Introduction
The integration of fish into irrigated rice cultivation (integrated rice–fish (IRF) farming is an ancient production system that can be traced back to the year ad 220 in ChinaReference Li1, where it was developed as an intensification strategy for overpopulated rural areas. IRF subsequently expanded throughout Asia to IndiaReference Saikia and Das2, BangladeshReference Gupta, Sollows, Mazid, Rahman, Hussain and Dey3, Reference Wahab, Kunda, Azim, Dewan and Thilsted4, VietnamReference Rothuis, Nam, Richter and Ollevier5, Reference Berg6, ThailandReference Middendorp and Vereth7, IndonesiaReference Purba8 and the PhillipinesReference Horstkotte-Wessler9. In Brazil, IRF commenced in the 1980s in the southern provincesReference Cotrim, Sacknies, Valente, Rojahn, Oliveira, Severo, Rojahn, Leal and Lara10, and is in its beginnings in the tropical north of Brazil, with pilot plots in Maranhão state established in 2006.
IRF farming is a promising management option especially for intensive smallholder agriculture, furnishing impressive gains in system productivity, resource use efficiency and ultimately in the sustainability of land use. In terms of per hectare productivity, irrigated rice grain production is associated with grain yields of up to 10 Mg ha−111. Fish farming ranks among the highest-producing livestock systems with fish production frequently exceeding 550 kg ha−1Reference Frei, Razzak, Hossain, Oehme, Dewan and Becker12. The integration of both components into one production system not only increases resource exploitation by niche partitioning but also creates a series of important synergistic interactions, notably plant and fish nutritionReference Panda, Ghosh and Sinhababu13–Reference Oehme, Frei, Razzak and Becker15, pest controlReference Berg6 and weed control.
Large areas of the Amazonian Atlantic coastline as well as those in other tropical regions are covered by marine hydromorphic alluvial soils. Such soils have a substantial potential to raise rice production via irrigated rice production, since they typically combine relatively elevated soil fertility with favorable hydrologic conditions and a flat terrain. This potential is turning into reality with a very rapid expansion of irrigated rice production in the study region. Large-scale (frequently>500 ha) semi-industrial operations predominate in the area and grain productivity averages 3 Mg ha−1. These systems are based on direct-seeding, are highly mechanized and involve intensive use of chemical fertilizers and pesticides, a concern for environmentally sensitive riverine areas. Smallholder rice production is traditionally based on cultivation of upland rice via slash-and-burn shifting cultivation of terra firme (not inundated) lands, and productivity is very low (usually well below 400 kg ha−1).
Smallholder irrigated-rice farming has been successfully promoted during the past decade in the study region, using low-density transplanting in continuously inundated fields, locally developed high-yielding varieties and chemical fertilizers. Rice productivity can be high (typically >7 Mg ha−1), but the need for financial inputs for fertilizers and pesticides has proven to be an obstacle, especially for resource-poor farmers.
Fish is a traditional component in the culture of this region, but overfishing of the Mearim River has seriously reduced fish yields. The integration of rice and fish into a single and interacting unit constitutes a form of development that unifies existing traditions and knowledge. This system may well constitute an environmentally sustainable and much-needed pathway out of poverty for resource-poor riverine farmers of the region.
Weed populations are considered a critical issue in irrigated rice culture, as populations can increase after several years of repeated rice cultivation. Continuous flooding eliminates many, but not all, weed species, with the remaining weeds requiring manual or herbicidal control. Manual weed control is extremely labor intensive and is often associated with unsatisfactory labor productivity (i.e., economic returns as compared to local wages). Chemical weed control has therefore largely substituted manual weedingReference Labrada16. However, the often considerable financial costs are problematic in the context of smallholder agriculture, since they may cause the exclusion of resource-poor smallholder farmers from this production system. Perhaps even more seriously, chemical weed control bears potentially serious environmental risks, since areas of rice irrigation are typically located in hydrologically sensitive areas, allowing potential contamination of riverine ecosystems and water reservoirs.
Since weed species differ widely in their aggressivity and competitivity, there does not exist any universally applicable critical threshold of acceptable weed density or biomass above which irrigated rice grain production would be negatively affectedReference Amaral17, Reference Erasmo, Costa, Pinheiro, Silva, Terra, Sarmento, Cunha and Garcia18.
Early stages of rice biomass can serve as indicators for future grain yieldsReference Ni, Moody and Robles19. Weed pressure and weed control are governed by timing, density and composition, since weed species and functional groups differ widely in their aggressivity and weed control requirementsReference Erasmo, Costa, Pinheiro, Silva, Terra, Sarmento, Cunha and Garcia18.
This paper investigates the potential of IRF cultivation for weed control in irrigated rice production in 3-year-old pilot plots in the eastern periphery of Amazonia.
Materials and Methods
Study region
Research was conducted between August 18 and December 5, 2008 in the Embrapa research station of Arari county, located 165 km from the state capital of São Luis in the ‘Maranhão lowlands’, in the Mearim River delta (3°27′30″S, 44°46′00″W), 15 m above see level. Soils are fertile alluvial vertisol aquerts, with a high content of montmorilonite and illite. The climate is classified according to the Köppen system as sub-humid equatorial (Aw) with 1500–2000 mm of annual precipitation. Irrigated rice production takes place during the dry season (August–November) when the absence of rain and the proximity to the Mearim River guarantee control of water regime. During the rainy season, fields lie fallow or are extensively grazed by cattle or waterbuffalo.
Treatments
The four IRF fields constituted 3-year-old pilot plots, which we compared with four adjacent fields with conventional irrigated rice (CIR) cultivation. Preceding land-use history on all fields had been two decades of continuous irrigated rice production. Each field plot was 8.5×20 m.
Two thousand seven hundred and twenty 25-day-old rice transplants (cv EPAGRI 108) were planted into puddled fields, with a plant density of 16 plants m−2 on all fields (25×25 cm). CIR was kept inundated until the grain filling stage after which irrigation was intermittent, based on need. Both rice production systems received standard doses of chemical fertilizers: 10 kg ha−1 N, 60 kg ha−1 P2O5 and 30 kg ha−1 K2O immediately before transplanting, and 45 kg ha−1 N as urea, split in two doses at 7 and 30 days after transplanting (DAT). Rice variety, water and transplant management as well as fertilizer rates were in accordance with high-intensity (i.e., 5–7 Mg ha−1 grain yields) smallholder irrigated-rice agriculture promoted during the past decade in the region. No pesticides were applied in any plot.
IRF fields contained by type 50% ‘Tambacu’ (female Colossoma macropomum Cuvier×male Piaractus mesopotamicus Holmberg) and 50% ‘Thai tilapia’ (Oreochromis niloticus L.) for a total of 4000 fish per ha or approximately 1.7 fish per m3. The fish were released into the rice fields after attaining sufficient size, at 30 DAT. The previous 2 years of IRF had been conducted with the same rice density and with similar fish densities, with ‘Tambacu’ in the first year and a mixture of Hungarian carp (Cyprinus carpio L.) and grass carp (Ctenopharyngodon idella Valenciennes) in the second year. The IRF fields contained in addition to the irrigated rice field a fish sanctuary, comprising about 5% of the area required for initial fish raising (protection of juveniles against predators).
Measurements
We quantified weeds on two occasions, at 20 and 40 DAT, corresponding to two distinct stages of rice ontogenesis, mid-growth and the onset of flowering. These periods are considered critical for rice developmentReference Piepho and Alkemper20–Reference Vega, Ona and Paller23. Weed infestation was not quantified in the later stages of rice development (grain filling and maturation stages) when weeds presumably had less influence on rice. Weeds were quantified in three randomly distributed 1 m2 samples per field, and field values were calculated as the means of these three samples.
We quantified weed density (number of plants m−2), aboveground biomass and species composition in each plot. We also quantified rice biomass at 40 DAT in the same plots. Weed and rice dry weight biomass were determined after drying at 70°C in a forced circulation oven for 1 week. Weed species were identified in the field or in the herbarium of Maranhão State University. We subsequently calculated species richness, Shannon–Weaver (H′) and Simpson (D) diversity indices and Jaccard similarity indexReference Magurran24. Dendrograms of floristic similarity were constructed with the program FITOPACReference Shepherd25. We also report on functional composition [monocotyledons versus dicotyledons and (main) species composition].
Statistics
We tested all data for normality of distribution (Kolmogorov−Smirnov and Lilliefors tests)Reference Lilliefors26, Reference Poole27. Weed and rice biomass followed normal distribution, whereas normality for abundance data was achieved with ln-transformation. We compared weed communities between systems (CIR versus IRF) and time of sampling (20 and 40 DAT) and related weeds at 40 DAT with rice aboveground biomass, both with individual t-tests and in a bi-factorial scheme. Statistical analyses were conducted with Statistica 7.028.
Results
Total weed populations
Total weed density and biomass differed between system at 20 and 40 DAT (Fig. 1a). Between 20 and 40 DAT, weed density declined overall (in both production systems) by 70.3% (P=0.06), whereas weed biomass increased overall by 64.0% (P=0.05), resulting in an average overall individual weed biomass increase of 332%.

Figure 1. (a) Total weed density (left) and weed biomass (right) at 20 and 40 DAT in CIR and IRF management systems. (b) Monocot weed density (left) and biomass (right) at 20 and 40 DAT in CIR and IRF management. (c) Dicot density (left) and biomass (right) at 20 and 40 DAT in CIR and IRF management (means±SE). Note scale differences between the three figures. All density data were ln-transformed for t-tests and ANOVAs.
Total weed density was lower in IRF than in CIR fields, as indicated by t-tests. Differences were significant at 20 and 40 DAT. Weed biomass did not differ between systems at 20 DAT, but at 40 DAT was significantly lower in IRF than in the CIR fields. Bi-factorial ANOVAs indicated significant effects of sampling time and rice production system and a tendency for interactions (P=0.10) between these factors for weed density, but no significant effect of treatments on total weed biomass was observed.
Weed type
Monocot density between systems and across sampling dates closely resembled that of total weed density (Fig. 1b), with significant differences at 20 and 40 DAT. Monocot biomass tended to be lower at 20 DAT in the IRF system, but did not differ at 40 DAT.
Bi-factorial analysis indicated significant effects of treatment and time on dicot density (Fig. 1c), with no interaction between factors, and no significant effect between treatments or sampling time for dicot weed biomass.
Bi-factorial analysis indicated significant effects of the rice production system on the relative growth-form composition of weeds between CIR and IRF at 20 and 40 DAT (Fig. 2a–d).

Figure 2. (a–d) Weed growth-form composition in CIR (left) and IRF (right) at 20 and 40 DAT: Abundance and biomass composition.
Weed species composition
Table 1 lists the most important weed species in CIR and in IRF at 20 and 40 DAT. Species ranking differed markedly with time and between the two production systems. Fimbristylis miliacea and Pontenderia cordata were most important in CIR, and P. cordata and Marsilea quadrifolia were the most important in IRF fields. Most noteworthy was the complete absence of F. miliacea (Cyperaceae) in the IRF system. This species is aggressive, disperses rapidly and causes significant yield reductions in irrigated rice agriculture of the regionReference Begum, Juraimi, Amartalingam, Rastan and Man29–Reference Begum30.
Table 1. Weed species ranking in CIR and IRF production systems at 20 and 40 DAT.

1 IV, importance values, calculated as averages of abundance shares (% abund), biomass shares (% biom) and frequency shares (% freq).
2 ‘Red rice’, a low-productivity ‘wild rice’ variety that tends to turn dominant in areas with repeated irrigated rice cultivation. Red rice is especially difficult to control with herbicides, due to its taxonomic similarity with cultivated rice.
Weed diversity and similarity
Weed species richness was significantly lower (29%) in IRF compared to CIR at 20 DAT, but not significantly lower (22%) at 40 DAT (Fig. 3).

Figure 3. Comparison of CIR and IRF systems on weed species richness at 20 and 40 DAT.
Weed species richness did not vary significantly over time in each system, and the interaction (time×system) was non-significant. Both Shannon–Weaver (H′) and Simpson (D′) indices did not differ significantly between systems at either sampling date (Table 2). This indicates that, in spite of the substantial reductions in weed biomass and abundance, this weed reduction caused by IRF management did not reduce weed species diversity to the same extent.
Table 2. Weed diversity at 20 and 40 DAT in IRF and CIR systems (means±SE).
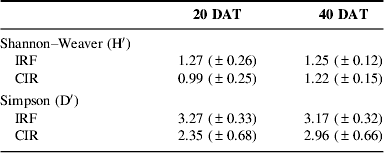
Figure 4 compares the floristic similarity (Jaccard index) of weed communities between the CIR and the IRF fields at 20 DAT (top) and 40 DAT (bottom). While floristic similarity was high within the CIR fields and within the IRF fields, the CIR and IRF fields were very dissimilar to one another, thus forming two floristically distinct groups, both at 20 and 40 DAT.

Figure 4. Dendrograms of floristic similarity (Jaccard index) between the eight study fields at 20 DAT (top) and at 40 DAT (bottom). CIR 1–4 are the four CIR fields, whereas IRF 5–8 are the four IRF fields.
Impact of weeds on rice biomass
Rice aboveground biomass at 40 DAT serves as an indicator of future grain yieldsReference Agrawal, Lal and Richharia31–Reference Saif-ur-Rasheed, Sadaqat and Babar34 and was negatively affected by total weed density (R 2=0.49, P=0.05) and by monocot weed density (Fig. 5), but not by weed biomass or dicot weed density.

Figure 5. Impact of monocot weed density on rice aboveground biomass at 40 DAT.
Discussion
IRF farming is a promising land-use alternative for smallholder riverine farmers of the eastern Amazonian lowlands. With a potential for high productivity in grain and fish yields, this system is capable of generating sufficient income for smallholders. The synergies between the rice and fish components may provide a potential reduction in financial inputs (chemical fertilizers and pesticides) and possibly labor, if manual weeding is reduced. The IRF system may also help eliminate weed seedbanks, as weed control is a key factor in irrigated rice grain productivity and economic returnsReference Fleck, Agostinneto, Rizzardi, Bianchi and Menezes35–Reference Navarro and Costa37.
Due to selectivity problems, chemical weed control is especially difficult for species related to rice cultivars, most notably ‘red rice’ (Oryza rufipogon Griff), a low-productivity ‘wild’ rice variety that causes serious management problems in the study regionReference Noldin and Corbucci38.
Compared to CIR management, 3 years of integrated rice–fish cultivation drastically reduced weed density. Thus, our study confirms previous findings reviewed by Frei and BeckerReference Frei and Becker39 on weed reductions associated with IRF. Differences in weed populations and weed reductions associated with IRF were greater in abundance than in biomass, and more apparent at 20 DAT than at 40 DAT. The reasons for this are unknown.
Since IRF effects on weeds are most expressed early (20 DAT) in rice development, this management alternative appears especially effective at the most critical stages of weed control for irrigated rice agriculture.
Weed reduction associated with IRF was also greater for monocotyledons than for dicotyledons. As weed floristic similarity between IRF and CIR fields was low, this may indicate that fish-weeding is species-specific. The underlying reasons for potential species-specificity are unknown. One possible explanation would be that 3 years of continuous IRF management causes a selective depletion of the seed bank of small-sized seeds, which in turn are predominantly monocotyledons. Alternatively, some fish may prefer monocotyledonsReference Xavier40, Reference Zanganini41, though feeding preference will vary not only with fish species but also with the relative availability of food sources. Since monocotyledonous weeds typically are the more aggressive species and more difficult to control both mechanically and with herbicidesReference Gelmini42, IRF appears to provide the best management option for the most important weed families, including CyperaceaeReference Keeley43, Reference Galon, Concenço, Ferreira, Silva, Ferreira, Noldin and Freitas44, in irrigated rice production.
Although the weed density reduction accomplished in a 3-year-old IRF system was significant, this was not accompanied by a reduction in weed species diversity. This may indicate that fish-weeding did not cause a simplification of the weed community.
In the future, we will conduct on-farm investigations of other key aspects of IRF related to the ecological and socioeconomic sustainability and adequacy for smallholder farmers: (i) the substitution of synthetic nitrogen with biological nitrogen fixation via Azolla, (ii) the ideal densities, species and management of the critical juvenile stage of fish, (iii) utilization of the field boarders (dikes) for irrigated horticulture, fruit production and for human and fish nutrition and (iv) monitoring of water quality and greenhouse gas emissions (CH4 and N2O).
Based on the data presented here, we conclude that IRF cultivation as a land-use system has important positive effects on weed control, apparently without the need for further mechanical or chemical weed control. In this important aspect of irrigated rice management, IRF is thus a land-use system ideally suited for resource-poor smallholder agriculture in environmentally sensitive riverine areas.