Introduction
One of the seminal discoveries made on the first landed mission to Mars was the unusual amount of Cl in the compositional profile of soils (Clark et al. Reference Clark, Baird, Weldon, Tsusaki, Schnabel and Candelaria1982), virtually the same at the two widely separated Viking landing sites and at concentrations far higher than in typical terrestrial soils or the lunar regolith. Subsequent measurements have shown the same unusual Cl concentrations at multiple, dispersed landing and rover sites (Gellert & Clark Reference Gellert and Clark2015). The general interpretation has been that the Cl must be in the form of one or more chloride salts (Clark & van Hart Reference Clark and van Hart1981; Yen et al. Reference Yen2006), in analogy with its widespread occurrences on Earth. Not until the wet chemistry experiments on the Phoenix mission in 2008, some 32 years after Viking, it was determined that not only there is chloride but also that a significant fraction of the Cl can be in the form of one or more perchlorates in the soil (Hecht et al. Reference Hecht2009; Kounaves et al. Reference Kounaves, Chaniotakis, Chevrier, Carrier, Folds, Hansen, McElhoney, O'Neil and Weber2014b). Speculation that perchlorate may be widespread across Mars seems to be borne out by the more recent evidence for oxychlorines from evolved gas analysis on the Curiosity rover mission (Ming et al. Reference Ming2014).
The Viking missions also detected one or more unexpected oxidized species in the soils. The first evidence was the release of O2 gas upon humidification or wetting of the samples (Oyama & Berdahl Reference Oyama and Berdahl1977). It was also found that a portion of amino acids in the nutrient of the Labelled Release experiment in the Biology investigation became oxidized, a possible indicator of metabolic activity (Levin & Straat Reference Levin and Straat1981) but also possibly due to an indigenous oxidant in the soils (Klein Reference Klein1978). These were clues that perhaps some or all of the chlorine and bromine compounds in martian soil are in oxidized forms (Clark et al. Reference Clark2005).
Other oxidized forms of chlorine such as chlorate, chlorite and hypochlorite may be present, either as intermediates in the progression of oxidation of Cl or as end-products (Fig. 1). The presence and variety of oxygen-rich forms of chlorine on Mars is important for a variety of reasons, including (a) as additional evidence for oxidative processes in the martian environment; (b) as agents to mimic metabolic activity and detection of life; (c) as reactants to destroy organic molecules; (c) as sinks for H2O; (d) as strong freezing-point depressants, enabling the formation of brines at very low temperatures; and (e) with the potential for reacting with other constituents during heating of soil, and thereby confounding the results of in situ thermally-evolved gas analysis investigations.
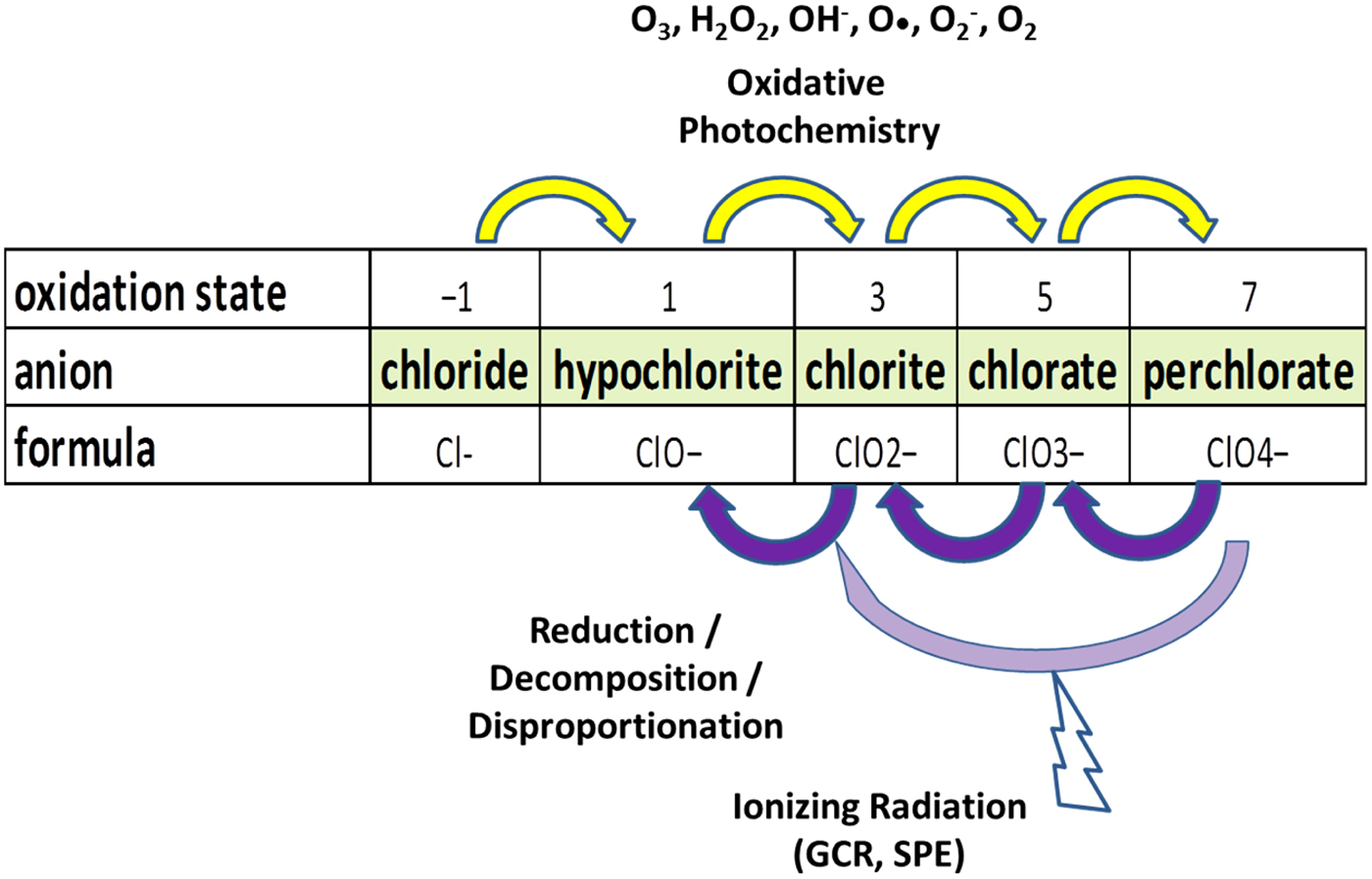
Fig. 1. The variety of plausible oxidation states for Cl on Mars. Pathways for progressive oxidation may be balanced by certain processes favouring the loss, rather the gain of oxygen, such as ionizing radiation.
Detection of Cl on Mars
A variety of techniques are available for the detection of Cl atoms, chemicals and minerals, ranging from X-ray to gamma ray spectroscopy; from wet chemistry to thermal gas evolution; and from reflection spectroscopy in the near and mid-infrared (IR) wavelength regions to laser-induced optical emissions. Each has, of course, its own strengths and limitations, including specificity, accuracy and spatial resolution.
X-ray based systems
The X-ray fluorescence (XRF) method of analyzing rocks and soils is quite sensitive and accurate for measuring the relatively high concentrations of Cl atoms in soils and sediments on Mars. From the 2.62 keV K-alpha-line fluorescence emission of Cl, nearly every sample of material analysed on Mars by in situ missions contained detectable chlorine. Regolith fines that encompass the global soil unit range from 0.4 to 0.8 wt.% Cl (Yen et al. Reference Yen, Ming, Gellert, Vaniman, Clark, Morris, Mittlefehldt and Arvidson2014). Although some cases of Cl may be simply due to contamination by soil or airfall dust, there are ample examples of exposed interiors of rocks (via grinding) with igneous compositional profiles that nonetheless have Cl at levels higher than in terrestrial analogs or martian meteorites.
The three plots of Fig. 2 are histograms showing the frequency of occurrence of different concentrations of Cl atoms as measured by the Mars Exploration Rover (MER) and Mars Science Laboratory (MSL) rover missions. It is noteworthy that the preponderance of Cl concentrations cluster between about 0.5 and 1.5 wt.% Cl for all three sites of exploration, although the MSL data at this point in its mission show more cases of higher levels of Cl than the MER rovers measured in Gusev crater and Meridiani Planum. Values above 2.5 wt.% Cl are relatively rare. This is surprising in view of the abundance of a wide variety of evidence for aqueous processes at all three sites because all the salts of Cl that are geochemically plausible based on the availability of appropriate cations are highly soluble, even at temperatures below the freezing point of pure H2O. In particular, in the multi-layered sediments of the ubiquitous Burns Formation in Meridiani Planum there are much higher concentrations of sulphates (3–5x higher than in soils and elsewhere on Mars), and though many of the inferred sulphates are highly soluble, the Cl concentrations are not strongly enhanced (Clark et al. Reference Clark2005), with the upper layers of the Burns Formation containing almost the same concentrations typical of soils, and the lower layers with only double that. The correlation between S and Cl in the Burns Formation is very weak, whereas in global soils observed at all landing sites, there are strong correlations between the two elements (e.g. Yen et al. Reference Yen, Ming, Gellert, Vaniman, Clark, Morris, Mittlefehldt and Arvidson2014).
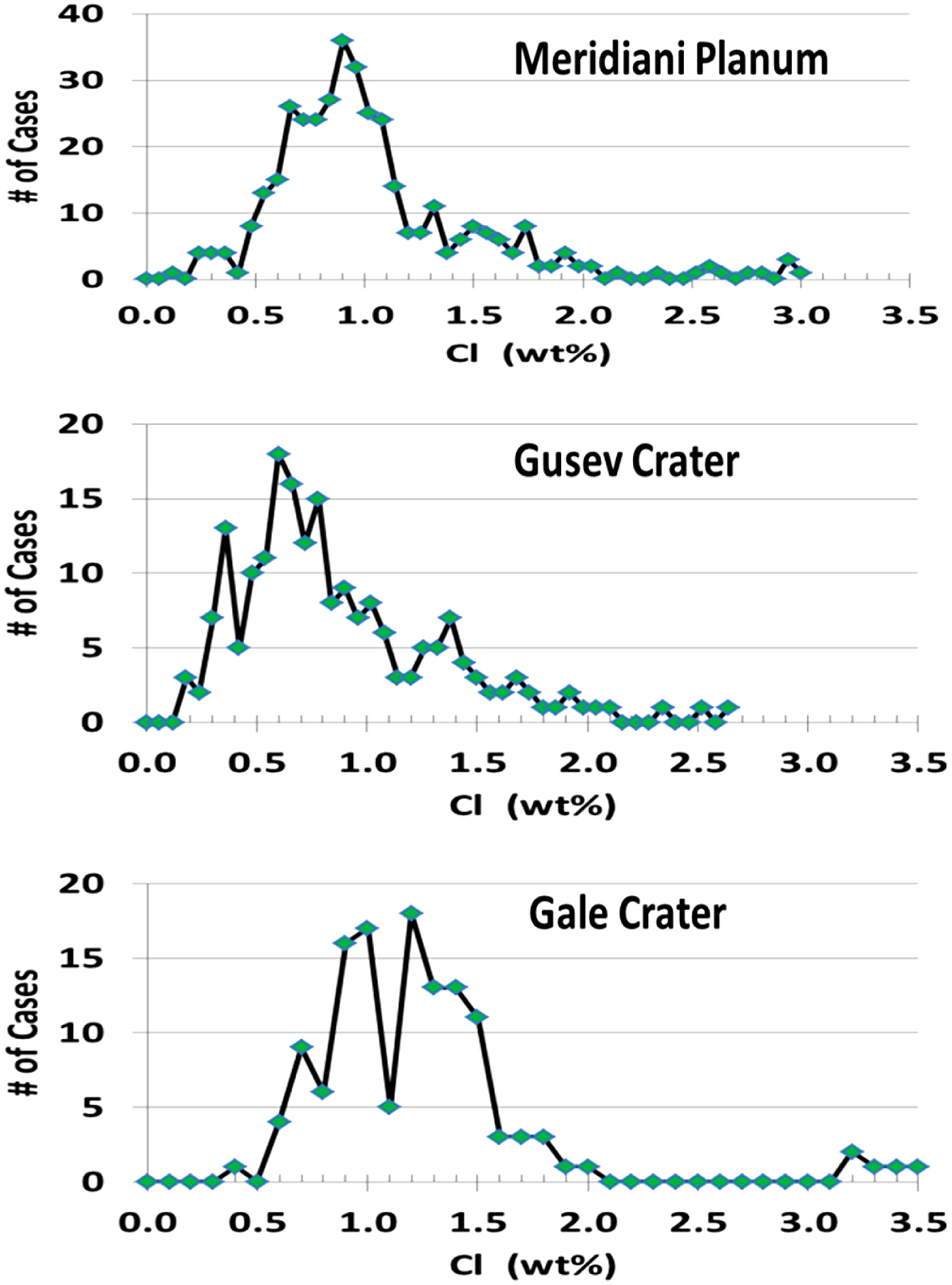
Fig. 2. Histograms of chlorine abundances for three rover missions (throughsol 3750 for Meridiani, and sol 854 at Gale crater).
Although X-ray fluorescence detects only the presence of atoms, the X-ray diffraction patterns from the CheMin instrument on the MSL rover Curiosity can, in principle, detect various mineralogical forms of Cl. However, the results to date have not revealed the presence of Cl minerals, with the exception of akaganeite (Vaniman et al. Reference Vaniman2014). This lack of detection of salts may be due to the relatively low concentration of the Cl minerals compared with the much more abundant minerals made up by the major elements. Alternatively, it could be that a significant portion, or all, of the Cl is in X-ray amorphous forms, such as sub-micron particles, or disordered material, or as thin rinds adsorbed or chemisorbed on individual silicate grains. Finally, there could be crystalline species but in multiple forms such that none is at a high enough concentration to be revealed by CheMin's X-ray diffraction patterns, compared with the patterns of the other constituent minerals. These forms could include not only multiple cation chemistries combined with Cl-bearing phases, but also different hydration states (stoichiometric numbers of H2O molecules of crystallization) occurring in different crystallographic forms.
Gamma ray and neutron techniques
Spectroscopy from orbit of energetic gamma rays stimulated by cosmic rays and their secondary particles allows Cl atoms to be detected (Keller et al. Reference Keller2006; Diez et al. Reference Diez2009). Systematic planet-wide measurements of Cl have been made by direct detection of the 1.95, 5.60 and 7.78 MeV gamma emissions by the Gamma Ray Spectrometer (GRS) on the Mars Odyssey orbiter. The spatial resolution of this technique is large, with ~300–400 km footprint on the surface. The neutron spectrometer portion of GRS has a similar footprint and also can infer the presence of chlorine atoms by their strong absorption cross-sections for thermal neutrons, relative to other rock- and soil-forming major and minor elements (Diez et al. Reference Diez2009).
The Cl abundances (Boynton et al. Reference Boynton, Taylor, Karunatillake, Reedy and Keller2007), as normalized to Pathfinder results, mostly range from 0.3 to 0.7 wt.%, close to the values for global soils. Lower average values (as low as 0.2 wt.%) tend to occur more often in southern latitudes, where bedrock may be more common. The highest values (up to 0.8 wt.%) occur in equatorial regions.
IR spectroscopy
Near-IR spectroscopy with the Phoenix mission camera was used to identify patches of higher concentrations of hydrated perchlorate (Mg- or Ca-) around the lander. The absorption band at 967 nm (Cull et al. Reference Cull, Arvidson, Catalano, Ming, Morris, Mellon and Lemmon2010) is prominent for these perchlorates. These authors acknowledge that several zeolites also produce this feature at this wavelength, as do some phyllosilicates and hydrated chlorides (bischofite), but provide various arguments why they conclude that the patches are perchlorates.
Also, from orbit the spectrum in the thermal IR region, as measured by the THEMIS instrument (also on the Odyssey spacecraft), has been interpreted as indicating the presence of chlorides. The absorption normally present in the region of 1260–900 cm−1 (7.9–11.1 µm) range is spectrally elevated by higher chloride-specific emissivity (Osterloo et al. Reference Osterloo, Hamilton, Bandfield, Glotch, Baldridge, Christensen, Tornabene and Anderson2008, Reference Osterloo, Anderson, Hamilton and Hynek2010). Spatial resolution of these observations is ~3 × 6 km2. Maps of these chloride concentrations show a preponderance in low albedo areas in the southern hemisphere, at latitudes mostly equatorward of 45°. Occurrences can be patchy, with a tendency to occur in topographic lows relative to nearby terrain, such as crater floors, and to be light-toned and exhibiting polygonal fracturing. In a critical review by Jensen & Glotch (Reference Jensen and Glotch2011), it was concluded that although certain sulphide minerals could partially mimic the spectral slope, there were sufficient differences between the spectra to rule them out, including the observation that alteration in the region is also indicated by the detection of phyllosilicates adjacent to the deposits in many cases.
Recently, Ojha et al. (Reference Ojha, Wilhelm, Murchie, McEwen, Wray, Hanley, Massé and Chojnacki2015) have discovered that four examples of the special type of gullies which change form with time (termed the Recurring Slope Lineae) show evidence of oxychlorines in their IR spectra, as measured by the Compact Reconnaissance Imaging Spectrometer for Mars instrument on the Mars Reconnaissance Orbiter. These implicated salts include Mg- and Na-perchlorate, and Mg-chlorate. As discussed below, oxychlorine salts in solution can significantly reduce the freezing point of water and hence produce brines that can flow under cold ambient conditions on Mars.
Laser-induced breakdown spectroscopy (LIBS)
The ChemCam (CCAM) instrument on the Curiosity rover includes a telescopic micro-imager and a LIBS system which analyses individual spots on rocks and soils as small as 300 µm in diameter (Wiens et al. Reference Wiens2012). Although the sensitivity is not high, there is evidence of detection of Cl using the emission line at 837.8 nm. Laboratory studies underway (A. Cousin 2015 personal communication) have shown that there are important matrix effects that will require different calibrations for rocks and soils. A search has also been made for detecting the molecular emission line of CaCl2, so far without success.
Wet chemistry analysis
Definitive identification and quantification of the perchlorate cation (ClO4−) in the Phoenix landing site soil was accomplished by two ion selective electrode sensors flown in the Wet Chemistry Laboratory (WCL) on the Phoenix polar lander (Kounaves et al. Reference Kounaves2010). One electrode originally labelled as a ‘nitrate’ sensor (often referred to as a ‘Hofmeister’ sensor), is known to be three orders-of-magnitude more sensitive to perchlorate than nitrate (i.e. it is also a perchlorate sensor). The other, a sensor specific for detection of calcium (Ca2+), is normally not responsive to anions, except to perchlorate, which causes a unique negative bias in its response to Ca2+. These two sensors definitively measured and identified ~2.7 mM ClO4− in all three Phoenix soil samples, equivalent to ~0.6 wt.% in the soil. The response of the Hofmeister sensor to ClO4− was so overwhelming that other species to which it responded would have to have been present in the soil at physically impossible amounts. It is also possible that the solution contained chlorate at concentrations ~3–6 mM, which would have been masked by the sensor's greater sensitivity to perchlorate. The unique behaviour of the Ca2+ sensor in the presence of ClO4− also allowed identification of the ClO4− parent salts as a 3 : 2 mixture of Ca(ClO4)2 and Mg(ClO4)2 (Kounaves et al. Reference Kounaves, Chaniotakis, Chevrier, Carrier, Folds, Hansen, McElhoney, O'Neil and Weber2014b; Quinn et al. This Issue).
Chloride was also measured directly with the WCL instrument. Values ranged from 0.24 to 0.6 mM in solution, equivalent to 0.03–0.05 wt.% in the soil (Kounaves et al. Reference Kounaves2010).
Evolved gas analysis
The MSL rover Curiosity targeted Gale Crater, well over 5600 km from Phoenix's landing site near Heimdall Crater. Gale's stratigraphy, mineralogy and landforms are starkly different from those that surrounded Phoenix. Yet, after four soil analyses by the Sample Analysis at Mars (SAM) instrument, the soils have been found to contain significant levels of an oxychlorine. The first clear evidence for the presence of ClO4−, or another oxychlorine phase, came from the SAM analysis of the Rocknest (RN) soil sample. Using both pyrolysis evolved gas analysis (EGA) by quadrupole mass spectrometry and gas chromatography mass spectrometry (GCMS), the samples also released several chlorine-bearing hydrocarbons at the same temperature where a rise in O2 and HCl were detected. The O2 is assumed to have evolved from the decomposition of a perchlorate or chlorate salt, with an equivalent concentration in the soil of 0.3–0.5 wt.%, Table 1 (Leshin et al. Reference Leshin2013). The Curiosity rover then traversed the Yellowknife Bay formation to Sheepbed, the lowermost stratigraphic unit, where it drilled two holes 3 m apart, designated John Klein (JK) and Cumberland (CB). These mudstone samples (formed in an ancient lake bed) were also analysed by the EGA and GCMS.
Table 1. Derived concentrations of ClO4 and Cl in MSL soil and rock samples

a Leshin et al. (Reference Leshin2013).
b Blake et al. (Reference Blake2013).
c Ming et al. (Reference Ming2014).
d McLennan et al. (Reference McLennan2014).
Both released O2, assumed to be from ClO4/ClO3 oxychlorines, with that from the CB sample (equivalent to 1.3 wt.% Cl2O7) on average about eight times that from JK (equivalent to 0.24 wt.% Cl2O7), and bracketing that from RN (Ming et al. Reference Ming2014). It should be noted, though, that the CB sample contained on average three times the total Cl of the JK sample as measured by APXS, 1.41 wt.% Cl versus 0.53 wt.% Cl for CB and JK, respectively (McLennan et al. Reference McLennan2014, Table S5). The JK sample though had two distinct peaks, implying a different O2 source or possibly consumption of O2 by organics or other material, and which could account for the lower abundance. Converting from the weight percentages for the two species, the fraction of Cl atoms detected by APXS which may be in the perchlorate form is ~10% (for JK) to 20% (for RN, CB). This and the Phoenix results demonstrate that the ratio Cl(perchlorate)/Cl(total) varies with the sample. Recently, Archer et al. (Reference Archer2015) also concluded this and pointed out that this ratio is linearly and positively related to the total Cl content, for the Gale crater samples.
Relative abundances of chemical and mineral forms of Cl
Ever since the time of discovery of oxygen release by simply wetting of martian soil (Oyama et al. Reference Oyama, Berdahl and Carle1977), the identification of oxidants in soils has been an important question. Oxidative processes involving atmospheric photochemical products under contemporary martian environmental conditions have been identified and continue to be studied (Hunten Reference Hunten1979; Yung & DeMore Reference Yung and DeMore1999; Krasnopolsky Reference Krasnopolsky2006; Lefevre et al. Reference Lefevre2008; Clancy et al. Reference Clancy2013). Two recent studies however indicate that the formation of oxychlorines may be predominately and globally occurring on Cl- bearing mineral surfaces. The first used a one-dimensional photochemical model to calculate the deposition rates of oxyanions and perchlorate from Mars’ atmosphere, and concluded that the modelled formation of perchlorate via purely gas-phase oxidation of volcanically-derived chlorine is insufficient by several orders of magnitude to explain the ~0.6 wt.% ClO4− measured by Phoenix (Smith et al. Reference Smith, Claire, Catling and Zahnle2014). The second experimental investigation showed that ClO4− and ClO3− can be produced photochemically on Cl-minerals without the presence of atmospheric chlorine or aqueous conditions, most likely due to SiO2 and metal-oxides acting as photocatalysts, generating O2− radicals from O2 which react with chloride (Carrier & Kounaves Reference Carrier and Kounaves2015).
A key question is whether the speciation of Cl among chloride and the various oxychlorines is a constant fraction of total Cl in the martian global soils, in Cl-containing coatings, and in sediments with enrichments in Cl. Without in situ experiments, such as the WCL on Phoenix, these determinations are very difficult. Elemental analysis has been performed on all landed missions except Phoenix, but does not discriminate between molecular forms of Cl. Remote sensing is difficult for oxychlorines, and the reported chloride deposits actually anti-correlate, in general, with the Cl trends revealed by GRS.
On Earth, perchlorate is rare relative to chloride: the relative concentration of Cl in chloride to Cl in perchlorate is very high and also quite variable by location, from 5 × 102 to over 1 × 106, with the lowest values occurring in the most arid location, the Atacama desert (Jackson et al. 2015). On Mars, the relative concentrations of perchlorate are generally much higher, as discussed above. This may be related to the greater importance of photochemical processes and the more extremely desiccated environment on Mars, as compared with Earth.
Another important and not fully resolved question is which cations are involved in the Cl salts. Major candidates include Na, Mg and Ca, and could involve Fe as well. The specific form of the salt affects its physicochemical properties, such as freezing point depression. The cations identified so far include Mg and Ca, with indications of a preponderance of the latter at both the Phoenix site (Kounaves et al. Reference Kounaves, Chaniotakis, Chevrier, Carrier, Folds, Hansen, McElhoney, O'Neil and Weber2014b) and the Gale site (Ming et al. Reference Ming2014). It also should be recognized that binary salts often exist in nature, such as combinations of Na, Mg, and/or Ca with anions such as Cl and sulphate. Several whose occurrences have been significant on Earth have mineral designations, such as tachyhydrite (Ca, Mg, Cl), tatarskite (Ca, Mg, S, Cl), D'Ansite (Na, Mg, S, Cl and replacements of Mg with Fe or Mn), kainite (K, Mg, S, Cl), sulphohalite (Na, S, Cl), and tatarskite (Ca, Mg, S, Cl). Most of these and the simple salts as well, are hydrated. Many more salt complexes are possible and the martian environment may uniquely favour assemblages that do not occur on Earth, especially for salts of oxychlorines.
Oxychlorines in martian meteorites
Aside from the Phoenix measurement, the only other direct measurement of perchlorate in martian material has been made in the EETA79001 Mars meteorite, recovered in Antarctica in 1979 at the Elephant Moraine ice ablation region. One of the largest, at 7942 g, it has been dated to ~ 170 Myr, with an ejection age of ~0.65 Myr, and terrestrial age of ~12 kyr (Jull & Donahue Reference Jull and Donahue1988). EETA79001 is composed of three lithologies: (A) a primary basaltic host of medium-grained, feldspathic pyroxenite; (B) a coarser-grained basalt similar to ‘A’ but free of olivine megacrysts, and (C) several shock-melted glass pockets and glass-filled veins (McSween & Jarosewich Reference McSween and Jarosewich1983). Within ‘A’ is a unique inclusion of white material comprised mainly of calcium carbonate (Martinez & Gooding Reference Martinez and Gooding1986).
Ion chromatography and isotopic analyses of cutting fines (‘sawdust’), that included the material from the inclusion, revealed the presence of approximately 0.6 ppm ClO4−, 1.4 ppm ClO3− and 16 ppm NO3− (Kounaves et al. Reference Kounaves, Carrier, O'Neil, Stroble and Claire2014a), with molar ratios of the NO3− to ClO4− of ~40 : 1 and Cl− to ClO4− of 15 : 1. As shown in Fig. 3, these ratios are very different than those for the Antarctic Dry Valley soils and ice near where the meteorite was recovered (10 000 : 1 and 5000 : 1, respectively). More interestingly, the nitrate oxygen and nitrogen isotopic ratios for the meteorite were found to give a δ 15N of −10.5 ± 0.3‰ and a δ18O of +51.6 ± 0.7‰. If the nitrate had been acquired from the ice which it was encased and transported through, the δ15N and δ18O should be the same or similar to the δ15N (+250‰) and δ18O (+30‰) of the Antarctic Dome-C ice and especially to the nearby Miller Range (MIL) blue ice which is similar to that where EETA79001 had been recovered. As can be seen in Fig. 4, the Dome-C ice values range from a δ15N of +350 to −10‰, while the MIL ice has a δ15N of about +102‰ and δ18O of about +43‰. If the meteorite had been contaminated with salts from the blue ice, the δ15N values should be the same or similar. These values and the location of the salts within the meteorite, make it difficult to reconcile with terrestrial contamination, leading to the conclusion that the salts are of martian origin. In conjunction with discoveries of perchlorate by Phoenix and Curiosity, as well as the evidence for oxidants at the Viking sites, these findings support the hypothesis that ClO4− is ubiquitous on Mars. In addition, the presence of ClO3− suggests the possible presence of other highly oxidizing oxychlorines such as ClO2− or ClO−, produced both by ultraviolet oxidation of Cl− (Kang et al. Reference Kang2009; Catling et al. Reference Catling2010; Schuttlefield et al. Reference Schuttlefield2012; Kounaves et al. Reference Kounaves, Carrier, O'Neil, Stroble and Claire2013; Carrier & Kounaves Reference Carrier and Kounaves2015) and the effects of ionizing irradiation of ClO4− (mimicking exposure to galactic cosmic rays, Quinn et al. Reference Quinn, Martucci, Miller, Bryson, Grunthaner and Grunthaner2013). The locations and age of the material analysed also suggests that perchlorate has been present on Mars for much of its history.
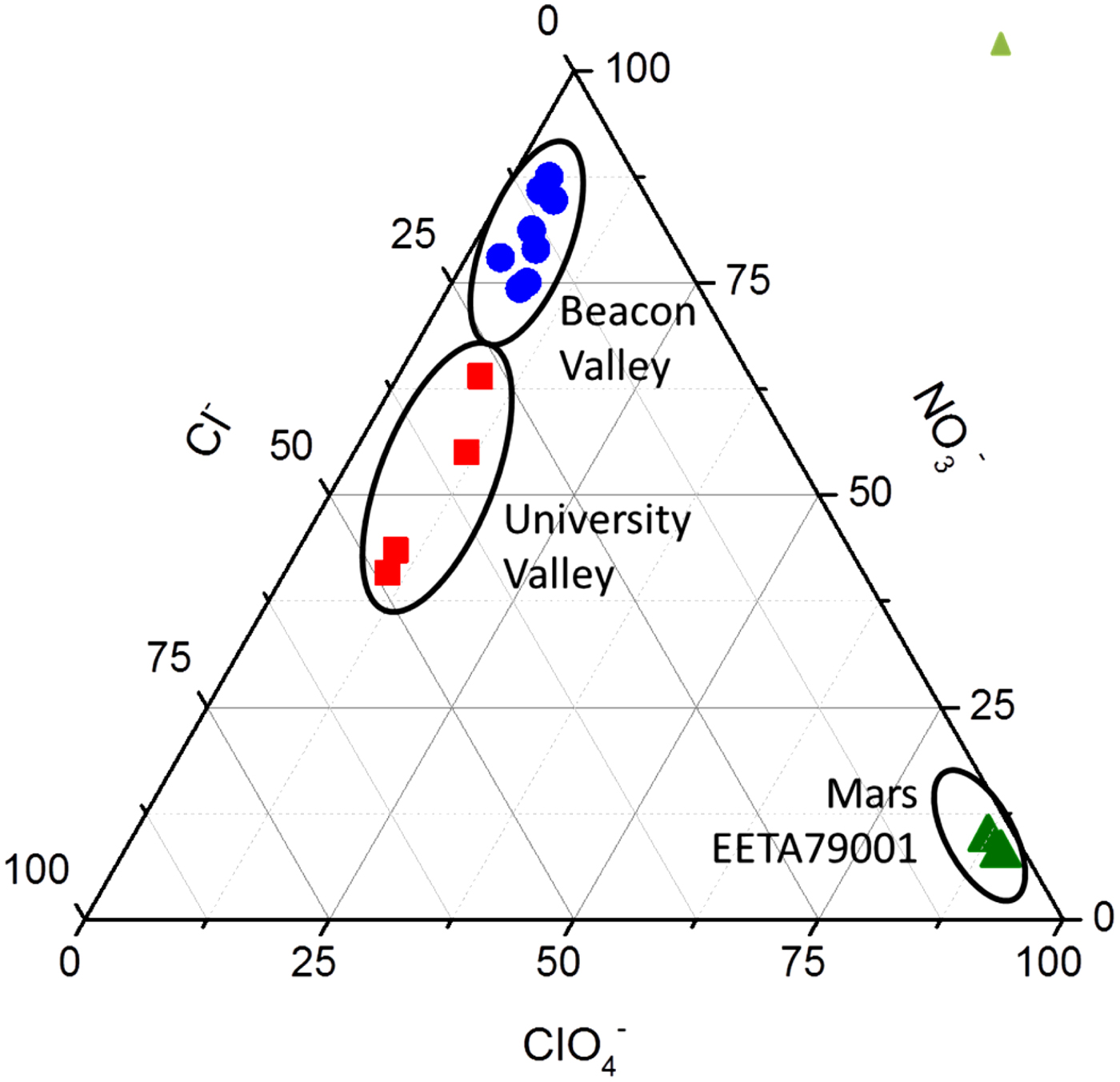
Fig. 3. A ternary plot for the concentrations of ClO4−, NO3− and Cl− in the Beacon (•) and University (■) Antarctic Dry Valleys (ADV), and the EETA79001 Mars meteorite (▲). The ADV soils show 2–3 orders of magnitude more NO3− and Cl− compared with the EETA79001.
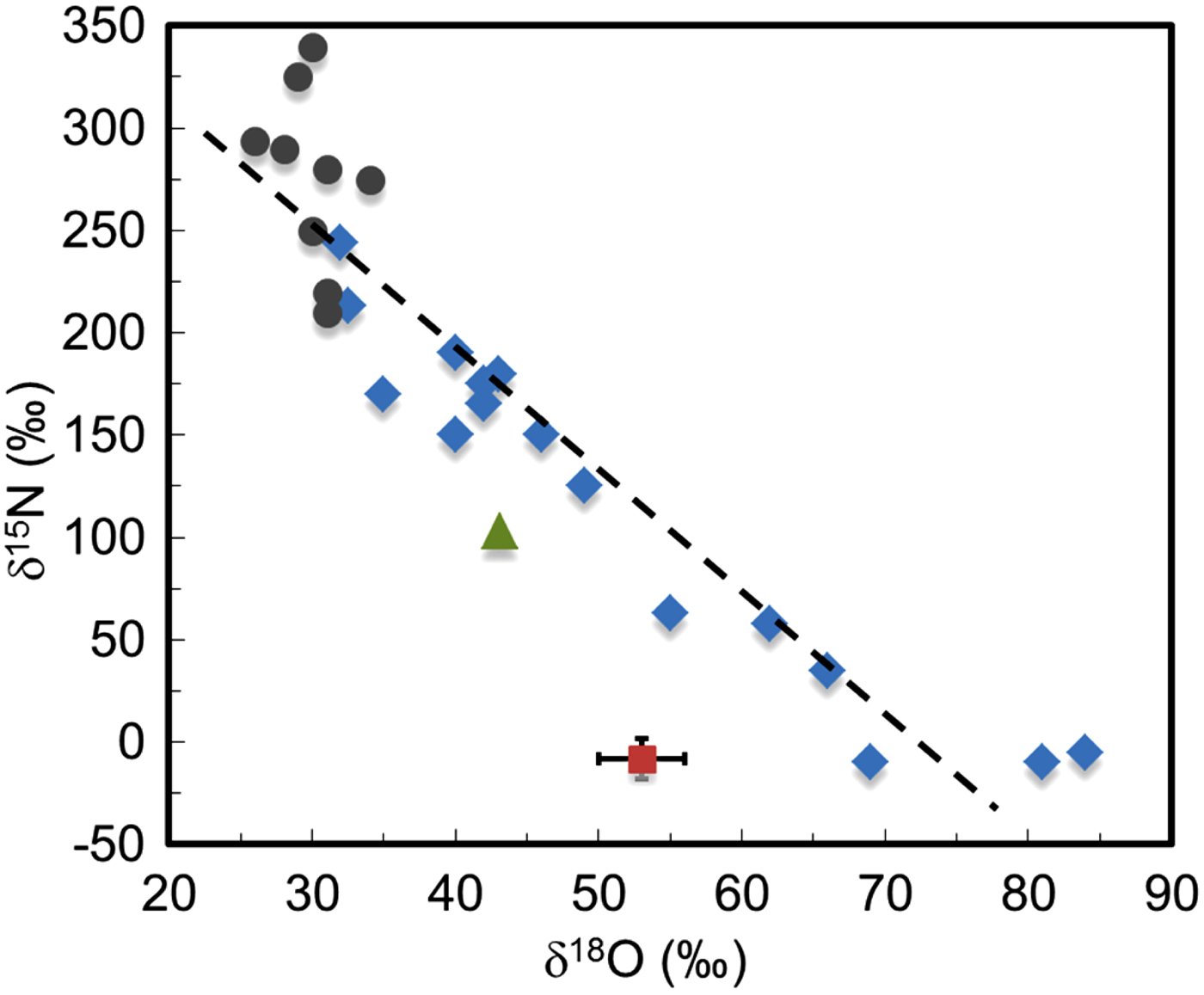
Fig. 4. The δ15N and δ18O of NO3− for EETA79001 in comparison with those for Miller Range and Dome-C ice. The Dome-C data are taken from Frey et al. (Reference Frey, Savarino, Morin, Erbland and Martins2009) and range in altitude from ~2000 to 3300 m and depths of 0–30 and 30–60 cm (♦ Dome-C ice 0–30 cm; • Dome-C ice 30–60 cm; ▲ Miller Range ice at 20 cm; ■ EETA79001).
Detection of other halogens
Because the elements comprising the halogen group share some chemical characteristics, it is of interest to consider whether they too may be present at unusually high concentrations and whether they are also susceptible to oxidation. For example, bromine is ubiquitous and at unexpectedly high levels on Mars. It has been suggested that Br may be present, at least in part, also in oxidized states, such as bromates and perbromates (Clark et al. Reference Clark2005). Bromine abundances are, however, highly variable on Mars and can range from less than one Br atom per 100 Cl atoms, to ten times that amount. Why the Br/Cl ratio should be so variable, especially considering that the Br content of soils is at trace levels to begin with, may be related to the relative partitioning into oxybromines and bromides, especially compared with its analog chlorine species. These various salts have different hydration levels, solubilities and eutectic points, and hence different mobility's under martian environmental conditions. Sensors for measuring soluble Br− and I− were included in the Phoenix WCL, but neither was detected at the respective sensor's limits of detection of 5 × 10−5 mol l−1, equivalent to 100 ppm in the soil samples (Kounaves et al. Reference Kounaves2010).
Fluorine has been detected via its molecular emission by Ca-F (Forni et al. Reference Forni2014). Fluorate and perfluorate salts may also exist on Mars, and their detection and quantification, combined with analogs of chlorine and bromine, may shed further light on the mechanisms which produced them. To date, none of the available spacecraft instrumentation techniques have demonstrated the identification of F-containing salts. Potentially limiting its usefulness as a diagnostic for salt, F can also occur in many other minerals not subject to dissolution under aqueous conditions.
Sources of chlorine
The explanation of why Cl is so ubiquitous in global soils has long been thought to reflect the origin of Cl as gases released by magmatic activity, especially as a product of the volcanism that has so importantly shaped the surface of Mars (Baird & Clark Reference Baird and Clark1981; Craddock & Greeley Reference Craddock and Greeley2009). The content of Cl in igneous rocks from Mars (SNC meteorites), 30 to 100 ppm (Lodders Reference Lodders1998), is less by factors of 40 to over 200 than in martian global soils. Deep-seated parental magmas on Mars may, however, contain as much as 0.3 wt.% Cl before devolatilization, greater than their actual H2O content (Filiberto & Treiman Reference Filiberto and Treiman2009). These magmas will lose much of their volatiles, whether through volcanic releases and outgassing of extruded lava, or indirectly through subsurface venting, fumerolic emissions, or formation of hydrothermal systems.
Infall of meteorites and interplanetary dust particles is another potential source but is not likely to have contributed significant Cl to the martian regolith. Most meteorites contain less than 0.03 wt.% Cl although some, such as the relatively rare enstatite chondrites and the carbonaceous meteorites, may contain up to 0.08 wt.% Cl (Mason Reference Mason1971). However, the S/Cl ratio across the broad range of meteorites is almost always at least 100 : 1, whereas in the martian global soil the chlorine is much higher and the S/Cl ratio (atom/atom) is typically 4 : 1 or somewhat less. Thus, unless undiscovered large reservoirs of Cl are found with low S concentrations, the contribution of meteorites to the regolith is small, in agreement with estimates by Flynn (Reference Flynn1996). Furthermore, the concentration of organic compounds in martian soil is so small that the influx of carbonaceous meteorites and interplanetary dust particles (IDP) should have been detectable if a significant fraction of the organic material or its relics had survived the oxidative pressure of the environment.
Recycling of chlorines
Although there have been several attempts at modelling the mechanism(s) of oxidation of chlorides on Mars, there has been little attention to processes which may reverse these reactions. It has been shown in experiments by Quinn et al. (Reference Quinn, Martucci, Miller, Bryson, Grunthaner and Grunthaner2013), however, that ionizing radiation can ultimately cause the loss of O atoms from perchlorate (also resulting in species whose reactivity is such that they can mimic the results of the various Viking life-detection metabolism experiments). The unrelenting bombardment of galactic cosmic rays and episodic energetic solar particle events provides a continuous pathway for converting chlorine valence states.
Another mechanism for reducing oxychlorines is heating to ~300°C. Such temperatures and higher can be attained with magmatic activity, especially when volcanic extrusives contact soils. Some of the target material during hypervelocity impact is also transiently heated to very high temperatures, which can induce decomposition reactions to evolve gases which quickly escape the milieu. If the global soils are ancient, a significant fraction may have been exposed to the impact heating events of primary target material and the consequences of emplacement of hot ejecta blankets, thereby causing the loss of O from oxychlorines, and reversion to lower oxidation states or HCl and chlorides.
Yet another mechanism is via reaction with organic compounds, endogenous or exogenous. The organics imported from asteroids, comets and IDPs (Flynn Reference Flynn1996) in one billion years could populate the soil to a depth of 10 m with a concentration of 0.2 wt.% (2000 ppm), approximately equal to the current estimates of perchlorate concentration. The apparent existence of some halogenated hydrocarbons (Navarro-González et al. Reference Navarro-González2006; Freissinet et al. Reference Freissinet2015) would be testimony to the interaction of organics with inorganic Cl in soil, since these compounds are rare in meteorites. Most of the organic constituents of the exogenous influx are presumably lost to oxidation by the perchlorates and/or photochemical mechanisms (Hunten Reference Hunten1979; ten Kate Reference ten Kate2010). In addition, endogenous sources should not be ruled out. Photosynthetic pathways for production of organics under contemporaneous martian conditions has been demonstrated in the laboratory (Hubbard et al. Reference Hubbard, Hardy, Voecks and Golub1973). Furthermore, past conditions on Mars may have been more reducing and hence much more favourable to large-scale synthesis of organic compounds. If so, then some organics could provide reducing power to react with oxidants such as the oxychlorines.
Whether by ionizing radiation, volcanic processing, impact heating, or reaction with organic compounds, pathways exist for interconversion in multiple directions of various oxychlorines and chlorides.
Salts of halogens can be potent freezing point depressants. Brines should readily form on Mars provided there is availability of liquid water, ice, frost, or a partial pressure of H2O vapour sufficiently high to induce deliquescence. As seen in Fig. 5, there are important differences in the effectiveness of various plausible salts (and acids). Combined with natural segregation processes due to differences in freezing point depression and hence transport by cold, aqueous processes, a cycling system for Cl could exist on Mars, resulting in spatial segregation of different salts and greater or lesser exposure to oxidizing mechanisms at the interface of the regolith with the atmosphere. These geochemical segregation and cycling mechanisms may contribute to the strong isotopic fractionation of Cl observed in some samples in Gale crater (Farley et al. 2015, in preparation).
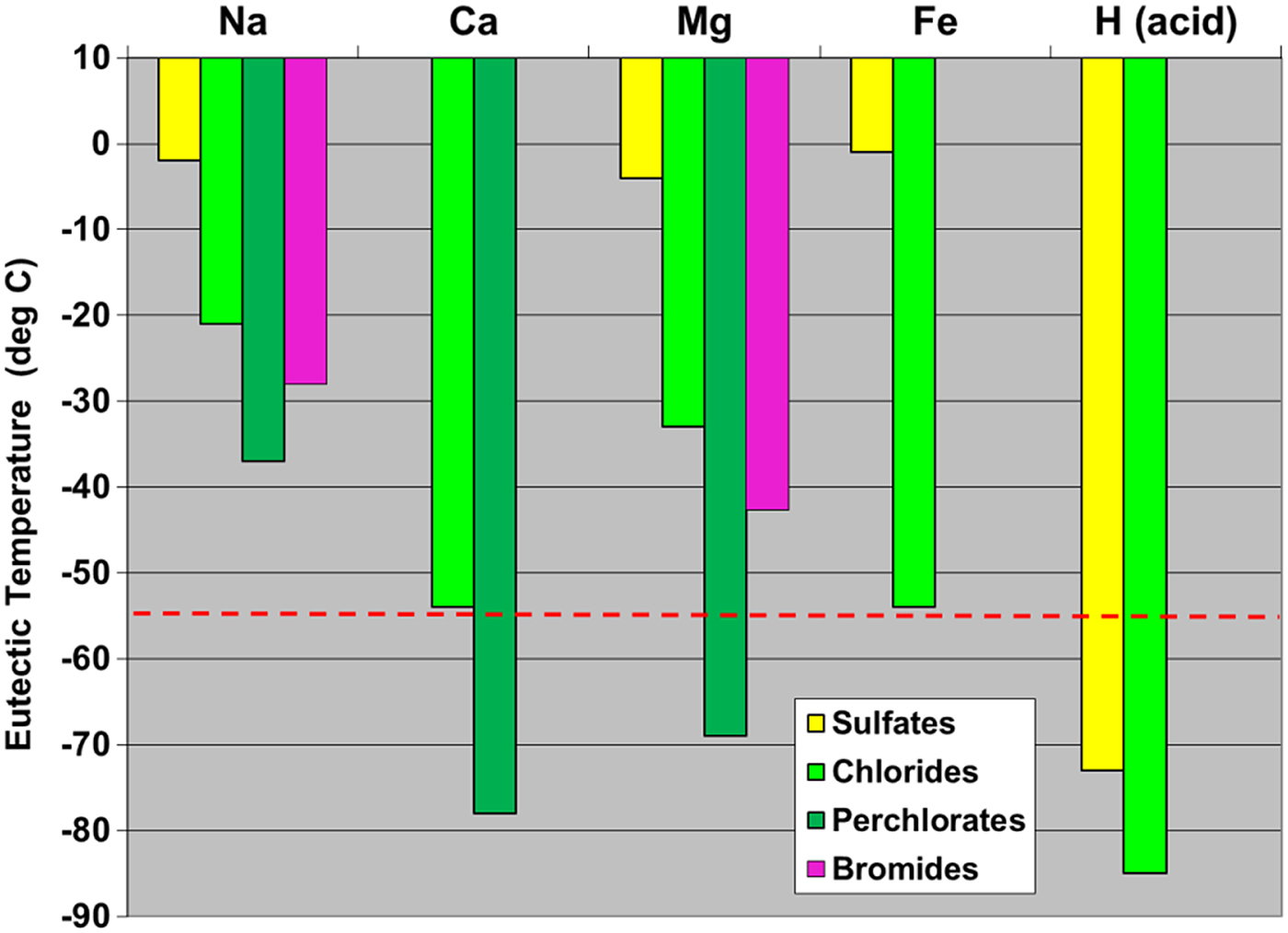
Fig. 5. Freezing point depression for aqueous eutectic solutions of the indicated cation salts with Cl and Br anions and oxyanions. Dotted red line indicates a typical average subsurface temperature on Mars at low to moderate latitudes. Several Cl salt brines do not freeze until below the average temperature, and for soils near the surface, temperatures often rise much higher. Note that although acids are very strong freezing-point depressors, their chemical reactivity with generally basic minerals would convert them to other species shown.
Future measurements
Several instruments comprising the science payload for NASA's 2020 Mars rover will have important detection capabilities, especially for mineral constituents. The XRF analysis is by the PIXL instrument, which can analyse the detailed structure of rocks, soils and sediments at spatial increments down to the 100 µm scale. It has good sensitivity for Cl and with it the potential for identifying specific salt grains or minerals containing Cl. The SHERLOC Raman spectrometric imager has the ability to identify perchlorate at low concentrations. The SuperCam is an expanded version of ChemCam, preserving all its capabilities but adding several others. As these instruments progress through development for flight, their calibration programs will now include a variety of Cl-containing minerals, including not only chloride salts but also the oxychlorine salts as well as chlorapatites, akaganeite, lawrencite, molysite and so forth.
The European Space Agency's 2018 ExoMars rover mission includes an infrared spectrometer (ISEM), a vis-IR spectrometer (MicrOmega), and the RLS Raman spectrometer, which may have detection capabilities for Cl-containing minerals.
The payload of the 2020 Al Amal ‘Hope’ mission by the United Arab Emirates includes EMIRS, a remote-sensing IR spectrometer, which could detect chloride enrichments that cover large areas ~300 km diameter.
Conclusion
The detection of chlorine abundances on Mars has been possible by a wide variety of techniques, both from orbit and on the surface. In almost all cases, this has been the consequence of a broader range of the measurements which only fortuitously included sensitivity to Cl atoms or minerals. No instrument has been specifically designed or tailored for investigating Cl or its forms, other than an electrode for detecting chloride ions in the WCL instrument. Yet, the variety and abundances of oxychlorines may be key to a deeper understanding of the martian physical-chemical environment, with importance to the search for organics, evidence of previous actions by liquid water, and the limits to life itself.
The global soil unit may have relative abundances of chlorides and oxychlorines that are similar everywhere, due to the widespread mixing caused by planet-wide dust storms. Wherever there has been processing to enrich (or deplete) Cl compared with that in the global soil, somewhat different profiles would be expected, and the relative amount of perchlorate could actually be enhanced in some circumstances. Perchlorates or other oxychlorines may exist wherever Cl is detected. This is the simplest hypothesis based on the disparate locations and geologic natures of the sites of exploration by Phoenix, Curiosity and Viking.
Acknowledgements
We wish to thank the reviewers for their comments and suggestions, especially by P. D. Archer Jr. This work was made possible in part by support from NASA and its JPL-led Mars missions, MER, MSL and Phoenix.








