Introduction
Recognising the importance of parasites and pathogens has recently become a major issue in animal ecology (Fox et al., Reference Fox, Roff and Fairbairn2001). The evidence of their crucial role comes from various aspects of ecology, ranging from pathogen-driven population cycles (Turchin et al., Reference Turchin, Wood, Ellner, Kendall, Murdoch, Fischlin, Casas, McCauley and Briggs2003) to parasite-mediated sexual selection on carotenoid based colouration (Hõrak et al., Reference Hõrak, Ots, Vellau, Spottiswoode and Moller2001; Saks et al., Reference Saks, Ots and Hõrak2003). However, it is often unclear how different parameters of the environment and those of the host individuals affect the outcome of the interaction. In particular, even if individual variability in resistance to pathogens is also frequently documented in herbivorous insects (Dwyer et al., Reference Dwyer, Elkinton and Buonaccorsi1997; Wilson et al., Reference Wilson, Cotter, Reeson and Pell2001; Cotter et al., Reference Cotter, Hails, Cory and Wilson2004; Singer et al., Reference Singer, Carriere, Theuring and Hartmann2004a,Reference Singer, Rodrigues, Stireman and Carriereb; Sanders et al., Reference Sanders, Scarborough, Layen, Kraaijeveld and Godfray2005; Klemola et al., Reference Klemola, Klemola, Rantala and Ruuhola2007), the sources of the variation are not sufficiently known to understand its potential role in ecological processes.
Host plant is a major component of the environment for an insect herbivore. The effect of the host plant on the interaction between the insect and its pathogen may, however, be realized via different routes (review in Cory & Hoover, Reference Cory and Hoover2006; Kepler & Bruck, Reference Kepler and Bruck2006; Ugine et al., Reference Ugine, Wraight and Sanderson2007). First, it appears natural to assume a positive effect of nutritional quality of the plant on pathogen resistance, which is mediated by individual condition of the herbivore (e.g. Hare & Andreadis, Reference Hare and Andreadis1983; Lampert & Bowers, Reference Lampert and Bowers2010). This requires only the assumption of immune defence being energetically costly (Freitak et al., Reference Freitak, Ots, Vanatoa and Hõrak2003; Schwarzenbach & Ward, Reference Schwarzenbach and Ward2006). On the other hand, there are reports of better pathogen resistance on lower quality host plants (Hoover et al., Reference Hoover, Washburn and Volkman2000; Klemola et al., Reference Klemola, Klemola, Rantala and Ruuhola2007), which must imply an effect of the plant not mediated by the condition of the insect. Different explanations for such occasional negative relationships between host quality and resistance have been proposed, and these are mostly based on proximate biochemical mechanisms. In particular, plant defences may be more effective against the parasites than the host insects (Reitz & Trumble, Reference Reitz and Trumble1997; Singer et al., Reference Singer, Rodrigues, Stireman and Carriere2004b). A similar effect can be observed when mild stress is caused, e.g. by suboptimal food which may induce the maintenance of elevated activity of immune system (Kapari, Reference Kapari, Haukioja, Rantala and Ruuhola2006; Mowlds et al., Reference Mowlds, Barron and Kavanagh2008).
In various insects, individual condition is modified by another environmental factor, the density of conspecifics during larval development. High rearing density frequently causes a presumably adaptive change in growth schedule leading to reduced adult weight and shorter larval development (Applebaum & Heifetz, Reference Applebaum and Heifetz1999; Tammaru et al., Reference Tammaru, Ruohomäki and Montola2000). Moreover, density may directly affect resistance to pathogens: a phenomenon called density dependent prophylaxis (DDP) has repeatedly been demonstrated in Lepidoptera and other insects (Goulson & Cory, Reference Goulson and Cory1995; Wilson et al., Reference Wilson, Thomas, Blanford, Doggett, Simpson and Moore2002; Cotter et al., Reference Cotter, Hails, Cory and Wilson2004; Wilson & Cotter, Reference Wilson, Cotter, Whitman and Ananthakrishnan2009).
Body size is a highly plastic condition parameter in most insects, which is typically well correlated with various components of fitness, fecundity in particular (Honek, Reference Honek1993; Tammaru et al., Reference Tammaru, Esperk and Castellanos2002). However, direct connections between body size and immunity have not been found in most studies on insects (Rantala et al., Reference Rantala, Koskimäki, Taskinen, Tynkkynen and Suhonen2000; Rolff, Reference Rolff2001; Cotter & Wilson, Reference Cotter and Wilson2002) although some examples of positive (Yourth et al., Reference Yourth, Forbes and Baker2002; Ots et al., Reference Ots, Freitak and Vanatoa2005) and negative (Yang et al., Reference Yang, Ruuhola, Haviola and Rantala2008) relationships exist.
To evaluate factors determining the resistance to pathogens, larvae of the moth Orgyia antiqua L. (Lepidoptera: Lymantriidae) were infected with the entomopathogenic fungus Metarhizium anisopliae Metschnikoff (Deuteromycotina: Hyphomycetes). O. antiqua, the vapourer moth, is a holarctic forest species which is highly dimorphic sexually; males are smaller, winged and pupate after four or five larval instars, while females are larger, wingless and go through four to six instars (see Esperk & Tammaru, Reference Esperk and Tammaru2006 for further details). The solitary polyphagous larvae feed externally on the leaves of various plants. The fungus M. anisopliae potentially affects a large number of insect taxa (Vestergaard et al., Reference Vestergaard, Cherry, Keller, Goettel, Hokkanen and Hajek2003). In susceptible individuals, death occurs two to ten days after the contact with the conidia (Boucias & Pendland, Reference Boucias and Pendland1998).
Survival patterns of the O. antiqua larvae experimentally infected by conidia of M. anisopliae were recorded. To create environmental variance, the moth larvae were reared on two host plants differing in nutritional quality. In addition to providing different host plants, the individual condition of the larvae was manipulated by imposing a crowding treatment. Furthermore, pupae were implanted with nylon filaments to measure their efficiency in encapsulating foreign objects. Encapsulation efficiency may provide information substantially different from the measurements of in situ survival as the expression of the pathogen resistance is separated in time from the insect-plant interaction, and a direct effect of plant chemistry can most probably be ruled out.
Materials and methods
Insect rearing
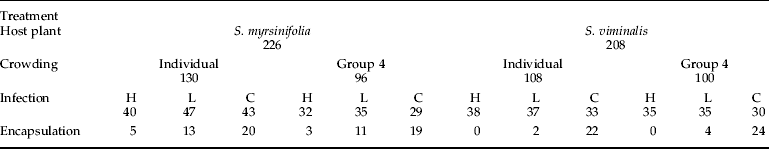
A total of 967 O. antiqua larvae representing six broods (offspring of an individual female) were reared in 50-ml clear plastic vials at 24°C and natural light regime (18L:6D). The insects were sourced from a non-inbred laboratory culture of Estonian and Finnish origin. To reduce the variation in development time and minimize larval mortality before the infection treatments, all larvae were fed on a good quality host plant – birch (Betula pendula Roth) – leaves ad libitum until the end of the 3rd instar.
For crowding manipulation, the larvae were reared either singly or in groups of four in a vial. Equal numbers of larvae from each brood were allocated to each experimental treatment (crowding and host plant). At the end of the 3rd instar, all larvae were transferred to individual vials. Crowding treatments were terminated before the infection treatments were applied to separate the possible adaptive responses to crowding from direct negative influences of mutual disturbance (Tammaru et al. Reference Tammaru, Ruohomäki and Montola2000).
The weight of each larva was recorded during the premoult period at the end of 4th instar. This parameter was included into statistical analyses as a continuous variable reflecting individual condition and will be referred to as initial weight (i.e. the weight measured before the treatments were applied). About half the individuals (533 of 967, 474 of them males) pupated at the end of their 4th instar, leaving 434 as the actual sample size (see table 1 for exact numbers used in data analyses in all treatments). Moulting of the remaining larvae to 5th instar was manipulatively synchronized. Synchronisation was achieved by transferring the larvae to 4°C as soon as they had initiated the moult into 5th instar, which arrested their development. It was confirmed in subsequent analyses that the survival of the larvae was not affected by time spent in the cold.
Table 1. Treatments and numbers of individuals tested.
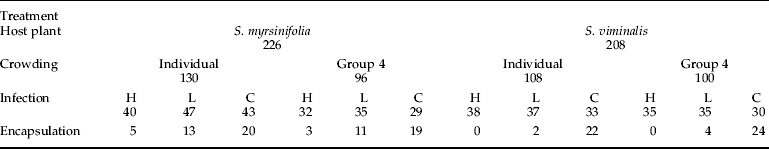
For each category, numbers represent number used in data analysis: H, L and C indicate high, low and control infection treatments, respectively.
Host plants
Two willow species, Salix myrsinifolia Salisb. and S. viminalis L., were used as host plants for the larvae. The larvae were equally divided between the two host plants in the beginning of the 4th instar. Fresh willow leaves were given to the larvae ad libitum, being renewed every other day.
The two species were selected because they are known to contain different amounts of phenolic glycosides, a group of defensive plant secondary chemicals (Julkunen-Tiitto, Reference Julkunen-Tiitto1986). Phenolic glycosides typically reduce plant quality for generalist insect herbivores (Lindroth et al., Reference Lindroth, Scriber and Hsia1988, Reference Lindroth, Roth, Kruger, Volin and Koss1997) and should also contribute to the lower quality of S. myrsinifolia as compared to S. viminalis. A previous experiment with O. antiqua has confirmed that these plant species differ in their quality as food for this species; the larvae reared on S. viminalis attained 1.65 times higher pupal weights than on S. myrsinifolia (Sandre et al., Reference Sandre, Tammaru, Esperk, Julkunen-Tiitto and Mappes2007).
Fungal infection
Three days after moulting to the 5th instar, larvae reared in groups versus alone, and on S. viminalis versus on S. myrsinifolia, were equally divided between the three fungal treatments: higher dose, lower dose and control treatment (0.05% Tween 80 in water). The strain of M. anisopliae used was V245A. It was originally isolated from agricultural soil in Finland in 1989 using Tenebrio molitor larvae as bait (Vänninen et al., Reference Vänninen, Hokkanen and Tyni-Juslin1999). For production of conidia, the entomopathogenic fungus was grown on PDA plates at 25°C. Fresh conidia were harvested from the plates with a scalpel and dissolved in 0.05% Tween 80 and water solution. The concentration of conidia was determined with Neubauer hemocytometer. Solutions with two concentrations of conidia were prepared: 5×107 and 1×107 conidia ml−1. To infect the insects, 10 μl of conidial solution was pippeted onto a Petri dish (Ø 5 cm). Larvae were placed into the fungal solution to ensure contact with conidia. A 1.5×1.5 cm birch leaf was added to each Petri dish to be consumed by the larvae. Birch was used instead of willows to equalize conditions during conidial germination. The larvae were left in the Petri dishes for 24 h and then transferred back to 50-ml plastic vials and fed the two willow species as before infection. Thereafter, the larvae were observed daily for death or pupation. The insects were sexed as pupae.
Encapsulation of foreign object in pupae
To measure the ability to encapsulate foreign objects, most (124) of the insects having survived the fungal infection trial were implanted with a nylon filament (Ø 0.1 and length 2 mm) 24 h after pupation as described in Vilmos & Kurucz (Reference Vilmos and Kurucz1998). The implants were inserted 1 mm above the 4th spiracle and removed exactly 24 h after the manipulation.
Encapsulated implants were kept in the dark prior to photographing them under the microscope. All implants were photographed under the light microscope from two sides together with a clean piece of equally sized nylon filament as the standard. The strength of the encapsulation response was estimated as darkness in the scale 0–255 using Adobe Photoshop 9. The darkness of the standard was subtracted from the value of encapsulated implant to avoid the possible effect of small variations in light conditions when attaining the value of darkness for statistical analysis.
Statistical analysis
The effect of treatments and condition parameters on performance indices was analysed either by ANOVA, or logistic regression, for binary response variables. Brood was included as an additional random factor. In the survival analyses, however, brood had to be treated as a fixed factor due to computational problems. Sex could not be included in the models as its value was not known for insects that were dead prior to pupation. However, most individuals in the infection trial were females because most males pupated already in the 4th instar. Full models, which included brood, crowding treatment, time spent in the cold, host plant species, infection treatment and weight, were fitted in preliminary statistical analyses but non-significant main effects and interactions were omitted from the final models unless they were of special interest. It was confirmed that retaining those effects in the models had no considerable influence on statistics associated with other effects. For statistical analyses, the development time of the larvae was measured from the beginning of the 4th instar (i.e. from the point when the crowding treatment was terminated and development could be followed individually). Statistical analyses were conducted with STATISTICA 8.0 and SAS 9 for Windows. In all ANOVA and ANCOVA analyses, type III sums of squares were used.
Results
Effects of experimental manipulations on development time and body size
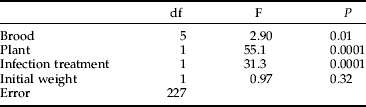
Pupal weights were higher and development times longer with the higher quality host plant, S. viminalis. Mean pupal weight of the uninfected O. antiqua on S. myrsinifolia was 114.1 mg (SE 2.0) for males and 339.2 (SE 10.0) for females, and on S. viminalis was 118.9 (SE 2.0) and 358.5 mg (SE 8.8) for males and females, respectively. The differences between pupal weights on the two host plants were statistically significant (effect of host when sex is accounted for: F1,625=8.4, P=0.004). Consistently, development time was shorter (GLM: F1,428=28.0, P=0.0001) on S. viminalis (LS means: 8.9 days SE 0.09) than on S. myrsinifolia (9.7 days SE 0.08), as based on a model with host plant and infection treatment as explanatory factors.
Rearing density (crowded vs. single during the three first instars) had a significant effect on body weight (ANOVA: F1,437=32.6, P=0.0001). In particular, larvae reared singly weighed more in the beginning of their 5th instar: 138.8 (SE 1.6) compared to 125.5 mg (SE 1.7) in the crowded ones. Moreover, the developmental time of the singly reared larvae (12.0, SE 0.082) was longer than that for the crowded ones (12.6, SE 0.074), and the difference was highly significant (F1,379=25.3, P=0.0001).
Survival of larvae after infection
From the beginning of the 5th instar onwards, i.e. after the infection, the overall larval survival to the pupal stage was 59.3% (i.e. 263 of 444 of the larvae died). Larval mortality was 88.1%, 70.4% and 22.4% in the higher dose, lower dose and control treatment, respectively. The majority of deaths occurred in the larval stage (89.8%), 28 insects died as pupae. All cadavers were covered with M. anisopliae conidiophores a week after death. No fungal growth was found in the control.
The survival of the larvae depended on treatment, brood and initial weight (table 2). The survival of larvae infected with a lower dose varied from 8.3 to 55.6% (median 23%) among different broods and of those with a higher dose from 4.2 to 16% (median 11.5%). Brood-specific means of survival and growth performance indices (development time and body weight) were not correlated (not shown). There was a significant interaction between host plant and infection treatment. In particular, survival of the infected larvae was higher on S. myrsinifolia, whereas the non-infected ones survived better on S. viminalis (fig. 1). The surviving larvae had been smaller in the beginning of the 5th instar, 127.4 (SE 1.87) mg, than the ones that died, 136.5 (SE 1.53) mg, during the treatment.

Fig. 1. Survival of O. antiqua larvae on different host plants depending on fungal infection level. (□, Salix myrsinifolia; ▪, Salix viminalis).
Table 2. Effects of various variables on survival of O. antiqua larvae in both fungal infection treatments and control group (n=429) in a multi-way logistic regression model.

Initial weight stands for weight in the beginning of 5th larval instar and characterizes individual condition before the application of infection treatments.
When analysing the importance of environmental vs. condition-related factors on survival after fungal infection, only the two infection treatments were included in the respective multi-way logistic regression model, i.e. the control individuals were omitted. Weight, infection dose, brood and host plant had an effect on survival paralleling those in the analysis with all infection treatments included (see previous paragraph). However, host plant species had clearly the strongest effect on survival of the larvae (χ2=19.7, P=0.0001, n=296).
Time to death after infection
Time to death after infection differed between the two infection treatments. The higher dose larvae died, on average, after 3.4 (SE 0.1); and the lower dose larvae died, on average, after 4.0 (SE 0.1) days after the infection. Those that survived until pupation were omitted from these analyses. Survival time depended on the host species and treatment (table 3). Survival time was longer on the inferior host, i.e. S. myrsinifolia being 4.1 (SE 0.09) days, as opposed to 3.3 (SE 0.08) days on S. viminalis.
Table 3. Effects of treatments on survival time of infected O. antiqua larvae in an ANCOVA (R2=28.4%).
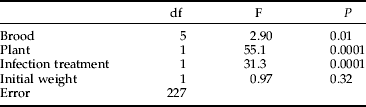
Survival time was measured as time to death after infection.
Encapsulation
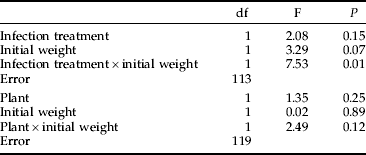
The strength of the encapsulation response against a nylon implant was not affected by any studied factor as a main effect in a multi-way ANOVA. Fitting one full model with all interactions was not considered meaningful because, in some treatment levels, the number of observations was too low (only six larvae reared on S. viminalis survived the infection). Therefore, two separate models were analysed to estimate the effects of host plant, infection treatment and initial weight on capsule darkness (table 4). Only the interaction between weight and infection treatment was significant; in the infected insects, there was a positive correlation between weight and capsule darkness (r=0.45, P=0.01, n=32; fig. 2); whereas, in the control group, there was no such relationship (r=0.093, P=0.39, n=85; fig. 2).

Fig. 2. Efficiency of implant encapsulation correlated with larval weight in lower fungal infection treatment but not in control treatment.
Table 4. Effects of various factors on capsule darkness (in females) in two ANOVA models applied to the same data set.
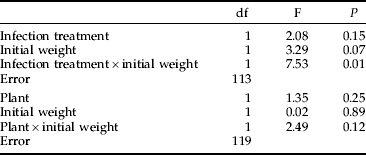
Analysing all factors in one model was not possible because, of larvae reared on S. viminalis, very few survived the infection and could be implanted as pupae. For both models, R2=0.5%.
Discussion
Dual effect of plant quality
Being infected with the fungus M. anisopliae, larvae of O. antiqua reared on an inferior host, S. myrsinifolia, had a higher survival rate (fig. 1) and a longer residual life span than those offered a superior host plant, S. viminalis. A likely reason for the better performance of the infected larvae on S. myrsinifolia is a marked difference in the concentration of phenolic glycosides in these hosts. S. viminalis has a very low concentration of phenolic glycosides (Julkunen-Tiitto, Reference Julkunen-Tiitto1986), which is also the likely reason for the good growth performance of generalist herbivores feeding on it. This study thus adds evidence of trade-offs between different aspects of host plant quality for generalist herbivores; a plant may be good in terms of enabling a high growth rate, or, alternatively, it may protect against natural enemies. Analogous trade-offs have been demonstrated in studies on the resistance to viruses (Hoover et al., Reference Hoover, Washburn and Volkman2000; McVean et al., Reference McVean, Sait, Thompson and Begon2002; Raymond et al., Reference Raymond, Vanbergen, Pearce, Hartley, Cory and Hails2002), bacteria (Navon et al., Reference Navon, Hare and Federici1993) and parasitoids (Singer et al., Reference Singer, Carriere, Theuring and Hartmann2004a). To our knowledge, the present study is, however, the first to demonstrate a trade-off between the effects of a host plant on resistance to a fungal pathogen and individual performance.
Phenolic glycosides could inhibit the growth or be otherwise toxic for M. anisopliae, which is consistent with the reports on the effects of purified plant secondary chemicals on entomopathogenic fungi in vitro (Vega et al., Reference Vega, Dowd, McGuire, Jackson and Nelsen1997; Klingen et al., Reference Klingen, Hajek, Meadow and Renwick2002). Moreover, the fact that the food plant affected only larval survival, but had no effect on encapsulation efficiency during pupal stage, appears to support this hypothesis; there was a suppressive effect on a live pathogen but no increase in insect immunity per se. Naturally, a further study, in which the quantity of phenolics in an otherwise identical diet (e.g. artificial diet) is manipulated, would be necessary to fully confirm the antifungal effect of ingested phenolic glycosides. Moreover, to eliminate possibility of strain specificity of the effect, future studies with other strains of M. anisopliae and with other fungal pathogens would be necessary.
The physiological mechanisms behind the host plant-based trade-offs between herbivore sensitivity to pathogens and individual performance indices are mostly unknown (Schoonhoven et al., Reference Schoonhoven, van Loon and Dicke2005), though a few have been proposed. For example, mechanisms associated with peritrophic matrix have been suggested to explain the resistance against ingested pathogens (Plymale et al., Reference Plymale, Grove, Cox-Foster, Ostiguy and Hoover2008). In particular, the thickness of the peritrophic matrix varies according to the properties of the food, which affects the ability of the pathogens to invade the larva. On the other hand, the thickness of the peritrophic matrix could affect the efficiency of nutrient absorption (Villalon et al., Reference Villalon, Ghosh and Jacobs-Lorena2003). Another mechanism explaining the trade-off is based on the ability of chemical defences to have an adverse effect on parasitoids and pathogens directly (Singer, Reference Singer, Carriere, Theuring and Hartmann2004a; this study).
Determinants of resistance: environment or individual condition
Our study emphasizes the importance of the effect of food plant traits rather than individual condition on the interaction between pathogens and the host insect. None of the studied traits, potentially reflecting the condition of the herbivore (body size or rearing density), showed a clear relationship with pathogen resistance. Similarly, most previous studies on insect immunity and individual body size show no connection between the two traits (Rantala et al., Reference Rantala, Koskimäki, Taskinen, Tynkkynen and Suhonen2000; Rolff, Reference Rolff2001; Cotter & Wilson, Reference Cotter and Wilson2002), and only some examples of positive connections exist (Yourth et al., Reference Yourth, Forbes and Baker2002; Ots et al., Reference Ots, Freitak and Vanatoa2005). In principle, large body size and related high fecundity may be attained at the cost of lower investments in the immune system (Zuk & Stoehr, Reference Zuk and Stoehr2002; Schmid-Hempel & Ebert, Reference Schmid-Hempel and Ebert2003), which may obscure the expected positive relationship.
Nevertheless, body weight still appears to have a role in determining immune parameters of the insects, though the interpretation is not necessarily straightforward at the present stage. On the one hand, smaller larvae had a higher survival rate after fungal infection, indicating a possible trade-off between resistance and fecundity. On the other hand, in the lower dose infection group, the larger larvae were more efficient at encapsulating the foreign antigen (fig. 2). In any case, in parallel with a number of similar studies (Ojala et al., Reference Ojala, Julkunen-Tiitto, Lindström and Mappes2005; Rantala & Roff, Reference Rantala and Roff2005; Yang et al., Reference Yang, Ruuhola, Haviola and Rantala2008), the results of the experiments with O. antiqua suggest that immune traits may well be involved in selective pressures determining the optimum of body size and may help to find the generally elusive costs of large size (Blanckenhorn, Reference Blanckenhorn2000; Tammaru et al., Reference Tammaru, Esperk and Castellanos2002). Nevertheless, the pattern appears to be complicated and far from clear. Unfortunately, the low number of broods that this study was based on did not allow us to explicitly address the question about genetic correlations between immune parameters and measures of body size. However, the remarkable among-brood differences in resistance to infection were not paralleled by corresponding differences in any of the parameters of larval growth performance measured.
Our results do not provide support to the density dependent prophylaxis hypothesis, which suggests that insects who experience high density conditions during their development invest more in immunity (Wilson et al., Reference Wilson, Thomas, Blanford, Doggett, Simpson and Moore2002; Cotter et al., Reference Cotter, Hails, Cory and Wilson2004). In the case of O. antiqua, rearing in crowded conditions had no effect on any of the immune traits tested, although the high density treatment was effective enough to reduce the size of the larvae. More specific immunological mechanisms (e.g. various humoral responses) behind the outcome of the infection were not investigated. However, if the outcome of summed immune reactions is not reflected in differences in performance, then these are not relevant in the evolutionary context.
The lack of DDP in O. antiqua larvae can be explained by the fact that the larvae of this species may not frequently experience extremely high density in nature even during the peaks of population cycles. Indeed, the density dependent prophylaxis is not ubiquitous among Lepidoptera and seems to require specific conditions to evolve (Wilson et al., Reference Wilson, Knell, Boots and Koch-Osborne2003). On the other hand, as we were specifically interested in adaptive responses to larval crowding rather than proximate effects, respective treatments were terminated before the larvae were experimentally infected. It could have been the case that any possible adaptive responses could have been lost during the instar when the larvae had been reared singly. However, at least some of the physiological and morphological responses of crowding have been shown to be maintained in the next instar or even longer after the transfer to solitary conditions (Gunn, Reference Gunn1998; Tammaru et al., Reference Tammaru, Ruohomäki and Montola2000).
Acknowledgements
We thank Külli and Hannes Lokko for help in the lab, and Anu Kollom for taking care of the fungus. The study was financially supported by the Estonian Science Foundation (no. 7522), the Estonian Ministry of Education and Science (target-financing project (no. SF0180122s08)) and the European Union through the European Regional Development Fund (Center of Excellence FIBIR).