in the connection between the auricle and the ventricle, and a semilunar valve can be found in the lumen of the connection between the ventricle and the aorta (Kodirov, Reference Kodirov2011). Such structures are rare but known within a few other molluscs (Brand, Reference Brand1972; Kodirov, Reference Kodirov2011; Seo et al., Reference Seo, Ohishi, Maruyama, Imaizumi-Ohashi, Murakami and Seo2014). Cuvier (Reference Cuvier1803) was the first to report the presence of an ‘aortic valve’ between the ventricle and the auricle (auriculoventricular) in A. punctata. Fujisawa et al. (Reference Fujisawa, Furukawa, Ohta, Ellis, Dembrow, Li, Floyd, Sweedler, Minakata, Nakamaru, Morishita, Matsushima, Weiss and Vilim1999) showed the innervation of the heart valves in A. kurodai. We, in fact, found a pair of auriculoventricular valves (av) and a semilunar valve (sv) in the lumen of the ventricle to aortic crest (Figure 2C) in A. depilans, which appears to be a unique character of the family.
Digestive system
The oesophagus connects the buccal mass with the stomach, clearly demarcated by the presence of the duct(s) to the digestive gland. The oesophagus is a simple tube in most mollusc classes and in the basal gastropods, but becomes more and more complex, bearing valve and glands, in more advanced gastropod forms (Simone, Reference Simone2011). In anaspideans, the crop, the gizzard and the filter chambers are located between the buccal mass and the stomach, which allows the interpretation that those structures may be homologous to the entire oesophagus of most gastropods. Due to that plesiomorphic state, we consider these three structures as modifications and adaptations of the oesophageal region to process food before it reaches the stomach and, subsequently, is forwarded to the digestive gland.
The crop of euthyneuran gastropods (including Anaspidea) is often observable as a dilatation of the oesophagus, but its definition and delimitation are unclear. In fact, it is often difficult to distinguish the crop from other oesophageal regions (Dayrat & Tillier, Reference Dayrat and Tillier2002). Klussmann-Kolb (Reference Klussmann-Kolb2004: Figure 7A) treated the oesophagus and the crop as a single structure, although it is possible to differentiate them in her figure of the digestive system of A. dactylomela. Our observations have shown that the oesophagus of A. depilans is a conspicuous tubular structure with thick walls. It is about the same buccal mass, and its internal surface is longitudinally folded (Figure 3C, D). It can be clearly separated from the crop by a posterior constriction, and clearly differs in having a greyish internal wall, while the crop is cream-yellowish and has taller longitudinal folds. The distinction between the oesophagus and the crop is well known among anaspideans (e.g. Akera bullata and Notarchus punctatus Philippi, Reference Philippi1836 (Guiart, Reference Guiart1901); A. punctata (Eales, Reference Eales1921; Howells, Reference Howells1942); A. fasciata (Bebbington & Thompson, Reference Bebbington and Thompson1968)). However, this issue is not clear in basal species of the genus Akera. A possible oesophageal crop was described as an aplysiid-exclusive structure by Morton & Holme (Reference Morton and Holme1955), as a crop in Akera bullata, and as an ‘oesophagus’ by Marcus & Marcus (Reference Marcus and Marcus1967) in the original description of Akera bayeri.
In the present paper, we describe the presence of a circular muscle layer in the crop walls (Figure 3D: cm) for the first time in the literature. This muscle is relatively easily observed in an external view of the crop. The crop of Aplysia depilans has two inflated parts, separated by a mild constriction. A similar organization, yet much more evident, was figured by Marcus & Marcus (Reference Marcus and Marcus1955: pl 4, Figure 30) for Aplysia juliana from Brazil and Klussmann-Kolb (Reference Klussmann-Kolb2004: Figure 7A) for Aplysia dactylomela (=A. argus) from Australia. Furthermore, Aplysia depilans and Aplysia juliana differ from A. dactylomela by having anterior and posterior chambers of similar volume separated by a constriction in the middle region. Marcus & Marcus (Reference Marcus and Marcus1955) have even illustrated the oesophagus twice; hence we understand that the ‘oesophagus’ figured in the middle of the crop is, in fact, the circular muscle of the crop (Figure 3D: cm). A similar approach can be applied to the constriction between ‘crop’ and ‘first gizzard’ reported by Rudman (Reference Rudman2004) in Aplysia depilans. We agree with the assumption of Dayrat & Tillier (Reference Dayrat and Tillier2002) that more data are needed to investigate the homology of the oesophagus and the crop, and our description of the circular muscle represents an additional step toward better understanding this structure.
The gizzard and the filter chamber (see Howells, Reference Howells1942) are located between the crop and stomach; and both are corneous structures (Mikkelsen, Reference Mikkelsen1996). Numerous terms have been employed to designate these structures, as well as the crop itself. They have been named (respectively): second and third region (Cuvier, Reference Cuvier1803; Rudman, Reference Rudman2004), first and second triturating stomach (Zuccardi, Reference Zuccardi1890; Mazzarelli, Reference Mazzarelli1893; Enriques, Reference Enriques1902), both as gizzard (Guiart, Reference Guiart1901), ingluvies or first stomach and muscular band of second stomach or grinding stomach (MacFarland, Reference Macfarland1909), anterior and posterior portion of the gizzard (Eales, Reference Eales1921, Reference Eales1944; Klussmann-Kolb, Reference Klussmann-Kolb2004), first and second gizzards (Morton & Holme, Reference Morton and Holme1955), and anterior and posterior gizzards (Marcus & Marcus, Reference Marcus and Marcus1957a, Reference Marcus and Marcus1961).
As is the case in the anaspideans, the gizzard is a pyramidal structure composed of a series of plates. The filter chamber contains the gastric hooks, which are smaller and more slender than the gizzard plates, with pointed tips and circular bases in most anaspideans. The filter chamber of Aplysia depilans, as is the case in all anaspideans, is located between the gizzard and stomach, and, by means of the gastric hooks, filters the crushed slurry of ingested food (Eales, Reference Eales1921; Fretter & Ko, Reference Fretter and Ko1979). However, the gastric hooks herein described for Aplysia depilans have a hexagonal-shaped base as tall as the pointed tip (Figure 3B), a unique feature to that species, since on most anaspideans they have a shallow and circular base with a long-pointed tip (e.g. Aplysia cervina (Dall & Simpson, Reference Dall and Simpson1901; MacFarland, Reference Macfarland1909); Aplysia punctata (Howells, Reference Howells1942: 377, figure 10; Eales, Reference Eales1921: pl. 3, figure 11c); Akera bullata (Morton & Holme, Reference Morton and Holme1955); Bursatella leachi guineensis Bebbington, Reference Bebbington1969: 329, figure 7; Phyllaplysia engeli Er. Marcus, Reference Marcus1955 (Marcus, Reference Marcus1955; Marcus & Marcus, Reference Marcus and Marcus1957a); Stylocheilus citrinus Rang, Reference Rang1828 (=S. longicauda (Quoy & Gaimard, Reference Quoy and Gaimard1825) Marcus & Marcus, Reference Marcus and Marcus1961: 17, figure 4). The gastric hooks are generally arranged in aligned rows (Bebbington, Reference Bebbington1969 (B. l. guineensis); Fretter & Ko, Reference Fretter and Ko1979 (Dolabella auricularia); personal observation (Dolabrifera dolafrifera)). However, in Aplysia depilans the filter chamber is unique in having many more hooks, crowding its entire surface (Figure 3D–E: fc).
Gizzard plates are generally described as arranged in rows (Howells, Reference Howells1942; Fretter & Ko, Reference Fretter and Ko1979) or tiers as referred to by Morton & Holme (Reference Morton and Holme1955), varying in number from 10 to 20 and having a pyramidal outline (Mikkelsen, Reference Mikkelsen1996). Our observations in A. depilans show that about 12 main gizzard plates are arranged in two lateral clusters (Figure 3D: gc) with about 6 plates each, with intercalary narrower and taller plates at the posterior edge of the gizzard (Figure 3E: gs). We observed that the gizzard plates detach easily from the gizzard wall in other aplysiids (e.g. Aplysia fasciata and Phyllaplysia engeli), leaving an adherence mark that permit the visualization of clusters (Figure 3D). Working with Aplysia brasiliana (referred to as Aplysia cervina) collected in Brazil, Marcus & Marcus (Reference Marcus and Marcus1957b) discarded the taxonomic value of the gizzard plates, which were not further used in taxonomic accounts on the Anaspidea since then (e.g. Marcus, Reference Marcus1972).
Klussmann-Kolb & Dinapoli (Reference Klussmann-Kolb and Dinapoli2006) considered the gizzard and gizzard plates as homologous apomorphic structures of Cephalaspidea s.s. and Pteropoda + Anaspidea. Jörger et al. (Reference Jörger, Stöger, Kano, Fukuda, Knebelsberger and Schrödl2010) indicated the presence of a gizzard as a synapomorphy of Euopisthobranchia. Some genera of Cephalaspidea (e.g. Bulla and Haminoea) bear three large plate-like structures within the gizzard (Malaquias & Reid, Reference Malaquias and Reid2008). The presence of a clustered arrangement with two lateral groups (Figure 3E: gc), and one narrower plate (gs), is another indicator of the hypothesis of homology of those structures.
Reproductive system
The gonad (hermaphrodite gland) of A. depilans is relatively well-studied (Mazzarelli, Reference Mazzarelli1891; Quattrini, Reference Quattrini1967; Thompson & Bebbington, Reference Thompson and Bebbington1969) and is considered similar to that of other aplysiids (e.g. A. punctata by Eales, Reference Eales1921; A. juliana by Marcus & Marcus, Reference Marcus and Marcus1955; A. fasciata by Thompson & Bebbington, Reference Thompson and Bebbington1969). The examined penis characters in A. depilans agree with previously published descriptions in being stout, black and having an internally armed sheath (Mazzarelli, Reference Mazzarelli1893; Bebbington & Thompson, Reference Bebbington and Thompson1968; Thompson, Reference Thompson1976). However, a distinction was found: the sheath is not a uniform structure, it indeed has two distinctive parts, each one internally armed with a characteristic arrangement.
The penis sheath was described by MacFarland (Reference Macfarland1909) as having both the ‘base’ and the ‘proximal part’ unarmed in A. cervina. The use of the name ‘base’ to differentiate the distal part of the penis sheath (e.g. Marcus, Reference Marcus1972) is understandable because that characteristic is notably present. According to Eales (Reference Eales1960), penis sheath is divided into a ‘proximal muscular portion’ and a distal part called ‘bulbous’. Here we use ‘muscle sheath’ (‘proximal end’ of MacFarland, Reference Macfarland1909) and ‘sheath sac’ (‘base’ of Marcus, Reference Marcus1972). The penis sheath in A. depilans has its thickest wall in the muscle sheath and on the right lateral side the folds are covered in spines; the sheath sac has thinner walls with radially organized spines. According to Marcus (Reference Marcus1972), this arrangement is partly due to the state of development of the organ. The same authors noticed that, in juvenile specimens of Notarchus punctatus armatus Baba, Reference Baba1938 from the Caribbean, the ‘male organ’ is covered by soft papillae, whereas older ones have spines lacking basal knobs. The same was observed here in A. depilans: examined juvenile specimens have inconspicuous papillae in the muscle sheath, devoid of spines, and in fact undeveloped. The same was described by Bebbington & Thompson (Reference Bebbington and Thompson1968) (and later reproduced by Thompson, Reference Thompson1976) about the penis sheath of A. depilans from Arcachon Bay, France. This could also indicate that this assembly can contain more than a single species, once the specimens studied by Bebbington & Thompson (Reference Bebbington and Thompson1968) were relatively larger (6–11 cm) than our juvenile specimens. Additionally, if the specimens studied by Bebbington & Thompson (Reference Bebbington and Thompson1968) are not juveniles, an unarmed muscle sheath agrees with what is known of related species of the subgenus Aplysia, as in Aplysia juliana and Aplysia dura (Eales, Reference Eales1960). The presence of spines at the sheath sac regardless of the sexual development stage remains to be investigated.
The armature of the penis and its sheath shows variable morphologies among anaspideans, for example: Phyllaplysia padinae Williams & Gosliner, Reference Williams and Gosliner1973 has unarmed sheaths (both muscle sheath and sheath sac) and an armed penis (Williams & Gosliner, Reference Williams and Gosliner1973); Petalifera petalifera has an unarmed muscle sheath with its sheath sac and armed penis (Martinez, Reference Martinez1996); and Aplysia winneba Eales, Reference Eales1957 (=A. fasciata) has an unarmed sheath (both muscle sheath and sheath sac) and penis (Martinez & Ortea, Reference Martinez and Ortea2002). Ontogeny could explain this variation, (e.g.) the ample variation in the armature of the penis and its sheath in Aplysia juliana stated by Engel & Eales (Reference Engel and Eales1957), in samples from Ambon, Indonesia (figure 2) with penis and sheath unarmed, sheath sac armed; and, South Africa (figures 13 and 15) with penis and sheath sac armed and muscle sheath unarmed. It is clear so far that the sheath is divided into two distinctive parts, and this is an important and useful tool for better understanding this character within the anaspideans.
Another point concerning the penis is the number of retractor muscles, described as variable from a single to a pair in anaspideans (Marcus & Marcus, Reference Marcus and Marcus1957b; Bebbington, Reference Bebbington1974; Thompson, Reference Thompson1976). Aplysia depilans was described as having two retractor muscles (Eales, Reference Eales1960; Bebbington & Thompson, Reference Bebbington and Thompson1968; Thompson, Reference Thompson1976). However, we found two pairs of muscles involved in the functional movement of the penis, a retractor pair and its protractor counterpart (Figure 5A). We verified this in Aplysia brasiliana, and this feature should undoubtedly be examined in another allied species.
Nervous system
There are no detectable differences in the nerve ring between A. depilans (Mazzarelli, Reference Mazzarelli1893; Wirz, Reference Wirz1952; this study) and other aplysiids. Nerves arising from the nerve ring and its branches were not treated here since they are similar to those of other heterobranch gastropods (e.g. Lemche, Reference Lemche1956; Garcia & Garcia-Gomez, Reference Garcia and Garcia-Gomez1988; Huber, Reference Huber1993; Jörger et al., Reference Jörger, Neusser, Haszprunar and Schrödl2008; Klussmann-Kolb et al., Reference Klussmann-Kolb, Croll and Staubach2013). The nervous system of Euthyneura has been discussed in detail by Dayrat & Tillier (Reference Dayrat and Tillier2000), who proposed the term ‘visceral ganglia’ to designate all the visceral loop ganglia. Additionally, the term ‘abdominal ganglia’ was proposed for the unpaired posterior ganglion of the visceral loop. However, we do not recognize ‘abdominal ganglion’ as the most appropriate term to designate this structure in aplysiids. It is more appropriately called ‘visceral ganglion’ by various authors (Mazzarelli, Reference Mazzarelli1893; Eales, Reference Eales1921; Marcus, Reference Marcus1972). Several authors have drawn attention to the fact that the ‘visceral ganglion’ is a fusion of multiple ganglia in Aplysia (Kodirov, Reference Kodirov2011). Nevertheless, the presence of the visceral ganglion is not often clearly evidenced in euthyneurans (Rosenbluth, Reference Rosenbluth1963), and the abdominal ganglion may be formed by the fusion of multiple ganglia (Kriegstein, Reference Kriegstein1977). Because of the apparent fusion, Jacklet et al. (Reference Jacklet, Peretz and Strumwasser1970) designated the abdominal ganglion as an aberrant parietovisceral ganglion in Aplysia. To improve the designation of that structure, we employ the term ‘abdominal ganglia’ for the posterior ganglion of the visceral loop, due to the highly concentrated and noticeably fused arrangement of its ganglia. Still regarding these ganglia, A. depilans has a similar arrangement to that reported in other congeners. The abdominal ganglia in A. depilans are clearly constituted of two hemispheres, which have also been reported by other authors (Guiart, Reference Guiart1901; Wirz, Reference Wirz1952). Its right side is the supraoesophageal ganglion, emitting the osphradial and pericardial nerves, while the left side consists of the fusion of the suboesophageal ganglion, which emits branches that innervate the mantle skirt, and the visceral ganglion, which emits the genital nerve (Guiart, Reference Guiart1901).
Dayrat & Tillier (Reference Dayrat and Tillier2000: 414, char. 43, 44) reported the lack of the left and right parietal ganglia in Aplysia. In A. depilans, however, the presence of both ganglia is evidenced by a bulge and by the concentration of tissue anteriorly to each hemisphere (both in young and adult individuals). Akera bullata, a basal anaspidean (Göbbeler & Klussmann-Kolb, Reference Göbbeler and Klussmann-Kolb2011), has a right parietal ganglion fused with the supraoesophageal, and the left parietal ganglion located midway along the pleurovisceral connective. According to Guiart (Reference Guiart1901), the presence of parietal ganglia is certain, with the abdominal ganglia in A. punctata showing two different variations: the right parietal ganglion is fused with the supraoesophageal ganglion. The left parietal ganglion can be seen anteriorly to the suboesophageal ganglion (Guiart, Reference Guiart1901: figure 70A) or both are completely fused with it (Guiart, Reference Guiart1901: figure 70B). The same arrangement is present in A. depilans, as demonstrated by Wirz (Reference Wirz1952).
Systematics
The morphological characters presented here are constant and, as such, are diagnostic for most of the subgenera of Aplysia and ensure the validity of these taxa. The subgenus Aplysia (type species A. depilans) shares the following characters with the subgenus Tullia Pruvot-Fol, Reference Pruvot-Fol1933 (type species A. juliana (=subgenus Metaplysia Pilsbry, Reference Pilsbry1951, type species Aplysia badistes Pilsbry, Reference Pilsbry1951)): the presence of a sole (=mesopodium) as large as the body width; metapodium with an indistinctly demarcated pedal sucker; and parapodia joined high up posteriorly. This indicates a close relationship or even a possible synonymy between these three taxa. On the other hand, these characters can be used to externally distinguish it from all other known subgenera: Phycophila, in having a slender body, broad cephalic tentacles and narrow foot with a long metapodium; Neaplysia, with a single species (A. californica) bearing a remarkable rectangular flattening described as an accessory plate of the shell; Pruvotaplysia in having a narrow foot, strongly concave shell, and similarly coloured border of the parapodia, foot, shell foramen, rhinophores and cephalic tentacles; and Varria Eales, Reference Eales1960, in having the parapodia joined low down posteriorly, filiform and unarmed penis, and usually a uniporous opaline gland that can be used to distinguish that subgenus from all others.
Some molecular studies have supported some Aplysia subgenera, such as Pruvotaplysia and Aplysia itself, but have shown a paraphyletic Varria (Medina et al., Reference Medina, Collins and Walsh2001), which becomes monophyletic if Neaplysia is included (Medina et al., Reference Medina, Collins and Walsh2005). Despite these interesting molecular results, the subgeneric division of Aplysia is still unclear, and mostly based on superficial morphological features (Eales, Reference Eales1960). A more detailed and ample morphological study is fundamental for a deeper analysis of this taxonomy, and even if these subgenera can be considered as full genera. The present paper, considering the type species of the main anaspideans genus, looks an important step in that direction.
Molecular approaches on anaspideans (Medina & Walsh, Reference Medina and Walsh2000) and specifically on Aplysia (Medina et al., Reference Medina, Collins and Walsh2001, Reference Medina, Collins and Walsh2005) have shown some interesting features that can be explored herein. An example is the homoplastic nature of the degree of fusion of the parapodia, which apparently appeared in different branches (Medina & Walsh, Reference Medina and Walsh2000). This feature has direct implications in the swimming capacity, which was inferred as appearing convergently in different branches (Medina et al., Reference Medina, Collins and Walsh2001: figure 7). However, in Medina et al.'s (Reference Medina, Collins and Walsh2001) hypothesis, the terminal branch Aplysia s.l. has ‘free parapodia fusion’. This, as seen above, cannot be considered true for all species of the genus Aplysia, since the subgenus Aplysia has parapodia ‘fused posteriorly’. In the same tree, this character appears as present in the genus Dolabella, which could be a homoplastic character between both terminal branches.
So far, a pair of parapodia well-fused posteriorly, as well as the pedal sucker and the sheath sac with thinner walls and spines radially organized are characteristics, as described by Eales (Reference Eales1960) for the subgenus Aplysia, that appear to be distinctive to the subgenus which includes A. depilans (type species) and allies (A. vaccaria, A. juliana, A. cedrosensis, A. dura, A. nigra and A. reticulopoda Beeman (Reference Beeman1960)) and can be used to help the generic/subgeneric distinction.
Medina et al. (Reference Medina, Collins and Walsh2001: figure 7), following Johnson & Willows (Reference Johnson and Willows1999) and Carefoot (Reference Carefoot1987), considered A. depilans as ‘swimming’ and A. juliana and A. vaccaria as ‘no swimming’. However, those species rise in the same branch, all sharing the distinctive characters of the subgenus Aplysia (as seen above). This divergence sounds to be a misidentification of A. depilans with a ‘no swimming’, like the swimming species A. fasciata, which belongs to the subgenus Varria, and has been misidentified as A. depilans (Quattrini, Reference Quattrini1967; Silva, Reference Silva2001; Rudman, Reference Rudman2007).
Finally, Medina et al. (Reference Medina, Collins and Walsh2005: table 1) described Subaplysia as a new subgenus of Aplysia, which includes A. depilans, type species of the subgenus Aplysia and A. juliana, the type species of subgenus Tullia (syn. of Aplysia), which must be considered a junior synonym of the subgenus Aplysia.
CONCLUSION
For the first time in the literature, the present morphological study provides an extensive comparative morphological assessment of the anaspideans. With this we demonstrate the importance of those characters in a phylogenetic context, their possible homologies and recommend novel, more accurate terminology for some important characters, such as parapodia lobation, cephalic tentacles, crop, penial sheath, gizzard plates and visceral ganglion.
Aplysia depilans, type species of genus Aplysia, is the most common aplysiid species in Europe. When compared to congeners from the same area, it can be easily distinguished by the following characters: low, bulky body, with a broad head and foot, metapodium producing a rounded sucker, and parapodia highly joined posteriorly. The anatomical study of A. depilans and the comparison with other known anaspidean species have brought new insights on their morphology. The penis, a widely used character in many taxonomic groupings, is now better understood within Aplysia. A standardization of names used to describe the penis sheath (muscle sheath and sheath sac) is proposed, as well as the description of two pairs of muscles (protractor and retractor) involved in penis functionality (previously, only one was reported). In the same way, the arrangement of the gizzard plates into two clusters and not in rows; the fusion of cephalic and oral tentacles; and the degree of attachment of the lateral columellar muscle to the hemocoel wall reported herein represent improvements in our understanding of Anaspidea. A refined anatomical knowledge will undoubtedly enrich future comparisons, not only among members of this order but within the Heterobranchia.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315418000528
ACKNOWLEDGEMENTS
We thank Philippe Bouchet (MNHN), Alexandre Pimenta (MNRJ) and Michael Schrödl (ZSM) for the loan of specimens. We thank M. Schrödl for suggestions on the manuscript. We thank Dimitris Poursanidis (Greece), Pedro Rosa (Portugal) and Jaime Jardim (MZSP) for collecting and sending samples. Daniel C. Cavallari (MZSP) and Anne DuPont (Florida, USA) for the review and corrections of English grammar and spelling. We also thank Marina Poddubetskaia (France) and Liam Faisey (UK) for photographing live specimens; Dione Seripierri, chief librarian of MZSP for her aid with the literature; Ênio Mattos and Phillip Lenktaitis from Laboratório de Miscroscopia Eletrônica da Universidade de São Paulo and Lara Guimarães, Laboratório de Microscopia Eletrônica (MZSP), for the SEM examinations. Three anonymous reviewers provided constructive comments and improved this manuscript. The comprehensive coverage of crucial literature associated with this study was only possible due to open-access initiatives focused on older scientific works, most notably the Biodiversity Heritage Library and the Internet Archive projects.
FINANCIAL SUPPORT
This project was partially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (to C.M.C., proc. 2010/11253-9), Capes Foundation proc. 8739/13-7 and the University of Bergen's Strategic Programme for International Research and Education, Norway (SPIRE) which granted a visiting scholarship to the Natural History Museum of Bergen.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.