Introduction
The use of electroconvulsive therapy (ECT) for depression is often limited by its cognitive side-effects (Semkovska and McLoughlin, Reference Semkovska and McLoughlin2010), but most of these usually resolve within two weeks of treatment, with the majority of cognitive functions improving beyond baseline scores after this period (Semkovska and McLoughlin, Reference Semkovska and McLoughlin2010). Age, sex, pre-morbid intellectual function, and form of ECT all impact on cognitive outcomes following a treatment course (Sackeim et al., Reference Sackeim, Prudic, Nobler, Fitzsimons, Lisanby, Payne, Berman, Brakemeier, Perera and Devanand2008; Tor et al., Reference Tor, Bautovich, Wang, Martin, Harvey and Loo2015; Semkovska et al., Reference Semkovska, Landau, Dunne, Kolshus, Kavanagh, Jelovac, Noone, Carton, Lambe, McHugh and McLoughlin2016; Kolshus et al., Reference Kolshus, Jelovac and McLoughlin2017), though to date we have no definitive predictors for risk.
Telomeres are DNA-protein complexes that cap the ends of chromosomes to maintain genomic integrity and are comprised of tandemly repeated hexameric sequences of TTAGGG repeats that form a scaffold to which telomeric proteins bind (Blackburn, Reference Blackburn2005). A multicomponent telomere homeostasis system exists to prevent telomere over-extension and promote extension whenever shortening occurs. Normal cells have limited replicative capacity, known as the ‘Hayflick limit,’ which can be explained by the progressive shortening of telomeres at every mitotic event (Hayflick and Moorhead, Reference Hayflick and Moorhead1961), leading eventually to cell arrest and senescence (Shalev et al., Reference Shalev, Entringer, Wadhwa, Wolkowitz, Puterman, Lin and Epel2013). Telomere length (TL) is thus suggested to represent a marker of a cell's biological, as opposed to chronological, age (Epel et al., Reference Epel, Blackburn, Lin, Dhabhar, Adler, Morrow and Cawthon2004). In the normal human population, TL is heterogeneous and ranges between 5–15 kilobases (Allsopp et al., Reference Allsopp, Vaziri, Patterson, Goldstein, Younglai, Futcher, Greider and Harley1992). TL is highly heritable, with a stronger maternal than paternal inheritance, and is positively associated with paternal age (Broer et al., Reference Broer, Codd, Nyholt, Deelen, Mangino, Willemsen, Albrecht, Amin, Beekman, de Geus, Henders, Nelson, Steves, Wright, de Craen, Isaacs, Matthews, Moayyeri, Montgomery, Oostra, Vink, Spector, Slagboom, Martin, Samani, van Duijn and Boomsma2013). TL is influenced by genetic background and environmental factors (Samassekou et al., Reference Samassekou, Gadji, Drouin and Yan2010), and can vary between tissue type, cells, and even between chromosomes within the same cell. TL dysfunction and accelerated shortening are associated with increased oxidative stress and increased inflammatory load, increased stress and hypothalamic-pituitary-adrenal axis dysregulation, metabolic imbalance, and decreased neurotrophic factors, e.g. brain derived neurotrophic factor, all of which have links to depression (O'Donovan et al., Reference O'Donovan, Pantell, Puterman, Dhabhar, Blackburn, Yaffe, Cawthon, Opresko, Hsueh, Satterfield, Newman, Ayonayon, Rubin, Harris and Epel2011; Barnes et al., Reference Barnes, Fouquerel and Opresko2018; Manoliu et al., Reference Manoliu, Bosch, Brakowski, Bruhl and Seifritz2018).
Depression is suggested to represent a state of accelerated biological aging (Wolkowitz et al., Reference Wolkowitz, Epel, Reus and Mellon2010), and is a risk factor for age-related disorders, e.g. cardiovascular disease (Van der Kooy et al., Reference Van der Kooy, van Hout, Marwijk, Marten, Stehouwer and Beekman2007) and Alzheimer's disease (Ownby et al., Reference Ownby, Crocco, Acevedo, John and Loewenstein2006), both of which are associated with shortened TL (Brouilette et al., Reference Brouilette, Moore, McMahon, Thompson, Ford, Shepherd, Packard and Samani2007; Liu et al., Reference Liu, Huo, Wang, Wang, Liu, Liu, Wang and Ji2016). Thus, researchers began examining the association between TL and depression in 2006. The first study to analyze TL in clinically diagnosed patients with depression showed shortened TL in patients compared to controls (Simon et al., Reference Simon, Smoller, McNamara, Maser, Zalta, Pollack, Nierenberg, Fava and Wong2006). However, since then results have been varied, with studies showing both shorter TL (Hartmann et al., Reference Hartmann, Boehner, Groenen and Kalb2010; Wikgren et al., Reference Wikgren, Maripuu, Karlsson, Nordfjall, Bergdahl, Hultdin, Del-Favero, Roos, Nilsson, Adolfsson and Norrback2012; Garcia-Rizo et al., Reference Garcia-Rizo, Fernandez-Egea, Miller, Oliveira, Justicia, Griffith, Heaphy, Bernardo and Kirkpatrick2013; Verhoeven et al., Reference Verhoeven, Revesz, Epel, Lin, Wolkowitz and Penninx2013; Szebeni et al., Reference Szebeni, Szebeni, DiPeri, Chandley, Crawford, Stockmeier and Ordway2014; Tyrka et al., Reference Tyrka, Parade, Price, Kao, Porton, Philip, Welch and Carpenter2016) and no difference in TL (Zhang et al., Reference Zhang, Cheng, Craig, Redman and Liu2010; Wolkowitz et al., Reference Wolkowitz, Mellon, Epel, Lin, Dhabhar, Su, Reus, Rosser, Burke, Kupferman, Compagnone, Nelson and Blackburn2011; Teyssier et al., Reference Teyssier, Chauvet-Gelinier, Ragot and Bonin2012; Hoen et al., Reference Hoen, Rosmalen, Schoevers, Huzen, van der Harst and de Jonge2013; Schaakxs et al., Reference Schaakxs, Verhoeven, Oude Voshaar, Comijs and Penninx2015) between clinically diagnosed patients with depression and controls. A number of meta-analyses have now been carried out to clarify the relationship between TL and depression (Schutte and Malouff, Reference Schutte and Malouff2015; Lin et al., Reference Lin, Huang and Hung2016b; Ridout et al., Reference Ridout, Ridout, Price, Sen and Tyrka2016). Overall, they show shortened TL in patients with depression v. controls, and this effect was greatest where patients were clinically diagnosed (Lin et al., Reference Lin, Huang and Hung2016b; Ridout et al., Reference Ridout, Ridout, Price, Sen and Tyrka2016). Ridout et al. (Reference Ridout, Ridout, Price, Sen and Tyrka2016) also showed that TL was significantly associated with depression severity. To date, only a few studies have examined the association between TL and response to treatments for depression (Martinsson et al., Reference Martinsson, Wei, Xu, Melas, Mathe, Schalling, Lavebratt and Backlund2013; Hough et al., Reference Hough, Bersani, Mellon, Epel, Reus, Lindqvist, Lin, Mahan, Rosser, Burke, Coetzee, Nelson, Blackburn and Wolkowitz2016; Rasgon et al., Reference Rasgon, Lin, Lin, Epel and Blackburn2016), with all suggesting that shorter TL is associated with poor treatment outcomes.
TL is suggested to be linked to cognitive performance (Kljajevic, Reference Kljajevic2011) and may act as a biomarker of both cognitive and physical aging (Harris et al., Reference Harris, Marioni, Martin-Ruiz, Pattie, Gow, Cox, Corley, von Zglinicki, Starr and Deary2016). One study investigating the relationship between TL and cognition showed a negative association with age and positive association with cognitive performance (episodic memory and associated learning, recognition memory for non-verbal patterns, working memory capacity) in healthy adults from the general population (Valdes et al., Reference Valdes, Deary, Gardner, Kimura, Lu, Spector, Aviv and Cherkas2010). TL accounted for 2.3% of the variance in cognitive ability, suggesting that it might act as a biomarker of cognitive aging. Other studies have also indicated that TL is associated with cognitive ability (Ma et al., Reference Ma, Lau, Suen, Lam, Leung, Woo and Tang2013), and shorter TL has been proposed as a prognostic factor for cognitive decline and dementia (Yaffe et al., Reference Yaffe, Lindquist, Kluse, Cawthon, Harris, Hsueh, Simonsick, Kuller, Li, Ayonayon, Rubin and Cummings2011; Martin-Ruiz et al., Reference Martin-Ruiz, Dickinson, Keys, Rowan, Kenny and Von Zglinicki2006; Devore et al., Reference Devore, Prescott, De Vivo and Grodstein2011; Honig et al., Reference Honig, Kang, Schupf, Lee and Mayeux2012). However, a recent meta-analysis found no association between TL and decline in general cognitive ability (Zhan et al., Reference Zhan, Clements, Roberts, Vassilaki, Druliner, Boardman, Petersen, Reynolds, Pedersen and Hagg2018). While a small pilot study (n = 53) showed a link between TL and cognitive ability in patients with schizophrenia (Vaez-Azizi et al., Reference Vaez-Azizi, Ruby, Dracxler, Rothman, Perrin, Walsh-Messinger, Antonius, Goetz, Goetz, Keefe and Malaspina2015), no study has examined the relationship between TL and cognitive performance in patients with depression or following ECT.
Here, we examined whether blood TL was associated with response to ECT, mood scores, and cognitive function in 100 depressed individuals before and after treatment with ECT. We also compared TL in depressed patients at baseline with that of healthy controls. We hypothesized that: (1) TL would be shorter in patients with depression compared to controls; (2) that shorter TL would predict poorer response to ECT; and (3) that shorter TL would predict the risk for cognitive side-effects post-ECT.
Material and methods
Subjects
This study was approved by St Patrick's University Hospital Research Ethics Committee and adhered to the Declaration of Helsinki (World Medical Association, 2013). All participants provided written informed consent.
Severely depressed patients were recruited as part of the EFFECT-Dep Trial between 2008–2012 in St. Patrick's Mental Health Services, Ireland (Semkovska et al., Reference Semkovska, Landau, Dunne, Kolshus, Kavanagh, Jelovac, Noone, Carton, Lambe, McHugh and McLoughlin2016). Healthy controls, with no history of psychiatric illness, were recruited through advertisement in local newspapers and social media.
Fasting peripheral blood samples were collected in K2EDTA tubes (BD, UK) between 07:30–09:30 and stored at −80 °C until analysis. Blood was collected from the patient on the morning of the first ECT treatment and from controls on the assessment day.
Electroconvulsive therapy
ECT was administered with hand-held electrodes using methohexitone (0.75–1.0 mg/kg) for anesthesia and succinylcholine (0.5–1.0 mg/kg) as muscle relaxant (Semkovska et al., Reference Semkovska, Landau, Dunne, Kolshus, Kavanagh, Jelovac, Noone, Carton, Lambe, McHugh and McLoughlin2016). Patients were randomly allocated to receive treatment twice-weekly with either moderate dose bitemporal (1.5 × seizure threshold) or high-dose unilateral (6 × seizure threshold) ECT in a real-world practice. Patients were maintained on pharmacotherapy as usual.
Inclusion criteria: >18 years old, referred for ECT for treatment of a major depressive episode as diagnosed by the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., Reference First, Spitzer, Gibbon and Williams1996), pre-treatment Hamilton Depression Rating Scale 24-item version (HAM-D24) score ⩾21 (Beckham and Leber, Reference Beckham and Leber1985).
Exclusion criteria: substance misuse in the previous 6 months, medically unfit for general anesthesia, ECT in the previous 6 months, dementia or other axis I diagnosis, involuntary status or inability/refusal to consent.
Clinical and cognitive assessments
Demographic and clinical data were documented for all participants. Depression severity and response to ECT were assessed using the HAM-D24. Response was defined as a 60% reduction in HAM-D24 and a score ⩽16 at end-of-treatment. Remission was defined as a ⩾60% reduction in HAM-D24 and a score ⩽10 for two weeks post-ECT.
Global cognition was assessed in all participants using the Mini-Mental State Examination (MMSE) (Folstein et al., Reference Folstein, Folstein and McHugh1975). Autobiographical memory was prioritized as a cognitive outcome in the EFFECT-Dep Trial and measured using the Columbia Autobiographical Memory Interview – Short Form (CAMI-SF) (McElhiney et al., Reference McElhiney, Moody and Sackeim2001; Semkovska et al., Reference Semkovska, Noone, Carton and McLoughlin2012). A prolonged time to recovery of orientation has also been linked to problems with autobiographical memory following ECT (Sackeim et al., Reference Sackeim, Prudic, Nobler, Fitzsimons, Lisanby, Payne, Berman, Brakemeier, Perera and Devanand2008; Tor et al., Reference Tor, Bautovich, Wang, Martin, Harvey and Loo2015). We assessed the immediate cognitive effects of ECT by documenting the time to recovery of orientation after each session using a 5-point scale over the 50 min post-ECT and calculating the mean time to recovery of orientation across the treatment course (Sackeim et al., Reference Sackeim, Prudic, Nobler, Fitzsimons, Lisanby, Payne, Berman, Brakemeier, Perera and Devanand2008; Semkovska et al., Reference Semkovska, Landau, Dunne, Kolshus, Kavanagh, Jelovac, Noone, Carton, Lambe, McHugh and McLoughlin2016). Thus, here we assessed the relationship between TL and global cognitive function (MMSE score) in patients and controls, and the relationship between TL and pre-ECT autobiographical memory performance, recovery of orientation following ECT sessions, and retrograde autobiographical amnesia post-ECT in patients, as these were the measures for which we had the most complete datasets (Semkovska et al., Reference Semkovska, Landau, Dunne, Kolshus, Kavanagh, Jelovac, Noone, Carton, Lambe, McHugh and McLoughlin2016).
DNA extraction
Whole blood DNA was extracted using an Autopure LS® (Qiagen) by Trinity College Dublin's Biobank facility, as previously described (Ryan et al., Reference Ryan, Dunne and McLoughlin2018). Purified DNA had an A260/A280 ratio of 1.7–1.9.
Telomere assay
Blood TL was measured using quantitative real-time polymerase chain reaction (qRT-PCR) (Cawthon, Reference Cawthon2002). A five-point standard curve of HeLa cell DNA ranging from 0.31–25 ng was included on each plate so that the quantity of targeted templates in each sample could be determined relative to the standard curve. TL is expressed as a telomere to single-copy gene (T/S) ratio.
The telomere primers used were tel1b (CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT), at a final concentration of 100 nM, and tel2b (GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT), at a final concentration of 900 nM. The primers for the single-copy gene (Hgb1) were hgb1 (GCTTCTGACACAACTGTGTTCACTAGC), at a final concentration of 300 nM, and hgb2 (CACCAACTTCATCCACGTTCACC), at a final concentration of 700 nM. Primers were salt-free purified (Eurofins Genomics, Germany). For the telomere qRT-PCR reaction, the cycling profile was 95 °C for 5 min followed by 30 cycles of 95 °C for 15 s and 54 °C for 2 min. For the single-copy gene, the cycling profile was 95 °C for 5 min followed by 40 cycles of 95 °C for 15 s, 58 °C for 20 s, and 72 °C for 28 s. Each sample was assayed in duplicate wells on three separate plates, with identical sample/well positions used across plates. The average r 2 of the PCR runs was ⩾0.99 and the average efficiencies of the TL and HGB runs were 105 and 92%, respectively. To control for inter-assay variability, five control DNA samples were included in each run. The intra-assay coefficient of variation (CV) was <3% and the inter-assay CV was <6%; assays above these %CVs were excluded from analyses.
Statistical analysis
SPSS, version 25 (IBM Corporation, NY, USA), was used to analyze all data. Data were assessed for normality using the Kolmogorov-Smirnov test. Differences in demographic and clinical characteristics between groups were determined using chi-squared tests or independent t tests, where appropriate. General linear models were used to determine differences between groups. Pearson's or Spearman's correlation tests were used to assess associations between continuous variables, while point-biserial correlations were used to determine associations between continuous and dichotomous variables. We used linear regression models to examine associations between TL and cognitive outcomes post-ECT. Logistic regression models were used to analyze the association between TL and therapeutic outcome. The EFFECT-Dep Trial showed that bitemporal ECT is associated with a longer time to recovery of reorientation post-ECT compared to unilateral ECT (Semkovska et al., Reference Semkovska, Landau, Dunne, Kolshus, Kavanagh, Jelovac, Noone, Carton, Lambe, McHugh and McLoughlin2016); therefore, we included electrode placement as a potential confounder in our analyses. Analyses were conducted as follows: (1) unadjusted or (2) adjusted for age and electrode placement (where appropriate). TL data are presented as mean T/S ratio ± standard deviation (s.d.). Differences with a p value < 0.05 were regarded as statistically significant.
An effect size (Hedges' g) of 0.422 for shorter leukocyte telomere length in patients diagnosed with depression compared to healthy controls was reported by Lin et al., Reference Lin, Huang and Hung2016b. Therefore, a post hoc analysis was performed to assess the power of our study to detect a similar effect size. Using 100 patients and 80 controls and setting alpha at 0.05 (two-sided), we had 80% power to detect an effect size of 0.422.
Results
Demographic and clinical characteristics of subjects
Table 1 depicts the demographic and clinical information for all subjects. Blood DNA samples were available from 131/138 patients from the EFFECT-Dep Trial. Owing to poor PCR amplification or for the purposes of age and sex matching with controls, 33 samples were excluded from final analyses. We thus compared samples from a total of 100 patients with depression to 80 age- and sex-matched healthy controls. The groups differed significantly with respect to the number of smokers (p < 0.001), with more smokers found in the depressed group, and educational attainment (p < 0.001), with the depressed group attaining a lower level of education overall.
Table 1. Demographic and clinical characteristics of the ECT and healthy control participants

BMI, body mass index; CAMI-SF, Columbia Autobiographical Memory Interview – Short Form; ECT, electroconvulsive therapy; HAM-D24, Hamilton depression rating scale, 24-item version; MAOI, monoamine oxidase inhibitor; MMSE, Mini-Mental State Examination; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
TL in patients with depression and controls
TL data were non-normally distributed. However, the data were normally distributed following natural log transformation and so log transformed data were used in further analyses.
As expected, TL was significantly negatively associated with age (ρ = −0.32, p = 0.000016) in the group as a whole, and in the depressed (r = −0.34, p = 0.001) and control (r = −0.33, p = 0.003) groups individually. TL was not significantly associated with sex, body-mass index (BMI), smoking status, or educational attainment (all p > 0.05). Therefore, all subsequent analyses were performed both unadjusted and adjusted for age as a confounder.
TL did not differ between controls and patients with depression (F (1,178) = 0.67, p = 0.42), with a mean TL (mean ± s.d.) of 2.43 ± 0.61 in the depressed group and 2.51 ± 0.68 in the control group (Fig. 1). Adjusting for age had no effect (F(1,177) = 0.11, p = 0.74). Moreover, removing persons with an inflammatory (n = 12) or neurological disorder (n = 6) from analyses did not alter results; therefore, we retained these people in all further analyses. The effect size of our study was −0.12 for shorter TL in depressed patients. This is below the threshold of 0.2 for a meaningful result.

Fig. 1. Unadjusted raw T/S ratio values in healthy controls compared to patients with depression. T/S ratio, telomere:single-copy gene ratio.
TL did not significantly differ (p > 0.05) between patients previously treated with ECT (n = 37) v. those who were not (n = 63) (mean ± s.d.: 2.34 ± 0.56 and 2.48 ± 0.63, respectively), or v. controls (p > 0.05), and adjusting for age had no effect. TL also did not differ between patients with unipolar or bipolar depression (mean ± s.d.: 2.41 ± 0.60 and 2.52 ± 0.65, respectively; F (1,98) = 0.50, p = 0.48), or psychotic or non-psychotic depression (mean ± s.d.: 2.28 ± 0.45 and 2.47 ± 0.64, respectively; F(1,98) = 1.69, p = 0.20), and adjustment for age had no effect. TL was not associated with the number of depressive episodes experienced by patients (ρ = −0.01, p = 0.92) or the duration of illness (ρ = −0.07, p = 0.53), and adjusting for age did not alter the results.
The relationship between TL and mood was also examined. We found no association between TL and baseline/pre-ECT HAM-D24 score in either the depressed (ρ = 0.11, p = 0.29) or control (ρ = −0.04, p = 0.71) groups, and adjusting for age had no effect.
TL in ECT responders and remitters
We next determined whether there were differences in TL between ECT responders/non-responders and remitters/non-remitters (Tables 2 and 3, respectively). ECT responders were significantly older than non-responders (p = 0.001) and had less ECT sessions (p < 0.001). There were significantly more smokers in the non-responder group (p = 0.02). ECT remitters were significantly older than non-remitters (p < 0.001) and had less ECT sessions (p < 0.001). No other differences were noted between responder/non-responder or remitter/non-remitter groups.
Table 2. Demographic and clinical characteristics of the ECT responders v. non-responders
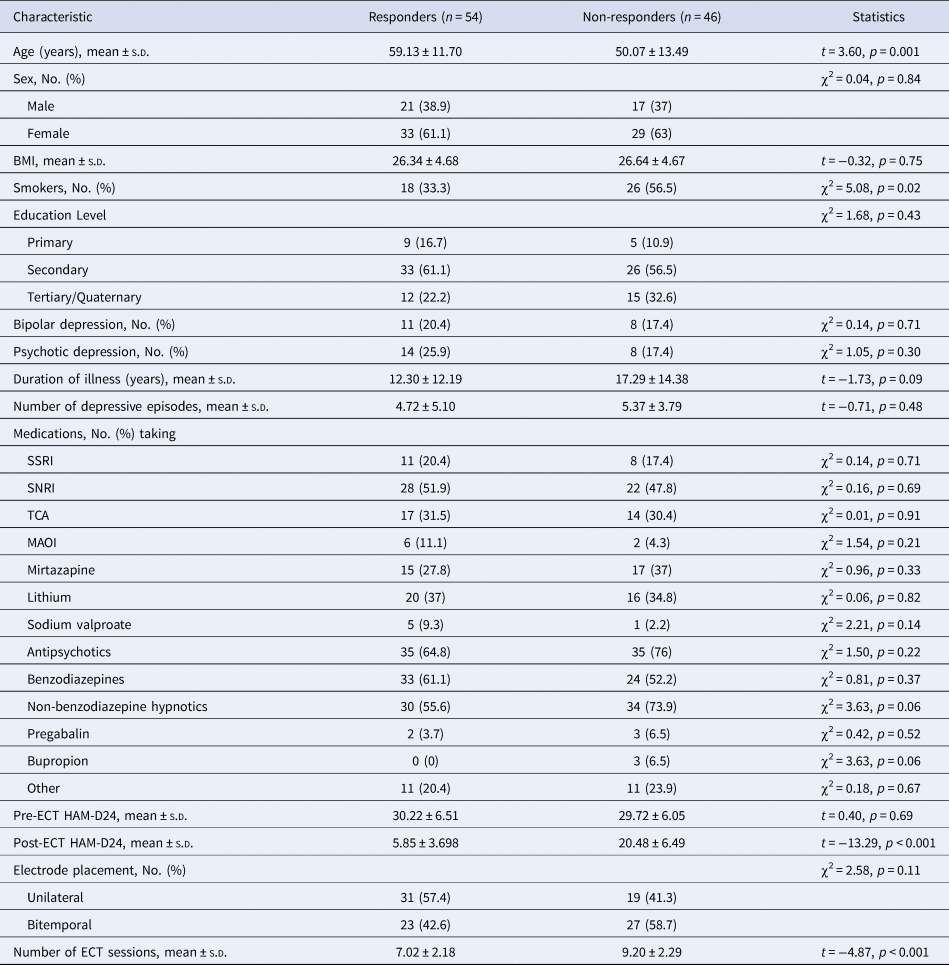
BMI, body mass index; ECT, electroconvulsive therapy; HAM-D24, Hamilton depression rating scale, 24-item version; MAOI, monoamine oxidase inhibitor; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
Table 3. Demographic and clinical characteristics of the ECT remitters v. non-remitters

BMI, body mass index; ECT, electroconvulsive therapy; HAM-D24, Hamilton depression rating scale, 24-item version; MAOI, monoamine oxidase inhibitor; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
Mean TL did not differ between responders and non-responders (mean ± s.d.: 2.33 ± 0.52 and 2.54 ± 0.69, respectively; F (1,98) = 2.16, p = 0.15) or between remitters and non-remitters (mean ± SD: 2.35 ± 0.51 and 2.49 ± 0.67, respectively; F (1,98) = 0.82, p = 0.37), and adjustment for age had no effect. Shorter TL did not confer increased odds for being an ECT non-responder or non-remitter, as assessed using unadjusted (β = 1.24, p = 0.15 and β = 0.40, p = 0.25, respectively) or fully adjusted (for age and electrode placement; β = 0.44, p = 0.64 and β = −0.07, p = 0.87, respectively) logistic regression models.
Correlation analyses assessed the association between TL and baseline HAM-D24 scores as well as the absolute and relative change in HAM-D24 score in responders/non-responders and remitters/non-remitters. There was no relationship between TL and mood at baseline in responders (ρ = −0.02, p = 0.92) and non-responders (ρ = 0.27, p = 0.08), or in remitters (ρ = 0.03, p = 0.83) and non-remitters (ρ = 0.15, p = 0.25). Moreover, we found no associations between TL and the absolute or relative change in HAM-D24 post-ECT, and controlling for age and electrode placement on the relationship between TL and the absolute or relative change in HAM-D24 did not alter these results (all p > 0.05).
TL and cognition
We collected mean time to recovery of orientation post-ECT for 100/100 patients with depression (mean time in min ± s.d.: 25.43 ± 11.57). A linear regression analysis was carried out to determine if baseline TL predicted the mean time to recovery of orientation post-ECT. The results of the regression model were non-significant (F (1,98) = 2.06, p = 0.15, R 2 = 0.02). However, the model was a significant predictor of mean time to recovery of orientation when age and electrode placement were added (F (3,96) = 3.96, p = 0.01, R 2 = 0.11), with age contributing significantly to the model (β = 0.28, p = 0.008), while TL (β = −0.04, p = 0.73) and electrode placement (β = 0.15, p = 0.13) did not.
We collected baseline CAMI-SF scores for 94/100 patients and percentage recall on the CAMI-SF at end-of-treatment for 92/100 patients. The baseline CAMI-SF score provides a measure of retrospective autobiographical memory performance with a maximum score of 60. There was no relationship between TL and baseline CAMI-SF score (ρ = 0.13, p = 0.21), and adjusting for age had no effect (Spearman's partial ρ = 0.05, p = 0.64). A linear regression analysis was performed to determine if TL predicted the percentage recall consistency of baseline memories post-ECT. The results of the regression model were non-significant (F (1,90) = 0.002, p = 0.99, R 2 = 0.000002), and the model remained non-significant when age and electrode placement were added (F (3,88) = 2.34, p = 0.08, R 2 = 0.07).
We collected MMSE scores for 85/100 patients with depression and 80/80 healthy controls. The mean baseline MMSE scores were 27.66 ± 2.15 for patients and 29.44 ± 0.86 for controls. The difference between the groups was significant (p < 0.001). Baseline MMSE score was significantly negatively associated with age (ρ = −0.281, p < 0.001). In the sample as a whole, MMSE score was significantly correlated with TL (ρ = 0.20, p = 0.01); however, this was no longer significant after adjusting for age (p = 0.14). There was no correlation between TL and baseline MMSE score in either the depressed (ρ = 0.18, p = 0.09) or control (ρ = 0.22, p = 0.05) groups individually, and adjusting for age had no effect (p = 0.83 and p = 0.05, respectively). In the depressed group, there was no correlation between TL and the absolute or relative change in MMSE score post-ECT (ρ = 0.14, p = 0.21 and ρ = 0.05, p = 0.59), and adjusting for age and electrode placement did not alter this result (p = 0.31 and p = 0.91, respectively).
Discussion
This study is the first to examine the relationship between TL and therapeutic response to ECT and selected cognitive outcomes post-ECT. In line with previous reports, our results show a significant negative correlation between TL and age. However, we found no difference in TL between healthy controls and medicated, hospitalized, clinically diagnosed patients with depression. There was no association between TL and chronicity of depression or mood scores. Contrary to our initial hypotheses, TL did not predict the response to ECT or selected cognitive outcomes post-ECT.
Previous results regarding the relationship between depression and TL have been varied. Our results are in line with those of some studies that showed no difference in TL between clinically diagnosed patients with depression and controls (Wolkowitz et al., Reference Wolkowitz, Mellon, Epel, Lin, Dhabhar, Su, Reus, Rosser, Burke, Kupferman, Compagnone, Nelson and Blackburn2011; Chen et al., Reference Chen, Epel, Mellon, Lin, Reus, Rosser, Kupferman, Burke, Mahan, Blackburn and Wolkowitz2014; Needham et al., Reference Needham, Mezuk, Bareis, Lin, Blackburn and Epel2015; Schaakxs et al., Reference Schaakxs, Verhoeven, Oude Voshaar, Comijs and Penninx2015; Simon et al., Reference Simon, Walton, Bui, Prescott, Hoge, Keshaviah, Schwarz, Dryman, Ojserkis, Kovachy, Mischoulon, Worthington, De Vivo, Fava and Wong2015). However, the results of three recent meta-analyses suggest that TL is significantly shorter in patients with depression v. controls overall, though, while significant, the effect sizes were small (r = −0.10 to −0.21) (Schutte and Malouff, Reference Schutte and Malouff2015; Lin et al., Reference Lin, Huang and Hung2016b; Ridout et al., Reference Ridout, Ridout, Price, Sen and Tyrka2016). These meta-analyses included studies utilizing different methodologies, both clinical diagnosis and self-report of depression, varied sample populations (with some studies including patients with chronic disease such as fibromyalgia and coronary artery disease), and DNA from different sample types (peripheral blood mononuclear cells, leukocytes, saliva, brain). In contrast to our results, Ridout et al. (Reference Ridout, Ridout, Price, Sen and Tyrka2016) showed a larger effect size for patients diagnosed clinically (r = −0.166) as opposed to by self-report instruments (r = −0.039). However, in keeping with our findings, the authors report no significant effect of the covariates age, sex, and smoking, which had previously been reported to impact on TL (Valdes et al., Reference Valdes, Andrew, Gardner, Kimura, Oelsner, Cherkas, Aviv and Spector2005; Gardner et al., Reference Gardner, Bann, Wiley, Cooper, Hardy, Nitsch, Martin-Ruiz, Shiels, Sayer, Barbieri, Bekaert, Bischoff, Brooks-Wilson, Chen, Cooper, Christensen, De Meyer, Deary, Der, Diez Roux, Fitzpatrick, Hajat, Halaschek-Wiener, Harris, Hunt, Jagger, Jeon, Kaplan, Kimura, Lansdorp, Li, Maeda, Mangino, Nawrot, Nilsson, Nordfjall, Paolisso, Ren, Riabowol, Robertson, Roos, Staessen, Spector, Tang, Unryn, van der Harst, Woo, Xing, Yadegarfar, Park, Young, Kuh, von Zglinicki and Ben-Shlomo2014; Muezzinler et al., Reference Muezzinler, Zaineddin and Brenner2014, Reference Muezzinler, Mons, Dieffenbach, Butterbach, Saum, Schick, Stammer, Boukamp, Holleczek, Stegmaier and Brenner2015, Reference Muezzinler, Mons, Dieffenbach, Butterbach, Saum, Schick, Stammer, Boukamp, Holleczek, Stegmaier and Brenner2016; Rode et al., Reference Rode, Nordestgaard, Weischer and Bojesen2014). The difference between our results and those reported previously for clinically diagnosed depression might be accounted for by the fact that the meta-analysis by Ridout et al. (Reference Ridout, Ridout, Price, Sen and Tyrka2016) included 18 clinically diagnosed case-control studies that used a range of methodologies (e.g. qPCR, Southern blot, qFISH) and different sample types (e.g. leukocytes, brain tissue). Among the studies that used the same method to clinically diagnose depression (i.e. SCID; n = 8) as we used here, those using Southern blotting, which additionally measures the subtelomeric region, were more likely to report differences in TL between patients and controls. Only one study using qRT-PCR to assess TL in samples from patients diagnosed using the SCID, in which the authors used absolute as opposed to relative quantification, reported a difference, with the remaining two studies, which used a methodology similar to the one used here, reported no difference in TL between patients and controls. The other two meta-analyses (Lin et al., Reference Lin, Huang and Hung2016b) showed that the type of assay used in the TL analyses moderated the effect size, with studies using Southern blot or fluorescent in situ hybridization (FISH) assays reporting greater associations between TL and depression than studies using PCR methods. Thus, methods other than PCR may be more suitable for detecting TL differences in case-control studies since the wide inter-laboratory differences in PCR methodology may be leading to the varied results between studies. An international collaborative study of TL assessment indicated that there is a 20% inter-laboratory CV for PCR analyses, while this averaged about 10% for other methodologies (Martin-Ruiz et al., Reference Martin-Ruiz, Baird, Roger, Boukamp, Krunic, Cawthon, Dokter, van der Harst, Bekaert, de Meyer, Roos, Svenson, Codd, Samani, McGlynn, Shiels, Pooley, Dunning, Cooper, Wong, Kingston and von Zglinicki2015). Interestingly, the meta-analyses also showed that the association between TL and depression was stronger in cohorts with a lower mean age (Schutte and Malouff, Reference Schutte and Malouff2015). Notably our total cohort had a mean age of ~54 years; however, when we performed our analysis using only the lower quartile of our sample set (mean age 37 years), we also found no difference in TL between patients with depression and controls (data not shown).
Reports regarding TL and the duration or severity of depressive illness have also been mixed to date. While we found no association between TL and the severity or chronicity of depression, in line with some previous reports (Wikgren et al., Reference Wikgren, Maripuu, Karlsson, Nordfjall, Bergdahl, Hultdin, Del-Favero, Roos, Nilsson, Adolfsson and Norrback2012; Rasgon et al., Reference Rasgon, Lin, Lin, Epel and Blackburn2016), others have reported a significant effect of lifetime exposure to depression on TL (Wolkowitz et al., Reference Wolkowitz, Mellon, Epel, Lin, Dhabhar, Su, Reus, Rosser, Burke, Kupferman, Compagnone, Nelson and Blackburn2011; Martinsson et al., Reference Martinsson, Wei, Xu, Melas, Mathe, Schalling, Lavebratt and Backlund2013), which was stronger in men than in women (Martinsson et al., Reference Martinsson, Wei, Xu, Melas, Mathe, Schalling, Lavebratt and Backlund2013). Importantly, another study suggested that TL shortening may occur in response to depression chronicity and is not involved in the pathophysiology of depression (Wolkowitz et al., Reference Wolkowitz, Mellon, Epel, Lin, Dhabhar, Su, Reus, Rosser, Burke, Kupferman, Compagnone, Nelson and Blackburn2011). TL attrition has also been linked to early life adversity (Tyrka et al., Reference Tyrka, Price, Kao, Porton, Marsella and Carpenter2010; Chen et al., Reference Chen, Epel, Mellon, Lin, Reus, Rosser, Kupferman, Burke, Mahan, Blackburn and Wolkowitz2014; Tyrka et al., Reference Tyrka, Parade, Price, Kao, Porton, Philip, Welch and Carpenter2016; Vincent et al., Reference Vincent, Hovatta, Frissa, Goodwin, Hotopf, Hatch, Breen and Powell2017), with the type and timing of exposure playing a significant role (Ridout et al., Reference Ridout, Levandowski, Ridout, Gantz, Goonan, Palermo, Price and Tyrka2018). It has been proposed that previous studies showing shorter TL in depression may have used patient groups that were enriched for individuals who had experienced adverse events during early life, and that this may account for the differences reported across studies (Vincent et al., Reference Vincent, Hovatta, Frissa, Goodwin, Hotopf, Hatch, Breen and Powell2017). We did not have data on early life adversity available for this study.
Only four studies have so far reported on TL and treatment outcomes for depression (Martinsson et al., Reference Martinsson, Wei, Xu, Melas, Mathe, Schalling, Lavebratt and Backlund2013; Hough et al., Reference Hough, Bersani, Mellon, Epel, Reus, Lindqvist, Lin, Mahan, Rosser, Burke, Coetzee, Nelson, Blackburn and Wolkowitz2016; Rasgon et al., Reference Rasgon, Lin, Lin, Epel and Blackburn2016), and ours is the first to examine the relationship between TL and response to ECT. In a small prospective study of 27 unmedicated patients with depression, pre-treatment TL predicted clinical response to selective serotonin reuptake inhibitors (SSRIs), with treatment non-responders having significantly shorter TL at baseline compared to responders (Hough et al., Reference Hough, Bersani, Mellon, Epel, Reus, Lindqvist, Lin, Mahan, Rosser, Burke, Coetzee, Nelson, Blackburn and Wolkowitz2016). In a double-blind placebo-controlled study of add-on pioglitazone (a peroxisome proliferator-activated receptors (PPAR-γ) agonist) v. treatment-as-usual in 37 patients with unremitted depression (Rasgon et al., Reference Rasgon, Lin, Lin, Epel and Blackburn2016), TL was strongly and significantly associated with mood improvement in the active but not the placebo arm of the trial. In a retrospective study of TL in bipolar disorder (n = 256), shorter TL was associated with poorer response to lithium treatment, with those responding well to lithium shown to have longer TL, and the duration of treatment with lithium correlated positively with TL (Martinsson et al., Reference Martinsson, Wei, Xu, Melas, Mathe, Schalling, Lavebratt and Backlund2013). This positive association between lithium and TL was subsequently confirmed in a large-scale study of patients with bipolar disorder (n = 200) (Squassina et al., Reference Squassina, Pisanu, Congiu, Caria, Frau, Niola, Melis, Baggiani, Lopez, Cruceanu, Turecki, Severino, Bocchetta, Vanni, Chillotti and Del Zompo2016). In contrast to these studies, we found that TL is not predictive of ECT treatment outcomes. Moreover, we did not find a difference in TL between those patients taking lithium at the time of inclusion in our study and those who were not (data not shown). In contrast to a previous report (Hartmann et al., Reference Hartmann, Boehner, Groenen and Kalb2010), patients treated with ECT prior to inclusion in our study did not have shortened TL when compared to patients who had never received treatment with ECT or v. controls. However, it remains plausible that since most of our patients had experienced prior depressive episodes and were taking pharmacotherapy for a prolonged period in most cases, that the use of pharmacotherapy over time may have assisted in TL maintenance. Thus, further studies on the relationship between pharmacotherapy use and TL are warranted.
Cognitive deficits are often reported in patients with depression (Gonda et al., Reference Gonda, Pompili, Serafini, Carvalho, Rihmer and Dome2015), and while antidepressant drugs appear to be, for the most part, associated with positive effects on cognition (Prado et al., Reference Prado, Watt and Crowe2018), the use of ECT for depression is often limited by its cognitive side-effects (Semkovska and McLoughlin, Reference Semkovska and McLoughlin2010). Here we found that TL is not associated with baseline cognitive function in patients with depression or controls, and that TL is not predictive of selected cognitive side-effects post-ECT. We previously showed that the type of ECT administered is important for cognitive outcomes, with bitemporal ECT shown to have more of an impact than high-dose unilateral ECT, in particular on time to recovery of orientation and autobiographical memory (Semkovska et al., Reference Semkovska, Keane, Babalola and McLoughlin2011; Kolshus et al., Reference Kolshus, Jelovac and McLoughlin2017). The findings with regard to TL and cognition have so far been mixed overall. Some studies have shown that, in healthy individuals, TL is associated with cognitive performance (Valdes et al., Reference Valdes, Deary, Gardner, Kimura, Lu, Spector, Aviv and Cherkas2010; Cohen-Manheim et al., Reference Cohen-Manheim, Doniger, Sinnreich, Simon, Pinchas, Aviv and Kark2016). In contrast, others found no association between TL and cognitive performance or age-related cognitive decline in two community cohorts with narrow age ranges (Mather et al., Reference Mather, Jorm, Anstey, Milburn, Easteal and Christensen2010). Two studies of healthy, non-demented individuals also showed that global cognition (MMSE) is not directly associated with TL (Harris et al., Reference Harris, Deary, MacIntyre, Lamb, Radhakrishnan, Starr, Whalley and Shiels2006; Ma et al., Reference Ma, Lau, Suen, Lam, Leung, Woo and Tang2013) and, in keeping with our results, one study of patients with late-life depression showed no association between global cognitive function (MMSE) and TL (Schaakxs et al., Reference Schaakxs, Verhoeven, Oude Voshaar, Comijs and Penninx2015).
There are some limitations to our study. First, using qRT-PCR, we assessed TL in whole blood, which contains a mixture of leukocytes, as opposed to assessing TL in individual cell populations. This is a common method used across the literature. Recent studies have shown that TL differs across blood cell types (Lin et al., Reference Lin, Epel, Cheon, Kroenke, Sinclair, Bigos, Wolkowitz, Mellon and Blackburn2010; Lin et al., Reference Lin, Cheon, Brown, Coccia, Puterman, Aschbacher, Sinclair, Epel and Blackburn2016a); however, we were unable to account for blood cell distribution here. Thus, future studies should employ cell sorting techniques or account for variations in cell distribution within blood samples. Moreover, inter-laboratory differences in methodology (e.g. qRT-PCR v. Southern blot, relative v. absolute PCR) can lead to different results; thus, standardization of the methodology for measuring TL is essential to provide definitive conclusions, in particular regarding TL in depression. Second, our TL measurements were cross-sectional, and so we were unable to examine changes in TL over time. Third, DNA and cognitive measurements were not available from all study participants. For instance, MMSE scores were missing from 14% of the depressed cohort, and this may have affected the results; however, we noted no significant differences between those patients included in the MMSE analyses and those for whom MMSE data were missing with regard to TL, depression severity, age, sex, BMI, smoking status, educational attainment, or electrode placement. Additionally, early life adversity data were not available for this cohort.
Overall, our results argue against the use of TL assessed by PCR as a biomarker for depression, response to ECT, or selected cognitive outcomes post-ECT.
Acknowledgements
The authors thank all subjects for their participation in this study.
Financial support
This work was supported by the Health Research Board, Ireland (Grant numbers TRA/2007/5, TRA/2007/5/R, & HPF/2010/17). The funding body had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Conflict of interest
Declan McLoughlin has received a speaker's honorarium from MECTA and an honorarium from Janssen for participating in an esketamine advisory board meeting. Karen Ryan has no interests to declare. Both authors have approved the final article.






