Introduction
In his classic paper on the comparative internal morphology of seeds, Martin (Reference Martin1946) examined seeds of 1287 genera of plants, and prepared a drawing (with scale) of a longitudinal- and of a cross-section of hundreds of species. He placed medium- to large-sized seeds with much endosperm and a small (i.e. rudimentary) embryo at the base of his family tree of seed phylogeny, and those with little or no endosperm and a large embryo at the top of the tree. Further, Martin viewed production of many small seeds to be a more advanced state than production of a relatively small number of medium- to large-sized seeds with much endosperm and a small embryo. Thus, he placed small (dwarf and micro) seeds on a side-branch of the tree in a relatively high position.
Martin's (Reference Martin1946) family tree of seed phylogeny continues to provide a foundation for enquiry into the evolutionary relationship between seed structure and seed dormancy (Forbis et al., Reference Forbis, Floyd and de Queiroz2002; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). Forbis et al. (Reference Forbis, Floyd and de Queiroz2002) used Martin's database, as well as data they collected from seeds of Amborellaceae, Chloranthaceae, Schisandraceae and Winteraceae, to address the question of evolution of embryo size in seed plants. They concluded that the underdeveloped embryo is primitive and that the embryo:seed (E:S) ratio has increased through evolutionary time in both angiosperms and gymnosperms. An underdeveloped embryo is small (low E:S ratio), has differentiated organs and grows inside the seed prior to radicle emergence (Grushvitzky, Reference Grushvitzky and Borriss1967).
In his 1946 paper, Martin devised a system of seed classification in which he recognized 10 types of seeds, based on various characters related to the embryo and endosperm, and two other types based primarily on size, i.e. dwarf (seed interior 0.3–2.0 mm long) and micro (seed interior ≤ 0.2 mm). (Note that the interval between >0.2 mm and < 0.3 mm is not included in either definition.) Martin divided the 12 types of seeds into three divisions: basal, peripheral and axile. However, in a note on p. 529 of his paper, Martin says that the basal division, except for the rudimentary type of seed, should be combined with the peripheral division. Dwarf and micro seeds belong to the miniature subdivision of the axile division. The miniature subdivision was described by Martin as ‘Seeds small to minute with embryos that are stocky or minute; seed coverings generally delicate and often cellular–reticulate; endosperm not starchy’ (p. 520).
The families listed by Martin as having dwarf seeds are Campanulaceae, Cephalotaceae, Clethraceae, Crassulaceae, Datiscaceae, Droseraceae, Elatinaceae, Ericaceae, Gentianaceae, Hypericaceae, Loganiaceae, Melastomataceae, Orobanchaceae, Penthoraceae, Podostemaceae, Saxifragaceae and Scrophulariaceae. Also, Martin listed 12 dwarf genera in the five non-dwarf families Hydrophyllaceae ( = Boraginaceae, sensu APG II, 2003), Polemoniaceae, Primulaceae, Rubiaceae and Solanaceae. However, there are many other species with small seeds that in whole, or in part, fit Martin's description of seeds belonging to the miniature subdivision, especially the dwarf-seed type. Martin knew about these additional dwarf-sized seeds and illustrated them in his paper, but for some reason(s) he did not include them on his list of dwarf-seeded families/genera. Thus, it is not clear how Martin's ‘dwarf seeds’ can be distinguished from many other seeds of the same size.
Our first objective in this paper was to evaluate various morphological characteristics, class of seed dormancy and phylogenetic position, to determine whether Martin's dwarf seeds could be distinguished from other small-sized seeds illustrated in his paper. We were unable to identify a set of characteristics that clearly differentiate ‘dwarf seeds’ from other small-sized seeds; consequently, our second objective was to revise Martin's key to the types of seeds, so that all seeds can be distinguished on the basis of embryo and/or endosperm characteristics. Finally, a third objective was to further revise Martin's key to the types of seeds so that it reflects: (1) the difference between linear underdeveloped (low E:S ratio) and linear fully developed (high E:S ratio) embryos; and (2) the difference between spatulate underdeveloped (low E:S ratio) and spatulate fully developed (high E:S ratio) embryos. Martin illustrated the two types of linear and of spatulate embryos but did not separate them in his key.
One important aspect of determining the phylogenetic relationship of plants is to describe their parts accurately so that comparisons can be made, and in the case of seeds, information on the internal morphology, especially embryo shape and embryo size in relation to the endosperm, is very useful (e.g. Forbis et al., Reference Forbis, Floyd and de Queiroz2002). However, keeping Martin's dwarf seeds as a separate category, and thus not considering their embryo morphology and/or E:S ratio, means that much information would be (has been?) excluded from phylogenetic studies. A revision of Martin's key to the types of seeds will make it possible to communicate clearly embryo information about all kinds of seeds, regardless of their size.
Evaluation of Martin's dwarf seeds
The first couplet in (Martin's Reference Martin1946) key for the types of seeds deals with endosperm and position of the embryo: (1) endosperm generally present and definitely starchy; embryo peripheral or partly so in relation to endosperm; or (2) endosperm, if present, not definitely starchy, except among a few linear-embryoed forms; embryo not peripheral. In Martin's key, the types of seeds with starchy endosperm and peripheral embryos are broad, capitate, lateral and peripheral, and a survey of the drawings in Martin's paper revealed that 5, 2, 1 and 10 families, respectively (with these types of embryos), have some species whose seeds are 0.3–2.0 mm long, i.e. they are the correct size to be called dwarf. Under non-starchy endosperm, Martin placed seeds with rudimentary, linear, spatulate, investing, bent and folded embryos, as well as dwarf and micro seeds. Since Martin placed his dwarf seeds in the non-starchy category, we will consider only non-starchy seeds in our evaluation of dwarf seeds. However, we will come back to the problem of small starchy seeds later. We will evaluate Martin's dwarf seeds on the basis of size, endosperm texture, seed coat, embryo morphology, class of dormancy and phylogenetic position.
Size of seeds
Among the non-starchy types of seeds in Martin's (Reference Martin1946) paper, we identified 37 families with one or more genera whose seeds are 0.3–2.0 mm long (Table 1) that were not included on Martin's list of dwarf families. Thus, clearly Martin did not place all small seeds with non-starchy endosperm into the dwarf-seed type.
Table 1 Plant families listed by Martin (Reference Martin1946) that have seeds 0.3–2.0 mm long, but were not listed by Martin as being dwarf, and characteristics of the (non-starchy) endosperm and kind of embryo in these families, as provided by Martin. See Fig. 2 for characteristics of each kind of embryo. Families are sensu APG II (2003)

Endosperm texture
In describing dwarf seeds, Martin emphasized the endosperm and said ‘With the exception of Drosera (crystalline-granular) the endosperm in all Dwarf seeds is fleshy and generally soft-fleshy. In Polypremum and Lindernia it is firm-fleshy as is also the case in most of the Linear and Spatulate genera that occur in Dwarf families’ (p. 584). The endosperm in non-dwarf families that have some dwarf genera was described as: Boraginaceae, fleshy to soft fleshy; Polemoniaceae, fleshy to firm fleshy; Primulaceae, hard or firm, semitransparent; Rubiaceae, firm to hard fleshy; and Solanaceae, fleshy and transparent (Martin, Reference Martin1946).
A survey of Martin's description of the endosperm in the 37 small-seeded non-dwarf families identified 20 families with fleshy or soft-fleshy endosperm, three with firm-fleshy or firm but watery-fleshy endosperm and three with watery-fleshy or soft and watery-fleshy endosperm; the other families have hard endosperm, or no information was provided by Martin (Table 1). Thus, many small-seeded (but non-dwarf) species belong to families with a type of endosperm that closely matches Martin's description of the endosperm in dwarf seeds. Consequently, it appears that Martin did not place all families with small seeds and non-starchy fleshy or soft-fleshy endosperm into the dwarf-seed type.
Seed coat
According to Martin (Reference Martin1946), dwarf seeds generally have a delicate and often a cellular–reticulate (net-like) seed coat. However, the testa (outer seed coat) in some members of some of Martin's dwarf families, including the Campanulaceae, Elatinaceae, Ericaceae, Gentianaceae, Hypericaceae, Loganiaceae, Melastomataceae, Saxifragaceae, Scrophulariaceae and Solanaceae, can have one or more layers of cells with part or all of the cell wall thickened or lignified (Netolitzky, Reference Netolitzky1926; Corner, Reference Corner1976; Takhtajan, Reference Takhtajan1996). Consequently, some members of these dwarf families have a very thin, cellular–reticulated (delicate) seed coat, while others may have a rigid, rather firm smooth seed coat. Examples of genera in dwarf families that may have non-delicate seed coats are Campanula (Campanulaceae), Heuchera (Saxifragaceae), Hypericum (Hypericaceae), Rhexia (Melastomataceae), Penstemon (Scrophulariaceae) and Vaccinium (Ericaceae) (Netolitzky, Reference Netolitzky1926; Corner, Reference Corner1976; Takhtajan, Reference Takhtajan1996; Baskin and Baskin, unpublished). Thus, not all dwarf seeds have a delicate seed coat.
The seed coat in some of the 37 small-seeded non-dwarf families listed in Table 1, including Apiaceae, Araceae, Betulaceae, Cyrillaceae, Loasaceae, Moraceae and Plantaginaceae, does not appear to have layers of thick or lignified cells (Netolitzky, Reference Netolitzky1926; Corner, Reference Corner1976; Takhtajan, Reference Takhtajan1991, Reference Takhtajan1992). Thus, although it is unlikely that any members of these seven non-dwarf families have a delicate seed coat, their seed coats may be comparable to those of the non-delicate seed coats of some species of Campanula, Heuchera, Hypericum, Rhexia and Penstemon. Thus, the type of seed coat is not consistent among all dwarf seeds, and some small non-dwarf seeds have a seed coat very similar to that of dwarf seeds.
Embryo morphology
Martin (Reference Martin1946) was aware of variation in embryo size among his dwarf seeds and wrote, ‘The Dwarf type, like the Micro, is complicated somewhat by two phases in embryo size, one inclining toward diminution of the embryo in relation to its endosperm concomitant with reduction of the seed as a whole and the other phase tending toward relatively large or total embryos. The former tendency (small embryo) can be seen in the Campanulaceae, Gentianaceae, Droseraceae and Orobanchaceae and the latter (larger embryo) is to be found in the rest of the Dwarf families’ (p. 526). In addition to variation in embryo size, various embryo morphologies are found among Martin's dwarf families, and also among the dwarf genera he recognized in non-dwarf families (Table 2). For some dwarf families, Martin did not illustrate the embryo, and information about these embryos has been obtained from the literature. A comparison of embryos in Martin's dwarf seeds (Table 2) with those in the 37 small-seeded non-dwarf families shows that, with one exception, there is complete overlap with regard to types of embryos (Table 3). The exception is that none of the 37 non-dwarf families have an undifferentiated embryo. The Orobanchaceae was included on Martin's list of dwarf families, but this family has seeds with an undifferentiated embryo (Teryokhin, Reference Teryokhin2001), which is characteristic of micro seeds.
Table 2 Families listed by Martin (Reference Martin1946) as having dwarf seeds and his non-dwarf families (*) that contain dwarf genera, and kind of embryo in each family (provided by Martin unless otherwise indicated). See Fig. 2 for characteristics of each kind of embryo. Families are sensu APG II (2003)

aSpongberg (Reference Spongberg1978); bBoesewinkel (Reference Boesewinkel1984), Compilation Committee (2000); cBaskin and Baskin (Reference Baskin and Baskin2005); d Watson and Dallwitz (Reference Watson and Dallwitz1992 onwards); e Jacques-Félix (Reference Jacques-Félix1977), Takhtajan (Reference Takhtajan1996), Compilation Committee (2000); fBaskin and Baskin (unpublished data); gda Silva et al. (Reference da Silva, Toorop, van Aelst and Hilhorst2004).
Table 3 Comparison of various types of embryos in Martin's (Reference Martin1946) dwarf and in his small-seeded, non-dwarf families; the size range for both dwarf and non-dwarf seeds is 0.3–2.0 mm. Numbers indicate families with each type of embryo
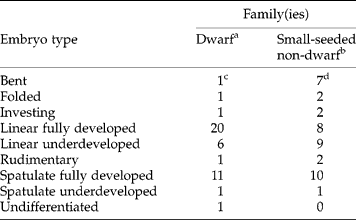
a Includes Martin's 17 dwarf families plus the dwarf genera in the five non-dwarf families he listed as having some dwarf genera.
b Includes the 37 small-seeded non-dwarf families plus the non-dwarf genera in the five non-dwarf families mentioned by Martin as having some dwarf genera.
c Numbers in this column add up to more than 22 because some families have more than one type of embryo.
d Numbers in this column add up to more than 37 because some families have more than one type of embryo.
Classes of seed dormancy
Although Martin was not concerned about seed dormancy or germination, we will evaluate classes of dormancy (sensu Baskin and Baskin, Reference Baskin and Baskin2004) to determine if there is a correlation between Martin's dwarf seeds and some class(es) of dormancy or of non-dormancy. If only one class of dormancy (or only non-dormancy) was found among Martin's dwarf seeds, this would be strong support for cohesiveness of the group.
Nikolaeva (Reference Nikolaeva2004) pointed out that ‘There hardly is a difference between Martin's rudimentary embryo in seeds of ‘medium size or larger’ and the embryo in some dwarf (0.3 to 2.0 mm in length) seeds’. She was correct. The embryos of dwarf seeds in some Campanulaceae and Gentianaceae are small and may increase by 57–182%, depending on the species, before the radicle emerges from the seed (Baskin and Baskin, Reference Baskin and Baskin2005; Baskin et al., Reference Baskin, Baskin and Yoshinaga2005). Thus, underdeveloped embryos and morphological (MD) and/or morphophysiological (MPD) dormancy occur in the Campanulaceae and Gentianaceae; however, some members of these families have seeds with fully developed embryos and either physiological dormancy (PD) or no dormancy. MPD is also found in some members of the dwarf families Ericaceae and Saxifragaceae (Nikolaeva et al., Reference Nikolaeva, Rasumova and Gladkova1985) and MD in seeds of Coffea arabica (Rubiaceae) (da Silva et al., Reference da Silva, Toorop, van Aelst and Hilhorst2004). Physiological dormancy (PD) occurs in seeds of Clethraceae, Crassulaceae, Elatinaceae, Hypericaceae, Melastomataceae, Penthoraceae, Polemoniaceae, Primulaceae, Scrophulariaceae and Solanaceae (for references related to PD in seeds of these families, see Baskin and Baskin, Reference Baskin and Baskin1998). None of Martin's dwarf families is known to have seeds with water-impermeable seed coats (Baskin and Baskin, Reference Baskin and Baskin2004); thus, the seeds do not have physical dormancy or combinational dormancy. With the exception of members of Martin's dwarf families that have MPD (or MD), PD is the only class of dormancy in the dwarf families.
Among the 37 small-seeded non-dwarf families, water-impermeable seed coats occur in the Cistaceae, Convolvulaceae, Fabaceae and Malvaceae (Baskin et al., Reference Baskin, Baskin and Li2000); therefore, these seeds have physical dormancy or, in some cases, perhaps combinational dormancy. MPD is found in eight of the 37 families. MPD has been investigated in the Apiaceae (Baskin and Baskin, Reference Baskin and Baskin1998), Caprifoliaceae (Hidayati et al., Reference Hidayati, Baskin and Baskin2000a, Reference Hidayati, Baskin and Baskinb, Reference Hidayati, Baskin and Baskinc), Fumariaceae (Baskin and Baskin, Reference Baskin and Baskin1994; Kondo et al., Reference Kondo, Okubo, Miura, Baskin and Baskin2005), Iridaceae (Morgan, Reference Morgan1990; Shipley and Parent, Reference Shipley and Parent1991; Coops and van der Velde, Reference Coops and van der Velde1995), Menyanthaceae (Hewett, Reference Hewett1964; Baskin et al., unpublished), Papaveraceae (Baskin and Baskin, Reference Baskin and Baskin1984; Baskin et al., Reference Baskin, Milberg, Andersson and Baskin2002; Karlsson et al., Reference Karlsson, Tamado and Milberg2003), Ranunculaceae (Horovitz et al., Reference Horovitz, Bullowa and Negbi1975; Forbis and Diggle, Reference Forbis and Diggle2001) and Sarraceniaceae (Ellison, Reference Ellison2001). Further, seeds of Ribes (Grossulariaceae) have an underdeveloped embryo and are highly dormant (Young and Young, Reference Young and Young1992), but MPD in this genus has not been studied in detail. Seeds in the remaining 24 (out of 37) families have PD, and some information related to dormancy-breaking and/or germination requirements for all the families except Resedaceae can be found in Baskin and Baskin (Reference Baskin and Baskin1998). Thus, the most common class of dormancy in Martin's dwarf families, as well as in the 37 small-seeded non-dwarf families, is PD, with MPD being the second most common.
Phylogenetic position
Can Martin's dwarf-seed families be distinguished from the small-seeded non-dwarf (37) families illustrated in his paper, when plotted on the APG II (2003) phylogenetic diagram? Further, if Martin's dwarf-seed families, and families with relatively large seeds and rudimentary embryos, are plotted on the APG II phylogenetic diagram, is there any support for Martin's idea that seeds with rudimentary embryos should be at the base of the family tree of seed phylogeny and dwarf seeds at a somewhat elevated position? To help answer these questions, we plotted Martin's 22 dwarf families, the 37 small-seeded non-dwarf families and the 39 families with relatively large seeds, known to have one or more members with rudimentary embryos (Baskin and Baskin, Reference Baskin and Baskin1998, unpublished data), on the APG II (2003) phylogenetic diagram for angiosperms.
Martin's dwarf families are restricted to the rosids, asterids and other (as yet unplaced to order) core eudicots (Fig. 1). The 37 small-seeded non-dwarf families occur in the rosids, asterids and other (as yet unplaced to order) core eudicots and also in the monocots and Ranunculales (eudicots). Thus, Martin's dwarf families and the small-seeded non-dwarf families occur in the same groups of plants, except for the presence of some non-dwarf families in the monocots and eudicots. Dwarf families certainly can not be separated from small-seeded non-dwarf families on the basis of phylogenetic position.

Figure 1 Placement of Martin's (Reference Martin1946) dwarf families (▲), 37 (non-starchy) small-seeded families not listed as dwarf by Martin (■) and relatively large seeds with a rudimentary embryo (Baskin and Baskin, Reference Baskin and Baskin1998) (●) on the APG II (2003) phylogenetic diagram for angiosperms. Each symbol for a taxon represents the occurrence of that embryo type in a family. The phylogenetic diagram has been reproduced from APG II (Angiosperm Phylogeny Group) (2003) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141, 399–436, with permission from Blackwell Publishing and the Botanical Journal of the Linnean Society.
Relatively large seeds with rudimentary embryos are quite common in the basal angiosperms and the magnoliids, but they also are found in the monocots, eudicots, core eudicots and euasterids; they are absent in the rosids. Thus, Martin's conclusion that dwarf seeds are more advanced than medium to large seeds with rudimentary embryos is generally true. However, dwarf and non-dwarf families, as well as families with rudimentary embryos, occur in the euasterids in the core eudicots.
Conclusions about dwarf seeds
Our evaluation of Martin's (Reference Martin1946) dwarf seeds reveals that, while they generally have fleshy or soft-fleshy non-starchy endosperm and thin cellular–reticulate seed coats, there are exceptions. Further, when comparing Martin's dwarf seeds with other small-seeded species illustrated in his paper, it is not clear why some of the latter species were not considered to be dwarf. Thus, there is no list of definitive characteristics that allows one to distinguish dwarf seeds from other seeds in the same size (0.3–2.0 mm) range, and given the diversity within Martin's dwarf seeds, it is not possible to make such a list.
We conclude that the ‘dwarf seed type’ per se is of limited value to seed biologists. This term does not convey any information about the embryo, and makes it difficult to compare the morphology of ‘dwarf’ seeds with that of other types of seeds, except on a size basis. Thus, we propose that use of Martin's dwarf type of seed be discontinued and that all types of seeds be named based on characteristics of the endosperm and embryo. Since Martin used endosperm and embryo characteristics to name 10 of his 12 seed types, our suggested change involves only his dwarf and micro seeds. For Martin's dwarf seeds, the change would mean not referring to the seeds as ‘dwarf’, and applying the name of one of Martin's other types that describes the embryo. For example, a member of the Gentianaceae with a small, short embryo would fit into Martin's rudimentary type. For micro seeds, the embryo is undifferentiated, thus these seeds could be referred to as ‘undifferentiated’ type.
Although, as mentioned above, Martin was not concerned about seed dormancy and germination, having accurate information about the embryo helps with classifying dormancy in seeds. However, embryo information will not necessarily place seeds into the proper class of dormancy, and it needs to be used along with other kinds of information in classifying dormancy. For example, seeds with a bent, investing or folded embryo might belong to members of Cistaceae, Fabaceae and Geraniaceae, respectively, be water-impermeable and thus have either physical dormancy or combinational dormancy. On the other hand, seeds with a bent, investing or folded embryo might belong to members of the Brassicaceae, Lauraceae or Burseraceae, respectively, be water-permeable and thus have PD or be non-dormant. Clearly, before classifying dormancy in seeds with bent, investing or folded embryos, imbibition tests need to be conducted. The other option is to consult the list of plant families known to have one or more genera with water-impermeable seed (or fruit) coats and thus physical dormancy (Baskin et al., Reference Baskin, Baskin and Li2000, Reference Baskin, Baskin and Dixon2006). The advantage of knowing that seeds have a bent, investing or folded embryo is that it excludes the possibility of them having MD or MPD.
If the names of all seed types were based on characteristics of the endosperm and embryo, we could talk about seeds of different sizes within each seed type and not have a category of dwarf seeds that included various embryo morphologies. For example, in this new system we would have large and small seeds with rudimentary embryos. As it is now, large seeds with a rudimentary embryo are placed in the rudimentary-seed type, and small seeds with a rudimentary embryo are placed in the dwarf-seed type, if the species is a member of one of Martin's dwarf-seed families. Another advantage of having all seed types based on endosperm and embryo characteristics is that we would not have to worry about properly categorizing the small seeds with starchy endosperm. For example, the many species in the Caryophyllaceae (with starchy endosperm and belonging to the peripheral type) with seeds of the same size as Martin's dwarf seeds would no longer be a problem. Finally, we presently have many small seeds that do not fit into any seed type according to Martin's classification system; thus, the revised system would allow us to put all seeds into a seed type.
We should not overlook the fact that seed size per se is of much interest from both an ecological and evolutionary perspective (Moles et al., Reference Moles, Ackerly, Webb, Tweddle, Dickie and Westoby2005). A discontinuation of use of Martin's dwarf seed type would not detract from these studies, because a person studying the phylogenetic relationships of seed size would not want to restrict their consideration of small seeds to Martin's dwarf seeds.
Modifications in Martin's key
We propose that the second portion of Martin's key to seed types be modified, so that all seed types are named based on characteristics of the embryo and endosperm; thus, the name ‘dwarf’ has been removed from the key (Fig. 2). ‘Micro’ has been replaced by ‘undifferentiated’ to indicate that the embryo in fresh seeds lacks organs. Using ‘undifferentiated’ solves the problem of what to do with relatively large seeds whose embryos lack organs at the time of seed maturity (e.g. Scatena and Bouman, Reference Scatena and Bouman2001). Further, Martin illustrated linear and spatulate embryos that are the full length of the seed, as well as linear and spatulate embryos that are only a fraction of the total length of the endosperm (seed). Linear embryos in mature seeds with a low E:S ratio can increase in size by 50 to >1000%, depending on the species, prior to radicle emergence in various families, including Apiaceae, 670%, 1433% (Baskin et al., Reference Baskin, Meyer and Baskin1995); Aristolochiaceae, 116%, 133%, 146% (Adams et al., Reference Adams, Baskin and Baskin2005); Campanulaceae 57–182% (Baskin and Baskin, Reference Baskin and Baskin2005; Baskin et al., Reference Baskin, Baskin and Yoshinaga2005); Caprifoliaceae, 294% (Hidayati et al., Reference Hidayati, Baskin and Baskin2005); Fumariaceae, 4433% (Kondo et al., Reference Kondo, Okubo, Miura, Baskin and Baskin2005); Liliaceae, 779% (Kondo et al., Reference Kondo, Sato, Baskin and Baskin2006); and Ranunculaceae, 251% (Walck et al., Reference Walck, Baskin and Baskin1999), 494% (Forbis and Diggle, Reference Forbis and Diggle2001). Also, spatulate embryos in seeds with a low E:S ratio in members of the Caprifoliaceae increased by 67, 158 and 208%, depending on the species (Hidayati et al., Reference Hidayati, Baskin and Baskin2000a, Reference Hidayati, Baskin and Baskinb, Reference Hidayati, Baskin and Baskinc). Thus, underdeveloped linear and underdeveloped spatulate have been added to the key, and Martin's original linear and spatulate now are referred to as ‘linear fully developed’ and ‘spatulate fully developed’. Since some dwarf seeds have an underdeveloped linear, fully developed linear, underdeveloped spatulate or fully developed embryo, these four types are needed if we are to accommodate all the seeds previously called ‘dwarf’ in the classification system.

Figure 2 The modified-Martin key to (mature, ripe) seed types. aPerisperm is present in some Cactaceae, e.g. Opuntia (Bregman and Bouman, Reference Bregman and Bouman1983). bOccasionally, the E:S ratio is >0.5, e.g. 0.67 in Sambucus spp. (Hidayati et al., Reference Hidayati, Baskin and Baskin2000b).
Many studies have been conducted on embryo growth in seeds with a low E:S ratio, and about 90 families have been identified in which at least one genus has underdeveloped embryos (Baskin and Baskin, Reference Baskin and Baskin1998, unpublished). There are additional families whose seeds have a low E:S ratio, but no studies have been done to determine if these small embryos are underdeveloped. However, not all seeds with small embryos and a low E:S ratio have an underdeveloped embryo. For example, seeds of Drosera anglica had a small (0.24 ± 0.01 mm) embryo in seeds with an E:S of 0.47, but embryos exhibited no growth prior to radicle emergence (Baskin and Baskin, Reference Baskin and Baskin2005). Exceptions like Drosera anglica may cause ‘problems’ if we wish to characterize seeds and embryos strictly from their physical appearance and have nice neat data for phylogenetic analyses. However, we should keep an open mind about these exceptions, because they may broaden our perspectives on the evolution of embryo size and of seed dormancy.
As a final note, we think Martin did a remarkable piece of work, and adding more detail to his key of the types of seeds should be viewed as recognition of his great legacy to seed biology, rather than a criticism.







