Introduction
Cognitive impairment is frequently observed in patients suffering from depression and is associated with poor response to treatment (Potter et al. Reference Potter, Kittinger, Wagner, Steffens and Krishnan2004; Story et al. Reference Story, Potter, Attix, Welsh-Bohmer and Steffens2008; Roiser et al. Reference Roiser, Elliott and Sahakian2012). Impaired cognition has been estimated to occur in around two-thirds of depressed patients (Abas et al. Reference Abas, Sahakian and Levy1990; Butters et al. Reference Butters, Whyte, Nebes, Begley, Dew, Mulsant, Zmuda, Bhalla, Meltzer, Pollock, Reynolds and Becker2004; Afridi et al. Reference Afridi, Hina, Qureshi and Hussein2011). Impaired ability to think, concentrate or make decisions is a DSM-IV-TR (APA, 2000) diagnostic criterion for major depressive episode. Consistent with this, several systematic reviews have demonstrated cognitive deficits in patients suffering from depression (Burt et al. Reference Burt, Zembar and Niederehe1995; Veiel, Reference Veiel1997; Zakzanis et al. Reference Zakzanis, Leach and Kaplan1998; Stefanopoulou et al. Reference Stefanopoulou, Manoharan, Landau, Geddes, Goodwin and Frangou2009; Snyder Reference Snyder2013), including first-episode patients (Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012).
Impairments in cognition have been found to persist beyond acute episodes of depression, and between one-third and one-half of remitted depressed patients are thought to be affected by cognitive deficits (Abas et al. Reference Abas, Sahakian and Levy1990; Bhalla et al. Reference Bhalla, Butters, Mulsant, Begley, Zmuda, Schoderbek, Pollock, Reynolds and Becker2006; Reppermund et al. Reference Reppermund, Ising, Lucae and Zihl2009). Furthermore, one study revealed that 94% of patients who had cognitive impairment while depressed continued to experience deficits in cognition when remitted from depression (Bhalla et al. Reference Bhalla, Butters, Mulsant, Begley, Zmuda, Schoderbek, Pollock, Reynolds and Becker2006).
To our knowledge, to date, only two groups have reviewed cognitive function in patients remitted from depression (Hasselbalch et al. Reference Hasselbalch, Knorr and Kessing2011; Bora et al. Reference Bora, Harrison, Yücel and Pantelis2013). The review by Hasselbalch et al. (Reference Hasselbalch, Knorr and Kessing2011) included 500 remitted patients (and 472 controls) and revealed impaired cognitive performance in nine of the 11 included studies. Their review also assessed the association between cognitive function and other clinical features such as residual depressive symptoms and current medication status. However, drawbacks of this review relate to the large number of different cognitive tests that were used across studies and the lack of implementation of standardized effect sizes to reflect magnitude of impairment. Meanwhile, the review by Bora et al. (Reference Bora, Harrison, Yücel and Pantelis2013) included 895 remitted patients (and 997 controls) from 27 studies and, using standardized effect sizes, revealed cognitive deficits in a composite measure of global cognition, in individual cognitive domain composites and in a subset of specific tasks. The review also separately assessed cognitive function in early-onset and late-onset patients and included a meta-regression to uncover the influence of other clinical and demographic factors on cognitive performance. Again, a minor drawback of this review is that task-specific analyses were limited to a subgroup of cognitive tests for which there were sufficient data; therefore, cognitive domain and global cognition meta-analyses necessarily included results from a variety of cognitive tests. A review of the longitudinal course of cognitive function in depression revealed that improvements in mood were most closely related to improvements in verbal memory, verbal fluency and psychomotor speed, whereas attention and executive function remained impaired across treatment (Douglas & Porter, Reference Douglas and Porter2009).
Our aim was to conduct a systematic review and meta-analysis to investigate the degree of cognitive impairment in patients with depression during symptomatic and remitted states, focusing on studies that used a single neuropsychological test battery, the Cambridge Neuropsychological Test Automated Battery (CANTAB). Our rationale for including only CANTAB studies was to enable assessment of a broad range of cognitive domains but with consistent tasks implemented across reviewed studies, thereby ensuring interstudy homogeneity. We predicted that cognitive deficits would be observable in both depressed and remitted states.
Method
Systematic review
Studies were identified by searching PubMed and Google Scholar using the following search terms: ‘Cambridge neuropsychological test automated battery’ or ‘CANTAB’ and any CANTAB test name (e.g. ‘Spatial Span’) or its acronym (‘SSP’) and ‘depression’ or ‘depressed’ during the period from 1980 to December 2012. The CANTAB neuropsychological tests included in the search involved the domains of executive function, memory, attention and reaction time, as follows.
Executive function
(One Touch) Stockings of Cambridge (OTS/SOC; Owen et al. Reference Owen, Downes, Sahakian, Polkey and Robbins1990)
This task was derived from the Tower of London test and assesses visual planning, reasoning and impulsivity. Outcome measures analysed were the number/percentage correct or number of moves above the minimum [for all problems or difficult (four/five-move) problems].
Spatial Working Memory (SWM; Owen et al. Reference Owen, Sahakian, Semple, Polkey and Robbins1995)
This self-ordered search task is based on foraging behaviour and assesses working memory and strategy use. Participants search for tokens without returning to previous token locations. Outcome measure analysed was between-search errors.
Intra-Extra Dimensional Set Shift (IED; Rogers et al. Reference Rogers, Blackshaw, Middleton, Matthews, Hawtin, Crowley, Hopwood, Wallace, Deakin, Sahakian and Robbins1999)
This test of cognitive flexibility, analogous to the Wisconsin Card Sorting Test (WCST), has multiple stages segregating cognitive processes that assess rule learning, rule reversal and attentional set-shifting. Outcome measures analysed were total errors, extra-dimensional shift errors (adjusted) or stages completed.
Spatial Span (SSP; Kempton et al. Reference Kempton, Vance, Maruff, Luk, Costin and Pantelis1999)
This is a task of spatial short-term memory based on the Corsi block-tapping task. Outcome measure analysed was spatial span.
Memory
Delayed Matching to Sample (DMS; Robbins et al. Reference Robbins, James, Owen, Sahakian, McInes and Rabbitt1994)
In this test participants remember the visual features of a complex, abstract target stimulus and select it from a choice of four target patterns after a variable delay. Outcome measures analysed were total/percentage correct (for all trials or 12-s delay trials).
Paired Associates Learning (PAL; Sahakian et al. Reference Sahakian, Morris, Evenden, Heald, Levy, Philpot and Robbins1988)
In this test participants learn the locations of a progressively increasing number of abstract stimuli. Outcome measures analysed were total errors (adjusted) or first trials correct.
Pattern Recognition Memory (PRM; Owen et al. Reference Owen, Sahakian, Semple, Polkey and Robbins1995)
This is a two-forced-choice test of abstract visual pattern recognition memory. Outcome measures analysed were total/percentage correct.
Spatial Recognition Memory (SRM; Owen et al. Reference Owen, Sahakian, Semple, Polkey and Robbins1995)
This two-forced-choice discrimination paradigm tests spatial recognition memory. Outcome measures analysed were total/percentage correct.
Attention
Rapid Visual Information Processing (RVP; Sahakian et al. Reference Sahakian, Jones, Levy, Gray and Warburton1989)
This is a continuous performance test that assesses sustained attention, signal detection and impulsivity. Participants monitor a stream of single digits for three-digit target sequences. Outcome measures analysed were target sensitivity or total hits/omissions.
Reaction time
Reaction Time (RTI; Sahakian et al. Reference Sahakian, Owen, Morant, Eagger, Boddington, Crayton, Crockford, Crooks, Hill and Levy1993)
This is a test of simple and five-choice reaction time. Outcome measure analysed was five-choice reaction time.
Inclusion criteria
The inclusion criteria for studies were: (1) used DSM or ICD criteria to diagnose major depressive disorder; (2) included a healthy control group; (3) used CANTAB to assess cognitive function in currently depressed patients and/or remitted depressed patients; and (4) reported sufficient data to estimate Cohen's d effect sizes, that is the group mean and either standard deviation or standard error data (and number of subjects in each group) were available for both patients and controls.
Our search revealed 24 studies including 784 currently depressed patients (and 727 controls) and six studies including 168 remitted depressed patients (and 178 controls) that met our inclusion criteria (see Table 1). The criteria for remitted depression varied across studies and are shown in Table 1.
Table 1. Study characteristics and patient demographics for currently depressed and remitted depressed comparisons
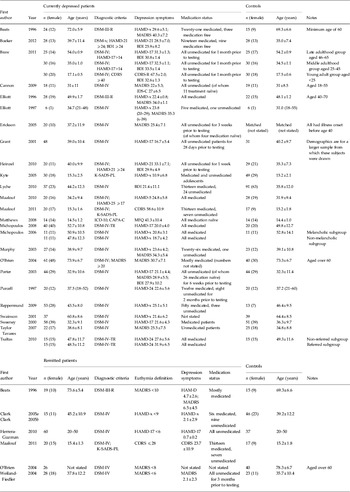
DSM-x, Diagnostic and Statistical Manual of Mental Disorders (APA, 2000); HAMD-17/-21/-24/-25/-x, Hamilton Depression Rating Scale (17-/21-/24-/25-item/unstated version) (Hamilton, Reference Hamilton1960); MADRS, Montgomery–Asberg Depression Rating Scale (Montgomery & Asberg, Reference Montgomery and Asberg1979); BDI, Beck Depression Inventory (Beck, Reference Beck1961); K-SADS-PL, Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (Kaufman et al. Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci, Williamson and Ryan1997); CDRS, Children's Depression Rating Scale (Poznanski et al. Reference Poznanski, Cook and Carroll1979); CAPA-C, Child and Adolescent Psychiatric Assessment – Child Version (Angold & Costello, Reference Angold and Costello1995); MFQ, Mood and Feelings Questionnaire (Angold et al. Reference Angold, Erkanli, Silberg, Eaves and Costello2002).
Values given as mean ± standard deviation or mean (range).
Meta-analysis
Meta-analysis was performed using Review Manager (RevMan, 2011). For each study, Cohen's d effect sizes (Cohen, Reference Cohen1988) were calculated as the mean difference between test performance scores for patients compared to controls divided by the pooled standard deviation; negative effect sizes reflected deficits compared to controls. Subsequently, for each test, effect sizes were weighted using the inverse variance method within a random-effects model and pooled across all studies with available data. Pooled effect sizes were reported for tests only when data from three or more studies were available. In addition to meta-analyses for currently depressed patients versus controls and remitted depressed patients versus controls, a separate subanalysis was conducted for currently depressed patients who were unmedicated at the time of assessment versus controls. There were insufficient studies of unmedicated remitted depressed patients to include a subanalysis of this population. Influenced by Cohen's convention regarding the magnitude of effect sizes (Cohen, Reference Cohen1988), a Cohen's d effect size in the range 0.2–0.35 was considered small, in the range 0.35–0.65 moderate and > 0.65 large. Statistical inferences were made based upon analysis of 95% confidence intervals (CIs).
Results
Profile of cognitive deficits in currently depressed patients
Cohen's d effect sizes were calculated based on data from 24 studies that used CANTAB tests in 784 currently depressed patients and 727 controls. Fig. 1 shows the weighted, pooled Cohen's d effect sizes for the comparison between depressed patients and healthy controls (black bars), and Table 2 presents detailed meta-analysis results.
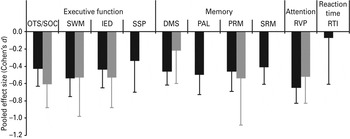
Fig. 1. Pooled, weighted Cohen's d effect sizes reflecting the performance of currently depressed patients (black bars) and remitted depressed patients (grey bars) compared to healthy controls on tasks of executive function [OTS/SOC, (One Touch) Stockings of Cambridge; SWM, Spatial Working Memory; IED, Intra-Extra Dimensional Set Shift; SSP, Spatial Span], memory (DMS, Delayed Matching to Sample; PAL, Paired Associates Learning; PRM, Pattern Recognition Memory; SRM, Spatial Recognition Memory), attention (RVP, Rapid Visual Information Processing) and reaction time (RTI, Reaction Time). Error bars represent 95% confidence intervals (CIs).
Table 2. Meta-analysis results
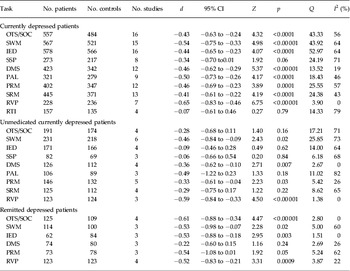
d, Weighted, pooled Cohen's d effect size; CI, confidence interval; Q, heterogeneity; I 2, percentage of total variability due to heterogeneity; OTS/SOC, (One Touch) Stockings of Cambridge; SWM, Spatial Working Memory; IED, Intra-Extra Dimensional Set Shift; SSP, Spatial Span; DMS, Delayed Matching to Sample; PAL, Paired Associates Learning; PRM, Pattern Recognition Memory; SRM, Spatial Recognition Memory; RVP, Rapid Visual Information Processing; RTI, Reaction Time.
Currently depressed patients showed significant moderate deficits compared to healthy controls across the cognitive domains of executive function (Cohen's d ranged from −0.34 to −0.54), memory (Cohen's d ranged from −0.41 to −0.50) and attention (Cohen's d was −0.65), and there was no significant deficit in reaction time (Cohen's d was −0.07). The non-significant finding for reaction time should be treated with caution because the results seem to have been affected by one study for which depressed patients showed significantly superior performance to controls. Indeed, when this study was excluded, currently depressed patients showed a nearly significant small deficit in reaction time compared to controls (d = −0.32, 95% CIs −0.59 to −0.05). Supplementary Fig. S1 (available online) presents forest plots depicting performance of currently depressed patients relative to controls.
Subanalysis: profile of cognitive deficits in unmedicated currently depressed patients
Cohen's d effect sizes were calculated based on data from eight studies that used CANTAB tests in 271 currently depressed patients who were unmedicated at the time of assessment and 267 controls. There were sufficient data to calculate weighted, pooled effect sizes for all executive function tasks, all memory tasks, and for the task of attention; insufficient data were available to calculate a weighted, pooled effect size for the reaction time task. Table 2 presents detailed meta-analysis results.
Unmedicated currently depressed patients showed significant moderate deficits compared to healthy controls on one executive function task (SWM; Cohen's d was −0.46), two memory tasks (DMS and PRM; Cohen's d ranged from −0.33 to −0.36) and the attention task (RVP; Cohen's d was −0.59). Although negative Cohen's d effect sizes (ranging from −0.06 to −0.49) were recorded for all remaining tasks, the 95% CIs crossed zero in all cases. Supplementary Fig. S2 presents forest plots depicting performance of unmedicated currently depressed patients relative to controls.
Profile of cognitive deficits in remitted depressed patients
Cohen's d effect sizes were based on data from six studies that used CANTAB tests in 168 remitted depressed patients and 178 controls. There were sufficient data to calculate weighted, pooled effect sizes for three (out of four) tasks in the domain of executive function, two (out of four) tasks in the domain of memory, and for the task of attention; insufficient data were available to calculate a weighted, pooled effect size for the reaction time task. Fig. 1 shows the weighted, pooled Cohen's d effect sizes for the comparison between depressed patients and healthy controls (grey bars), and Table 2 presents detailed meta-analysis results.
Patients remitted from depression showed significant moderate deficits compared to healthy controls across the cognitive domains of executive function (Cohen's d ranged from −0.53 to −0.61) and attention (Cohen's d was −0.52). There was a tendency towards moderate deficits in the domain of memory (Cohen's d ranged from −0.22 to −0.54). Although the 95% CIs crossed zero in both cases, they only just crossed zero for PRM (95% CIs were from −1.08 to 0.01). Supplementary Fig. S3 presents forest plots depicting performance of currently depressed patients relative to controls.
Discussion
Our systematic review and meta-analysis revealed that impairments in cognitive function, assessed with a single neuropsychological test battery (CANTAB), were exhibited by currently depressed patients and by patients remitted from depression. Current depression was associated with significant moderate deficits across all tasks within the domains of executive function, memory and attention, with the exception of the SSP task of executive function, for which there was a tendency towards a moderate deficit. Although the systematic review and meta-analysis revealed no reaction time deficit in currently depressed patients, exploratory reanalysis excluding one anomalous study (in which depressed patients showed significantly superior performance relative to controls) revealed a tendency towards a small deficit in reaction time. Analysis of only unmedicated currently depressed patients showed a significant moderate deficit in the domain of attention and significant small and moderate deficits in some, but not all, tasks within the domains of executive function and memory. Meanwhile, remitted depressed patients showed significant moderate deficits within the domains of executive function and attention. However, in the domain of memory, remitted depressed patients showed only a tendency towards small/moderate deficits. In summary, our systematic review and meta-analysis demonstrated that cognitive impairment, particularly affecting the domains of executive function and attention, is a core feature of depression that persists during remission in the absence of clinically relevant symptoms of low mood.
The present systematic review and meta-analysis included only studies that had used CANTAB tasks to assess cognitive function in symptomatic or remitted depressed patients relative to controls. To our knowledge, this is the first systematic review and meta-analysis that has focused on studies using a single neuropsychological test battery. The magnitudes of cognitive deficits recorded in the current investigation are broadly in line with those that have been recorded previously. However, our finding of a non-significant deficit in reaction time in currently depressed patients relative to controls contrasted notably with the literature. Nevertheless, following exclusion of one anomalous result, a tendency towards a small deficit on the RTI task was recorded, and the size of this deficit (Cohen's d = 0.32) was similar to the deficit recorded on the psychomotor speed composite (Cohen's d = 0.33) in the Snyder (2012) meta-analysis.
Impaired cognitive functioning has been linked with poor response to antidepressant treatment (Potter et al. Reference Potter, Kittinger, Wagner, Steffens and Krishnan2004; Story et al. Reference Story, Potter, Attix, Welsh-Bohmer and Steffens2008). However, the potential clinical relevance of cognitive deficits in depression also depends upon their impact on psychosocial functioning. Impaired psychosocial functioning is a core feature of depression (Weissman et al. Reference Weissman, Bland, Canino, Faravelli, Greenwald, Hwu, Joyce, Karam, Lee, Lellouch, Newman, Rubio-Stipec, Wells, Wickramaratne, Wittchen and Yeh2010). It persists in up to 60% of individuals with depression even after mood symptoms of depression have remitted (Jaeger et al. Reference Jaeger, Berns, Uzelac and Davis-Conway2006), indicating that severity of depressive symptoms cannot fully account for impaired functional ability. For example, patients with subsyndromal depressive symptoms have been found to manifest similar levels of psychosocial dysfunction to those of patients with clinically relevant symptoms (Judd et al. Reference Judd, Paulus, Wells and Rapaport1996). One possible explanation is that persisting cognitive impairments may contribute to poor quality of life and psychosocial functioning in patients whose depressive symptoms have remitted. In support of this, psychosocial functioning has been shown to be associated with performance on measures of attention, executive function, paired associates learning and visuospatial ability in depression (Jaeger et al. Reference Jaeger, Berns, Uzelac and Davis-Conway2006). Importantly, the association between cognitive deficits and poor psychosocial functioning has been shown to remain significant even when taking into account residual, subclinical depressive symptoms (Jaeger et al. Reference Jaeger, Berns, Uzelac and Davis-Conway2006).
Another study revealed that severity of cognitive impairment and severity of low mood associate independently with different measures of psychosocial functioning (McCall & Dunn, Reference McCall and Dunn2003). Furthermore, in bipolar disorder, psychosocial functioning has been shown to be predicted by both cognition and residual depressive symptoms (Mur et al. Reference Mur, Portella, Martinez-Aran, Pifarre and Vieta2009; Solé et al. Reference Solé, Bonnin, Torrent, Balanzá-Martinez, Tabarés-Seisdedos, Popovic, Martinez-Arán and Vieta2012).
Overall, these findings suggest that remediation of cognitive impairment and alleviation of depressive symptoms may both be involved in improving psychosocial functioning in depression. We therefore argue that cognitive impairment in depression is clinically relevant and may be a valuable target for intervention.
Although there are relatively few published studies assessing the cognitive enhancing effects of pharmacological treatments in depression, one potential augmentation therapy is the wakefulness-promoting agent modafinil. Indeed, 4-week adjunctive treatment with modafinil was shown to improve performance on a task of executive function in currently depressed patients with only partial response to antidepressant therapy (DeBattista et al. Reference DeBattista, Lembke, Solvason, Ghebremichael and Poirier2004). However, further research is required to delineate coincidental improvements in mood and fatigue from true improvements in cognitive function.
Limitations
One limitation of the current systematic review and meta-analysis relates to lack of assessment of the association between cognitive deficits and depressive symptoms. The importance of consideration of this association was highlighted in a meta-analysis that revealed that severity of depressive symptoms correlated significantly with impairment across domains of cognition including executive function, episodic memory and processing speed (McDermott & Ebmeier, Reference McDermott and Ebmeier2009). However, only a small portion (at most around 10%) of the variability in cognitive function is accounted for by variability in depressive symptom severity (McDermott & Ebmeier, Reference McDermott and Ebmeier2009). Therefore, there remains considerable separation between symptoms of depressive mood and cognitive impairment in patients suffering from depression, indicating that cognitive impairment cannot be considered entirely as a secondary feature of low mood in depression. Overall, although there is some evidence of an association between depressive symptomatology and cognitive function, this association does not account for the majority of variability in cognitive performance in depressed patients.
A further limitation of this study relates to most patients in the included studies being medicated. However, our subanalysis demonstrated significant cognitive deficits in unmedicated currently depressed patients on the SWM, DMS, PRM and RVP tasks, which span the domains of executive function, memory and attention. These findings support the idea that cognitive impairment is at least in part separable from medication effects in currently depressed patients.
The final limitation relates to the range of criteria used to define remission from depression within the remitted samples. Therefore, it is possible that our results may have been affected by the presence of low levels of persisting depressive symptoms in the remitted depressed group.
Conclusions
This review has demonstrated that cognitive impairment across the domains of executive function and attention, and to an extent memory, represents a core and clinically relevant feature of depression that persists beyond symptoms of low mood. Cognitive impairment is exhibited by depressed patients during current and remitted states, including in unmedicated samples. Previous research has demonstrated that cognitive impairment cannot be fully accounted for by severity of depressive symptoms and, along with symptoms of low mood, is associated with poor psychosocial function. We argue that cognitive impairment may represent a valuable target for new therapies for depression because remediation of cognitive impairment in addition to depressive symptoms will be important in improving functional outcome for patients with depression.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291713002535.
Declaration of Interest
Drs Rock, Riedel and Blackwell are full-time employees of Cambridge Cognition, and Dr Blackwell holds shares in Cambridge Cognition. Dr Roiser is a paid consultant for Cambridge Cognition.





