INTRODUCTION
Several parasite species that are transmitted trophically from prey to predators alter the behaviour or other phenotypic traits of their intermediate hosts (reviewed by Moore, 2002). Since the probability of individual parasites completing their life-cycles is typically very small (e.g. Dobson, Hudson and Lyles, 1992), the ability of parasites to manipulate host phenotype may increase parasite transmission efficiency to the target hosts (the next host in the life-cycle), and thus be favoured by natural selection (Rothschild, 1962; Holmes and Bethel, 1972). In most systems, however, host manipulation has been studied only under laboratory conditions, and altered host behaviour (e.g. Levri and Lively, 1996; Thomas and Poulin, 1998; McCarthy, Fitzpatrick and Irwin, 2000; Poulin and Latham, 2002) or susceptibility to predation (e.g. Moore, 1983; Lafferty and Morris, 1996; Thomas and Poulin, 1998; Voříšek et al. 1998; Mouritsen and Poulin, 2003) have rarely been demonstrated in the field. Furthermore, most studies have used field-collected animals instead of experimentally infected. Thus, their results may be confounded, for example, by other infections, and physiological or behavioural differences between hosts before infection takes place, which might determine their susceptibility both to parasitism and predation. In the present study, we used the parasite Diplostomum spathaceum (Trematoda) and its fish intermediate host as a model system to study host manipulation in the field. The parasite has a 3-host life-cycle with a bird definitive host, and snail and fish intermediate hosts (see e.g. Chappell, Hardie and Secombes, 1994). The parasite infects a variety of fish species (Valtonen and Gibson, 1997), and is transmitted trophically from fish to birds. In fish, metacercarial stages of the parasite lodge themselves in the lenses of the eyes, and, together with the opacity of the lenses caused by their metabolic wastes, reduce or even destroy the host's vision (Rushton, 1937, 1938; Shariff, Richards and Sommerville, 1980; Karvonen, Seppälä and Valtonen, 2004a). In our earlier laboratory studies (Seppälä, Karvonen and Valtonen, 2004, 2005a,b), we found that fully developed D. spathaceum metacercariae manipulate fish phenotype by reducing their escape response (Seppälä et al. 2004) and crypsis (Seppälä et al. 2005a), and increase their susceptibility to simulated avian predation (capture with a dip-net, Seppälä et al. 2004, 2005b). The aim of this study was to expand this set-up into natural conditions by investigating how the parasite affects the vulnerability of experimentally infected fish to predation by natural bird hosts.
MATERIALS AND METHODS
For the experiment, we used juvenile rainbow trout (Oncorhynchus mykiss) because they are relatively susceptible to infection (Betterton, 1974) and easy to maintain under laboratory conditions. Fish were obtained from a commercial fish farm where they had been reared in indoor tanks supplied with ground water, which ensured that they had no eye flukes or other helminth parasites. We recognize that the behaviour of farmed fish may not be fully comparable to that of free-living fish. However, wild fish are commonly infected with several parasite species, which favours the use of fish farmed in ground water. The experimental set-up was designed to mimic natural transmission dynamics of the parasite. Haphazardly chosen fish were infected with D. spathaceum cercariae under laboratory conditions in late summer (10 months before the predation experiment) when the parasite transmission from snails to fish occurs naturally (Karvonen, Seppälä and Valtonen, 2004b). Since complete development of metacercariae usually takes 1–2 months, depending on water temperature (see Sweeting, 1974), this procedure ensured that the parasites were fully developed by the time of the predation experiment and capable of manipulating fish (Seppälä et al. 2005b). Cercariae were released by 10 naturally infected Lymnaea peregra snails. We pooled all cercariae into one suspension, and estimated the cercarial density from 10×1 ml samples. Fish were exposed to the parasites concurrently in 6 tanks each containing 150 fish in 65 litres of water. Three randomly chosen tanks received an infection dose of 250 cercariae per fish. The purpose of this exposure procedure was to produce fish with infection intensities (intensity indicates the number of parasites in an infected host; Bush et al. 1997) high enough to induce possible effects but still corresponding to natural intensities (see Valtonen and Gibson, 1997; Marcogliese et al. 2001). Fish in the remaining 3 tanks were sham exposed with water and retained as uninfected control fish. During the exposure, the water flow through the tanks was turned off and the water, at 17·1 °C at the time of exposure, was aerated with aquarium pumps. After 30 min exposure, the water flow was turned on, and the water volume in each tank was increased to 185 litres. Fish were maintained under these conditions until the predation experiment and fed daily with commercial fish pellets. During the maintenance period, water temperature decreased to 4–5 °C over the winter. All fish appeared healthy to the human eye during the study.
The predation experiment was conducted in next spring and early summer (10 months after parasite exposure) in 3 floating cages, each with a diameter of 2·0 m and a depth of 2·4 m. Cages, made of soft black net and left open from the top to allow free entrance for the birds into cages, were placed near the shoreline into Lake Konnevesi in Central Finland (62 °37′N, 26 °21′E). The experiment was conducted in late May and early June when parasite transmission from snails to fish does not yet occur in our study site (Karvonen et al. 2004b). During the experiment, water temperature increased from 6·5 to 15·7 °C. We haphazardly selected fish from all the storage tanks and placed them into the cages so that each cage received 20 infected and 20 control fish. The fish were allowed to recover from the transfer for 5 days before the experiment, at which time they were fed daily and the cages were covered with a net to prevent predation by birds. At the start of the experiment, the net covering the cages was removed and wild birds were allowed to feed freely on the fish from the cages. We followed progress of the experiment from the shore with binoculars, and recorded the bird species utilizing the cages and the number of fish taken. We terminated the experiment after 13–15 fish were taken from each cage. Since the experiment lasted only a few days each time, the fish were not fed during that time. We repeated the experiment 3 times in each cage using a new set of fish every time. Three fish died during the adjustment periods and were excluded from the data.
At the end of the experiment, the remaining fish were removed from the cages and killed with an overdose of 0·01% MS 222 fish anaesthetic (Sigma Chemical Co., St Louis, USA). We measured the coverage of parasite-induced cataracts from the lenses of each fish with a Kowa Portable Slit Lamp SL-14 microscope (see Wall and Bjerkås, 1999; Karvonen et al. 2004a) using a subjective scale: 0=no cataracts; 1=cataracts covering less than 50%; 2=cataracts covering 50–100%; 3=cataracts covering 100%; and 4=opaque cataracts covering 100% of the lens area. The thickness of cataracts was considered only when whole lens was occluded. We counted D. spathaceum metacercariae by dissecting the lenses, and measured the length (±1 mm) and mass (±0·1 g) of each fish. Since the intensity of infection, cataract coverage and body size could not be measured from the fish that were removed by birds during the experiment, we estimated these parameters from samples of 30 infected and 30 control fish taken haphazardly from the storage tanks. We killed the fish and studied them as mentioned above.
We analysed the data by calculating the values of Manly's α (Manly, 1974) for each group of fish to assess the relative predation susceptibility of infected and control fish:
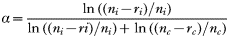
where ni is the number of infected fish at the beginning of the experiment, and ri is the number of infected fish caught in the experiment. Correspondingly nc presents the number of control fish in the beginning of the experiment, and rc the number of control fish caught. The values of α range between zero and 1, and values higher than 0·5 indicate that infected fish were caught more often than control fish. Observed values of α were compared to a situation of equal susceptibility (α=0·5) using a one-sample t-test. Possible change in the relative susceptibility of fish to predation during the experiment was analysed by comparing the values of α between different rounds of the experiment using ANOVA. Distributions of infection intensity and cataract coverage between the haphazard sample of infected fish taken from the storage tanks and those not eaten in the experiment were compared using independent-samples Kolmogorov-Smirnov tests. Body length and mass of infected and control fish were compared using independent-samples t-tests. The experiment was conducted with permission of the Lab-Animal Care and Use Committee of the University of Jyväskylä.
RESULTS
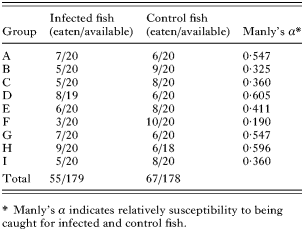
Four different bird species, common gull (Larus canus), lesser black-backed gull (L. fuscus), herring gull (L. argentatus) and common tern (Sterna hirundo) visited the cages during the experiment. Since these were unmarked wild birds, we were unable to determine the number of different individuals feeding on the cages. Common gulls caught 64%, lesser black-backed gulls 31%, herring gulls 2% and common terns 3% of the total of 122 fish that were eaten during the experiment. The susceptibility of infected fish to predation did not differ from that of control fish (Table 1; t-test: t8=−1·30, P=0·229). Either relative susceptibility of fish to predation did not change between the rounds of the experiment (ANOVA: F2,6=0·371, P=0·705). Possible differences between bird species in their preference for infected and uninfected fish could not be analysed. In the haphazard sample of exposed fish taken from the storage tanks, all fish were parasitized and the mean (±S.E.) intensity of D. spathaceum metacercariae was 64·0±4·6. This caused cataract formation in the lenses of fish with coverage from 1·5 to 4·0 (median 3·0; mean for both lenses, see the scale above). Control fish were free of eye flukes and no cataracts were observed. Distributions of infection intensity and cataract coverage did not differ between the haphazard sample of infected fish and those not eaten in the experiment (Kolmogorov-Smirnov test: intensity of infection: Z=0·735, P=0·653, n1=30, n2=124; cataract coverage: Z=0·433, P=0·992, n1=30, n2=124). The average (±S.E.) body length and mass for infected fish in the haphazard sample were 118±2·4 mm and 15·9±1·0 g, respectively. Corresponding values for control fish were 114±2·2 mm and 14·0±0·8 g, which did not differ from the infected fish (t-test: length: t58=−1·21, P=0·230; mass: t58=−1·46, P=0·149).
Table 1. Numbers of infected and control fish eaten from each group and values of Manly's α (During the adjustment periods, 1 infected fish died from group D and 2 control fish from group H.)
DISCUSSION
Several trophically transmitted parasites alter the phenotype of their intermediate hosts, which has been suggested to enhance parasite transmission to target hosts through increased host susceptibility to predation (reviewed by Moore, 2002). However, in most study systems, the effect of the parasites has been investigated only under laboratory conditions, which is why it is difficult to interpret how host manipulation affects parasite transmission efficiency in the wild. In this study, we predisposed fish infected with the trematode D. spathaceum and equivalent controls to predation by wild birds in cages placed into a lake. Contrary to our expectations, we did not find any effect of the parasite on the vulnerability of fish to predation as both infected and control fish were taken from the cages in equal proportions. This result appears to contradict our earlier laboratory studies where we have shown the parasite to reduce fish escape response (Seppälä et al. 2004) and crypsis (Seppälä et al. 2005a), and increase their vulnerability to simulated avian predation (capture with a dip-net, Seppälä et al. 2004, 2005b). Thus, the result suggests that the laboratory experiments may have overestimated to some extent the effect of altered fish behaviour on their vulnerability to predation.
However, the result of this study may have been confounded by the experimental set-up, because the cages had an apparent effect on foraging behaviour of gulls, which caught most of the fish (97%) that were eaten in the experiment. Usually gulls stood on the edges of the cages (ca. 15 cm above the water surface) waiting until the fish swam close to them, and then either jumped into the water or picked up the fish while still standing on the edge. Both of these techniques were very effective probably because these short distance attacks were very fast and also excluded some of the stimuli to fish caused by the movement or shadow of an approaching predator. In particular, this may have increased the predation vulnerability of control fish (Seppälä et al. 2004). Only terns caught fish in their usual way by striking into the water from the air. This technique may have allowed fish to escape attacks more effectively, which is supported by our observations on the lower percentage of successful predation attempts made by terns, although this was not quantified in detail. However, only a minority of fish (3%) were caught by terns compared to gulls (97%) and therefore their effect on the result was only marginal. This may be because terns were rare in the area and may have preferred smaller fish in their diet (see Bugoni and Vooren, 2004; Mauco and Favero, 2004). To sum up, these factors may have contributed to equal predation susceptibility of infected and control fish in this study.
Diplostomum spathaceum parasites can infect several different bird species such as gulls (Karvonen et al. 2006), common terns (personal observations), great cormorants (Phalacrocorax carbo, personal observations) and even domestic chickens (Field, McKeown and Irwin, 1994). This suggests that D. spathaceum is a broad host generalist in its definitive host use, and may rely on periodic ingestion by a wide variety of definitive hosts in its transmission. Under such circumstances, the relative role of different bird species in the maintenance of the parasite life-cycle becomes an issue of exposure rather than of specificity. In this study, most of the fish were caught by gulls, although in nature, except for lesser black-backed gulls, fish caught by the birds themselves generally comprise only a low proportion in the diet of Finnish gull species (reviewed by Götmark, 1984). Usually gulls prefer mainly terrestrial food (e.g. earthworms and other invertebrates) or refuse and fish offal from human activities (Götmark, 1984). This suggests that the exposure of gulls to infected fish may be low except for locations such as fish docks and fish farms where they can easily feed on considerable amounts of fish, and thus the parasite numbers can locally be very high (Karvonen et al. 2006). Alternatively, it may be that bird species consuming mainly fish are more frequently infected with the parasite in natural conditions, although detailed data on D. spathaceum infections in several bird species in Finland are currently lacking. From such species, terns caught only a minority of fish in this study, and although arctic loons (Gavia arctica), red-breasted mergansers (Mergus serrator) and common mergansers (Mergus merganser) were common in the area, these birds did not enter the cages.
In general, examination of the effect of host manipulation on parasite transmission efficiency in the wild would require experiments considering predation also by other fish eating bird species than gulls. To attract diving birds, experiments could be conducted in larger cages, possibly bordering up on shoreline (see Lafferty and Morris, 1996). In the case of terns, cages, possibly with smaller fish, could be located closer to tern colonies. These studies could also evaluate possible differences between bird species in their preference for infected and uninfected fish and help to resolve which species act as ‘required hosts’ (sensuHolmes, 1979) maintaining parasite populations for D. spathaceum. However, we emphasize that the importance of different bird species may vary both temporally and spatially depending on differences in local bird community structures and host ecology.
We thank J. Hannula for assistance in the field. We are grateful to R. Poulin and the anonymous referees for their helpful comments on the manuscript. Konnevesi Research Station provided the facilities for the experiment. The study was funded by the SUNARE research programme of the Academy of Finland, Ella & Georg Ehrnrooth Foundation, Biological Interactions graduate school, and the Finnish Cultural Foundation.



