Introduction
The Antarctic is known as a krill-based ecosystem; therefore, fluctuations in the distribution and abundance of Antarctic krill (Euphausia superba) may have strong implications on dependent predator populations. Some examples of responses of Antarctic predator populations being strongly related to these fluctuations have been recorded and future scenarios of risks are considered, indicating certain species and populations, especially penguins, to be more sensitive than others (e.g. Trivelpiece et al. Reference Trivelpiece, Hinke, Miller, Reiss, Trivelpiece and Watters2011, Klein et al. Reference Klein, Hill, Hinke, Phillips and Watters2018). Thus, observation of the feeding habits and ecology of these predators is very important, especially in the age of climate change and stronger human pressure in polar ecosystems (Trivelpiece et al. Reference Trivelpiece, Hinke, Miller, Reiss, Trivelpiece and Watters2011). The Pygoscelis penguins amount to ~70% of the total Antarctic avian biomass (see Trivelpiece et al. Reference Trivelpiece, Trivelpiece and Volkman1987), and as a major consumer of Antarctic krill, they are an important indicator species included in the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) Ecosystem Monitoring Programme (CEMP) aimed at detecting changes induced by harvesting. The King George Island (South Shetland Islands) area is a breeding site of three penguin species (the Adélie (Pygoscelis adeliae), gentoo (Pygoscelis papua) and chinstrap (Pygoscelis antarcticus)), which tend to exhibit specific reactions to environmental shifts (Forcada et al. Reference Forcada, Trathan, Reid, Murphy and Croxall2006). Studies of their populations were carried out by, for example, Trivelpiece et al. (Reference Trivelpiece, Trivelpiece and Volkman1987, Reference Trivelpiece, Hinke, Miller, Reiss, Trivelpiece and Watters2011), Korczak-Abshire et al. (Reference Korczak-Abshire, Węgrzyn, Angiel and Lisowska2013), Sierakowski et al. (Reference Sierakowski, Korczak-Abshire and Jadwiszczak2017) and Juáres et al. (Reference Juáres, Casaux, Corbalán, Blanco, Perchivale, Coria and Santos2018). There are several studies reporting relationships between changes in climate, krill abundance and penguin population size (e.g. Trivelpiece et al. Reference Trivelpiece, Hinke, Miller, Reiss, Trivelpiece and Watters2011, Hinke et al. Reference Hinke, Trivelpiece and Trivelpiece2017a, Reference Hinke, Cossio, Goebel, Reiss, Trivelpiece and Watters2017b). The population dynamics of Adélie, chinstrap and gentoo penguins appear to be influenced by interannual variability in sea-ice extent and E. superba biomass. For example, Trivelpiece et al. (Reference Trivelpiece, Hinke, Miller, Reiss, Trivelpiece and Watters2011) and Korczak-Abshire et al. (Reference Korczak-Abshire, Węgrzyn, Angiel and Lisowska2013, Reference Korczak-Abshire, Zmarz, Rodzewicz, Kycko, Karsznia and Chwedorzewska2019) noted that the populations of these three species in the South Shetland Islands have been undergoing rapid changes. In general, penguin breeding populations of both chinstrap and Adélie penguins have declined in the last 30 years in the Antarctic Peninsula region, while gentoo populations are stable or increasing (Trivelpiece et al. Reference Trivelpiece, Hinke, Miller, Reiss, Trivelpiece and Watters2011).
The Antarctic krill E. superba is an important element of the Antarctic food web and the main component of the diet of Antarctic predators. Its abundance is fluctuating, which is often associated with the sea-ice reduction during winter and with fluctuations in phytoplankton availability during summer (Siegel Reference Siegel2000). In the case of penguins, E. superba may constitute between 84.5% (gentoo) and 99% (Adélie and chinstrap) of their food mass (Volkman et al. Reference Volkman, Presler and Trivelpiece1980, Juáres et al. Reference Juáres, Casaux, Corbalán, Blanco, Perchivale, Coria and Santos2018).
Current catches of mid-water trawl fishery for Antarctic krill within the region of coastal waters of the Scotia Arc archipelagos and the northern Antarctic Peninsula reach almost 300 000 metric tons (https://www.ccamlr.org/en/document/publications/krill-fishery-report-2018). At the same time, this area serves as a feeding and breeding ground for the penguins analysed in this study (Weimerskirch et al. Reference Weimerskirch, Inchausti, Guinet and Barbraud2003). Due to the density of rookeries, Hinke et al. (Reference Hinke, Cossio, Goebel, Reiss, Trivelpiece and Watters2017b) expected increased overlap of fishing activities and foraging areas of Antarctic predators to occur in the vicinity to Admiralty Bay. High rates of overlap were found near to the shores of Livingston Island and the entrance of Admiralty Bay (Hinke et al. Reference Hinke, Cossio, Goebel, Reiss, Trivelpiece and Watters2017b). Knowledge regarding the feeding ecology of a species at each breeding site is essential in order to determine the relationship between the fluctuations in the local marine resources (prey) and the population dynamics of predators (from Juáres et al. Reference Juáres, Casaux, Corbalán, Blanco, Perchivale, Coria and Santos2018).
In this study, our main goal was to provide an insight into the Pygoscelis penguin diet in the 2012–13 breeding season by describing the E. superba composition of their stomachs in order to provide data concerning food selectivity.
Material and methods
Penguin population
Fieldwork was carried out on King George Island (South Shetland Islands), situated ~125 km north of the tip of the Antarctic Peninsula, within the Antarctic Specially Protected Area (ASPA) No. 128 at the western shore of Admiralty Bay (62°01'21"S, 58°15'05"W), during the 2012–13 season. ASPA 128 is an ice-free area of ~16.8 km2 where three penguin species (i.e. Adélie, chinstrap and gentoo) breed in five colonies (Fig. 1). During the breeding season, the population size expressed in the total number of occupied nests and breeding success (chick counts) was calculated according to the procedure followed the CCAMLR CEMP standards. Ground-level images of the breeding groups (> 500 nests) were used for a nest census in some cases (for more details, see Korczak-Abshire et al. Reference Korczak-Abshire, Węgrzyn, Angiel and Lisowska2013).
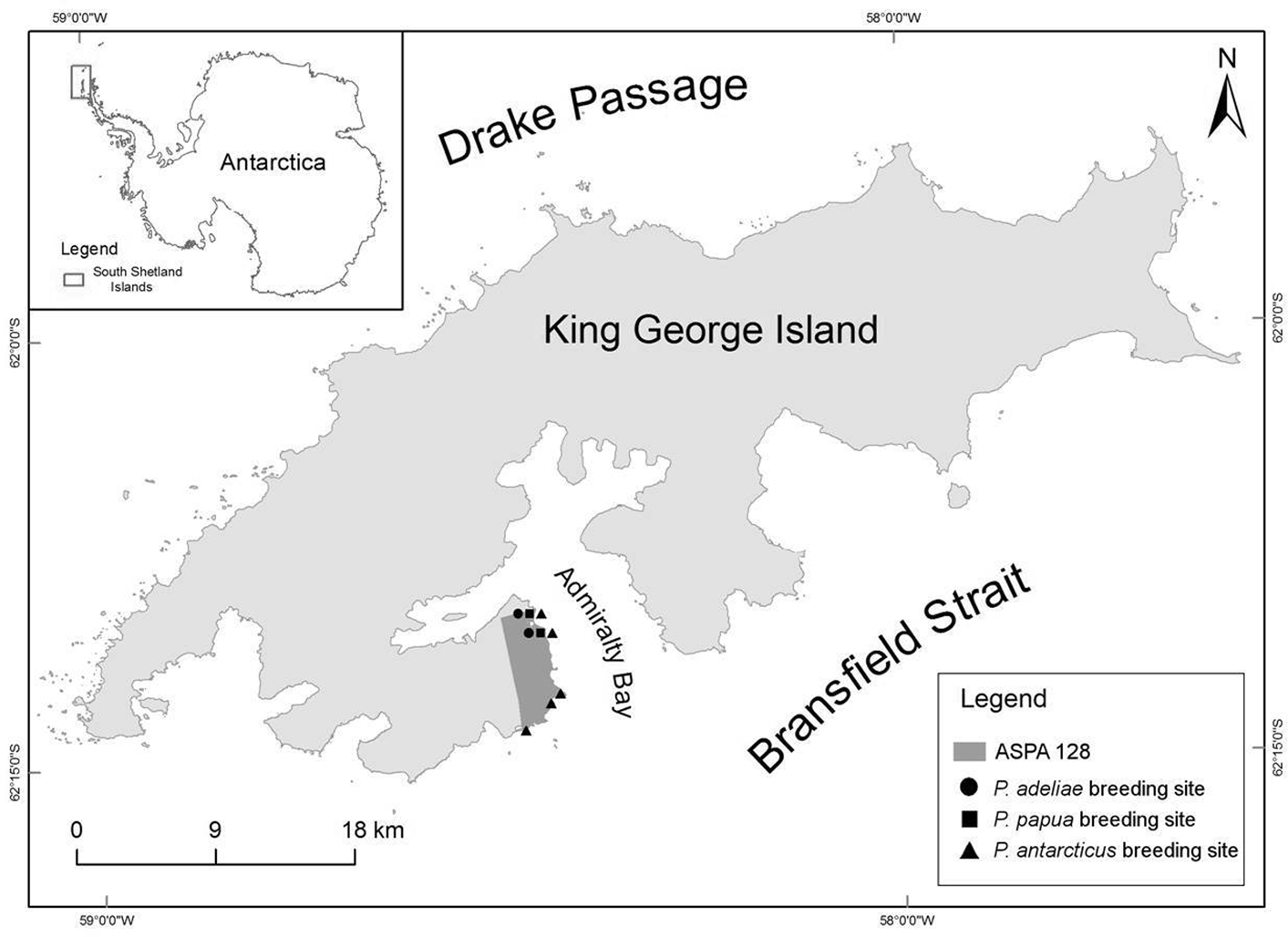
Fig. 1. Research area at King George Island (South Shetland Islands), with marked breeding sites of Adélie penguins (Pygoscelis adeliae), chinstrap penguins (Pygoscelis antarcticus) and gentoo penguins (Pygoscelis papua) within the Antarctic Specially Protected Area No. 128 (ASPA 128) at the western shore of Admiralty Bay (contour of the Antarctic continent coast source: http://www.marineregions.org/gazetteer.php?p=details&id=1926, contour of the King George Island coast source: Scientific Committee on Antarctic Research (SCAR) Antarctic Digital Database, version 6.0, 1993–2015).
Stomach contents sampling
Stomach contents sampling took place in December 2012 and January/February 2013. Samples were collected during the chick-rearing period after chicks had reached the crèche stage (> 2.5 weeks of age) by sampling breeding adults returning from foraging trips between 15h00–17h00 local time, using the water-offloading technique (Wilson Reference Wilson1984) following a modification of the CCAMLR CEMP standard methods. Wilson's technique involves flooding the penguin's stomach with warm water via a tube and pump and inverting the penguin over a bucket while applying pressure to the stomach to induce regurgitation. Penguins are usually pumped several times until clear water is expelled (after Robertson et al. Reference Robertson, Williams, Green and Robertson1994). Determining sex in penguins was performed by taking morphometric measurements (e.g. Scolaro Reference Scolaro1987, Polito et al. Reference Polito, Clucas, Hart and Trivelpiece2012).
In total, 60 stomach samples were collected: 30 from Adélie penguins, 20 from gentoo penguins and 10 from chinstrap penguins. The wet weight of the food so acquired was taken, all organisms in the samples were counted in total and intact E. superba individuals were measured to the nearest millimetre from the anterior side of the eyeball to the tip of the telson according to the CCAMLR standard protocol (https://www.ccamlr.org/en/document/publications/ccamlr-ecosystem-monitoring-program-standard-methods). If possible, sex of krill was determined visually based on the presence or absence of thelycum and petasma. In the case of part of the material being digested, pairs of eyes were counted, as these stay intact much longer than the rest of the krill body (Lishman Reference Lishman1985).
Statistical analysis
Multivariate statistical analysis of euphausiid abundance data was carried out using the PRIMER 7 software package (http://updates.primer-e.com/primer7/manuals/User_manual_v7a.pdf). Obtained abundances of taxa were fourth root transformed prior to analysis. Similarities between the samples were examined using the Bray–Curtis index, depicted as a non-metric multidimensional scaling. The method was used to reveal proportions and similarities in the consumed amount of E. superba female and male individuals for all penguin species. Constrained ordination techniques were applied in CANOCO 5 (Ter Braak & Šmilauer Reference Ter Braak and Šmilauer2012). For this purpose, we used canonical correspondence analysis (CCA) following a fourth root transformation of the abundance data for evaluation of the relationship between size distribution of consumed E. superba specimens and penguin species, sex and date of the sampling treated as functional traits. Additionally, redundancy analysis was used to explain the differences between penguin species and amounts of digested/undigested parts of the diet. For all of the statistical analyses, we assumed a significance level of P < 0.05.
Results
During the 2012–13 season, the penguin breeding population size reached ~5620 occupied nests of Adélie penguins, ~5460 of gentoo penguins and ~760 of chinstrap penguins in the five investigated colonies in ASPA 128. The breeding success rates expressed as number of chicks fledged per pair reached 0.47, 1.09 and 0.85, respectively.
The results of our diet study show that the stomach contents of all the three penguin species consisted of nearly 100% E. superba (both by number and by weight) (Table I). Some individuals of Euphausia frigida and Thysanoessa macrura were also found; however, they constituted < 0.1% of counted and recognized euphausiids in only five penguin stomach contents (Adélie and chinstrap penguins) due to numbers as low as 4 individuals of T. macrura and 12 of E. frigida (for over 1000 animals counted). Additionally, amphipod species were noted (e.g. Eusirus sp. and Parathemisto gaudichaudii). The abundance of other euphausiids and amphipods was not statistically significant (P > 0.24). Frequently, algal fragments, stones and small pebbles were also found, but they were not considered to be part of the diet. Moreover, small ingested plastic debris was also present in the stomach contents of four Adélie penguin individuals. In two cases, the plastic found took the form of strands (probably fragments of fishing gear), and in the other two it took the form of unidentified debris (Fig. 2).

Fig. 2. Example of ingested plastic debris found in penguin diets in the summer season of 2012–13 during our study. Upper left corner: strong plastic strand. Upper right corner and middle left part: green, thin, hard and easily breakable plastic debris. Lower part: think, strong, inextensible bundle of strands.
Table I. Composition of pygoscelid stomach contents (by weight and number; no statistically significant differences were found, P > 0.05).

a Other euphausiids, amphipods, pebbles, algae and/or debris.
In order to investigate the variation in the lengths of eaten specimens of E. superba, a CCA was used (Fig. 3). The results of this analysis show that the penguin species were selective in their prey. Adélie penguins fed on the smallest euphausiids (mean length 15–18 mm), chinstrap penguins chose crustaceans of mean length 39–42 mm (although this was not statistically significant) and gentoo penguins preferred E. superba of mean length 56–58 mm (Fig. 3). Penguin species explained a total of 8.8% of the variability of krill abundances in stomach contents. The mean length of all investigated (consumed) krill was 40 mm (SD = 5.25). There were also significant differences between both sexes of penguins and time (month) of sampling (Fig. 4). The largest krill individuals (> 40 mm) were consumed in February, and the smallest one was observed in December (Fig. 4). Variables such us penguin species, sex and time of sample collection accounted for > 18% of the variability between samples, and all (with an exception of the month of January) were statistically significant (P < 0.002). Moreover, in all of the samples from Adélie, chinstrap and gentoo penguins, krill females dominated in the diet (Fig. 5). In almost all of the analysed stomachs, females carrying spermatophores were found.

Fig. 3. Ordination plot from canonical correspondence analysis on the abundances of euphausiid group sizes in relation to penguin species (P < 0.05). Explanatory variables are indicated as arrows (Adélie, chinstrap and gentoo penguins). The length of Euphausia superba is indicated in millimetres (e.g. Es55 mean E. superba of 55 mm in length).

Fig. 4. Ordination plot from canonical correspondence analysis on the abundances of euphausiid group sizes in relation to penguin sex and time period of sampling (P < 0.05). Explanatory variables are indicated as arrows. The length of Euphausia superba is indicated in millimetres (e.g. Es55 mean E. superba of 55 mm in length).

Fig. 5. Non-metric multidimensional scaling analysis of male and female Antarctic krill abundances in penguin diets (A = Adélie, G = gentoo, C = chinstrap). Light grey indicates female krill and dark grey indicates male krill. Significance level of sample statistics: 0.9%.
A difference between the amounts of the digested and undigested parts of the diet was noted between Adélie and gentoo penguins (P < 0.008). Redundancy analysis showed that more digested material could be found in the stomachs of Adélie penguins, while the stomachs of gentoo penguins contained more E. superba individuals, which were preserved well enough for identification, and often for measurements (Fig. 6). The differences between these two penguin species and chinstrap penguins were not statistically significant (P > 0.3).

Fig. 6. Redundancy analysis of the digested and undigested Antarctic krill in penguin diets in relation to penguin species (A = Adélie, G = gentoo, C = chinstrap, P < 0.05).
Discussion
Our research shows that E. superba represented almost 100% of the stomach contents of three investigated penguin species (Table I), which is similar to the results of Volkman et al. (Reference Volkman, Presler and Trivelpiece1980), Lishman (Reference Lishman1985), Trivelpiece et al. (Reference Trivelpiece, Trivelpiece, Geupel, Kjelmyr, Volkman, Kerry and Hempel1990), Lynnes et al. (Reference Lynnes, Reid and Croxall2004), Rombolá et al. (Reference Rombolá, Marschoff and Coria2010) and Juáres et al. (Reference Juáres, Casaux, Corbalán, Blanco, Perchivale, Coria and Santos2018), among others, who investigated populations in the West Antarctic Peninsula and the Scotia Sea region. During our study, we observed small fragments of fish (not statistically significant), but only in the stomachs of gentoo penguins (0.5%) and Adélie penguins (0.1%) (Table I). It should be mentioned that Volkman et al. (Reference Volkman, Presler and Trivelpiece1980), Miller et al. (Reference Miller, Kappes, Trivelpiece and Trivelpiece2010) and Xavier et al. (Reference Xavier, Trathan, Ceia, Tarling, Adlard and Fox2017) observed greater amounts of fish fragments in the gentoo penguin diet (~15% of diet), which was not noted in our study. This can be explained by there being sufficient krill amounts within the feeding area. Although in the area of King George Island E. superba is the primary prey for penguins, we expected to find larger amounts of smaller euphausiids in the samples. The other euphausiid species, such as T. macrura and E. frigida, represented only minor fractions of penguin diets in our study. Although we cannot assess how many unidentified individuals from the genus Euphausia were in the digested parts of the samples, which can create a bias, we are certain in stating that no T. macrura individuals were omitted due to the visible differences in their eye structure. Overall, during the study, we observed that a greater amount of digested material was found in Adélie penguin stomachs than in the gentoo penguin diet (Fig. 6). This can be explained by the foraging ranges of those two species, as gentoo penguins are known to take shorter trips than Adélie penguins (Trivelpiece et al. Reference Trivelpiece, Trivelpiece and Volkman1987, Cimino et al. Reference Cimino, Lynch, Saba and Oliver2016). Ainley et al. (Reference Ainley, Wilson, Barton, Ballard, Nur and Karl1998) observed that when penguins travelled long distances, they were no longer foraging optimally, as they were mainly spending their resources on self-maintenance, and the adults brought back smaller amounts of undigested food for their chicks.
The largest difference between the penguins was noted in the length of consumed E. superba. Adélie penguins consumed mainly smaller individuals (Fig. 3). The same conclusions were drawn by White and Conroy (Reference White and Conroy1975), Lishman (Reference Lishman1985) and by Pickett et al. (Reference Pickett, Fraser, Patterson-Fraser, Cimino, Torres and Friedlaender2018). Lynnes et al. (Reference Lynnes, Reid and Croxall2004) noted on the South Orkney Island that the mean length of krill individuals taken by chinstrap penguins was larger than that taken by Adélie penguins. The same authors highlighted that when only large krill are available, all of the krill-eating species, including penguins, seals and fish, are likely to be feeding on the same part of the krill population. Lishman (Reference Lishman1985) and Wilson (Reference Wilson2010) suggested that different foraging ranges may be a consequence of this competition, although Trivelpiece et al. (Reference Trivelpiece, Trivelpiece and Volkman1987) highlighted that the major factor responsible for the ecological segregation of Adélie and chinstrap penguins, especially during the summer, is their asynchronous breeding cycles. Adélie penguin chicks fledge in late January, which is just as chinstrap penguin chicks enter crèches and both parents begin foraging simultaneously, and nearly 70% of the krill consumed by chinstrap chicks is caught after Adélie chicks have fledged (Trivelpiece et al. Reference Trivelpiece, Trivelpiece and Volkman1987). During this study, the largest specimens of krill were present in the gentoo penguin diet. Previous results for populations from King George Island also suggest that gentoo penguins take the largest-sized krill that are available to them (Volkman et al. Reference Volkman, Presler and Trivelpiece1980, Miller & Trivelpiece Reference Miller and Trivelpiece2007, Dimitrijević et al. Reference Dimitrijević, Paiva, Ramos, Seco, Ceia and Tiago2018, Pickett et al. Reference Pickett, Fraser, Patterson-Fraser, Cimino, Torres and Friedlaender2018). The larger krill found in gentoo penguin stomachs might be explained by their longer dives and their searching for krill swarms at greater depths (Kokobun et al. Reference Kokobun, Takahashi, Mori, Watanabe and Shin2010) due to the Antarctic krill behaviour of dividing into two layers during the day, with adult krill remaining in deeper water than younger individuals (Siegel Reference Siegel2000). The difference in the size of krill taken may reflect the seasonal pattern of growth of Antarctic krill, with rapid growth in autumn and early summer (Siegel Reference Siegel2000). In our study, the mean length of the consumed krill was 40 mm. Similar results were obtained by Volkman et al. (Reference Volkman, Presler and Trivelpiece1980), and the mean lengths of euphausiids consumed by the three Pygoscelis species on King George Island during the breeding season oscillated between 37.6 and 45.3 mm. Notably, during our research, females of all three penguin species fed upon smaller crustaceans (Fig. 4), which was not observed in previous studies. This suggests that there exist some sex differences in food selectiveness and foraging areas, which has been previously demonstrated by Clarke et al. (Reference Clarke, Manly, Kerry, Gardner, Franchi, Corsolini and Focardi1998), as there are also occasional differences in the provision of food for female and male chicks (Jennings et al. Reference Jennings, Varsani, Dugger, Ballard and Ainley2016). However, such a hypothesis should be further investigated through the implementation of tracking of penguin foraging trip data.
Volkman et al. (Reference Volkman, Presler and Trivelpiece1980) observed that female euphausiid individuals were more abundant in the diet samples of all three penguin species, which was confirmed in our study (Fig. 5). This is to be expected, especially in summer, because of the importance of summer for krill reproductive cycle. The presence of spermatophores attached to the thelycum of krill females confirms that penguins fed upon mature krill females that were ready for reproduction. Similar results were obtained by Reid et al. (Reference Reid, Trathan, Croxall and Hill1996). During a study in February 1986 (at South Georgia), Reid et al. observed that the most abundant maturity/sex stage of krill in net samples was not strongly represented in predator diets (both gentoo and macaroni penguins). Moreover, in the stomach contents of both penguin species, sexually active krill females were the most dominant, which suggests strong selectiveness in their diet and preference for larger krill individuals (as krill females swell at reproductive stage) (Reid et al. Reference Reid, Trathan, Croxall and Hill1996). Our results demonstrating the greater amount of female krill in the penguin diets are a corroboration of their selective feeding.
The presence of plastic debris contents in the penguin stomachs (Fig. 2) could be alarming, although this was not statistically significant. While some strands of plastic were found within the debris, which could be fragments of fishing nets, we also noted the presence of flat, colourful plastic pieces, which implies a different origin. It should be highlighted that plastic debris in the Antarctic has been considered to be rare (Waller et al. Reference Waller, Griffiths, Waluda, Thorpe, Loaiza and Moreno2017), although Bessa et al. (Reference Bessa, Ratcliffe, Otero, Sobral, Marques and Waluda2019) in the regions of Bird Island (South Georgia) and Signy Island (South Orkney Islands) have found that a total of 20% of gentoo penguin scats contained microplastics, consisting mainly of fibres and fragments of different sizes and polymer compositions. To our knowledge, this is the first of this kind of observation in the stomach contents of the Pygoscelis penguins in the area of South Shetland Islands. Previously, plastic debris in penguin stomachs has been recorded on the Brazilian coast (Pinto et al. Reference Pinto, Siciliano and Di Beneditto2007). Although the presence of seagrass and marine remains in penguin stomachs is common and may reflect incidental or secondary ingestion (e.g. in case of the Spheniscus magellanicus from the northern distribution limit on the Atlantic coast of Brazil; Pinto et al. Reference Pinto, Siciliano and Di Beneditto2007), the plastic ingestion reported by us and Bessa et al. (Reference Bessa, Ratcliffe, Otero, Sobral, Marques and Waluda2019) may suggest increasingly strong human activity in the research area.
It is still unclear how penguins will react when only small amounts of krill are available or when there are changes in the krill species structure. With that in mind, it is crucial to continue the sampling of penguin diets and to continue behavioural observations of penguins feeding at sea. Moreover, the reports of the presence of artificial ingested plastics in penguin stomachs are alarming and point towards a strong need for the continuation of such research in the Antarctic marine system.
Acknowledgements
The authors would like to thank the Antarctic Ecosystem Research Division, Southwest Fisheries Science Center, National Marine Fisheries Service National Oceanic and Atmospheric Administration (USA) for providing Pygoscelis penguin diet samples and research data on the Llano Point breeding site. This work was supported by the Polish National Science Centre, Project Miniatura 1 no. 2017/01/X/NZ8/01181, received by Anna Panasiuk. Special thanks are given to Dr Jefferson Hinke and the anonymous reviewers, whose comments and suggestions substantially improved the manuscript. The data on penguin populations used in the paper were collected at the Henryk Arctowski Polish Antarctic Station.
Author contributions
AP was responsible for the management and preparation of the manuscript, as well as for coordinating of the sample collection and organizing the dataset and interpretation of the results. JW-B managed the laboratory analysis, dataset preparation, maps and results analysis and interpretation, and she provided assistance during manuscript preparation. AM was responsible for the laboratory analysis. MK-A provided field data and interpretations of the results regarding the investigated penguin populations, delivered a map of penguin population distributions and contributed during manuscript preparation.
Data deposit
The analysed material is available at the Department of Marine Plankton Research Storage, Institute of Oceanography, University of Gdansk, Poland.









