Published online by Cambridge University Press: 03 May 2005
All newborn infants have limited pulmonary reserve compared with older children. This puts them at increased risk of respiratory complications, such as those associated with infection by the respiratory syncytial virus. Young children with congenital cardiac disease are particularly likely to suffer severe disease related to infection by the virus. In these children, the extreme vulnerability of the lung to pulmonary oedema is compounded by the additional burden caused by the respiratory syncytial virus.
In addition to the well-documented acute pulmonary effects of infection with the respiratory syncytial virus, there may also be consequent long-term respiratory morbidity. Clinical studies have shown that infection by the virus in infancy is associated with a higher risk of developing subsequent bronchial obstructive disease. Much debate surrounds the mechanisms underlying this association. It is thought that a combined immunological and neurogenic response mechanism is likely. Prevention of severe respiratory disease in infants and young children with congenital heart disease due to infection by the virus may, therefore, offer both immediate and long-term benefits. Indeed, an increasing body of evidence supports this hypothesis, indicating a clinical rationale for prophylaxis against the virus in infancy, in order to reduce the chance of developing reactive airways disease and asthma in later life.
Infants and young children with congenital cardiac disease are more likely to be hospitalised, and have greater morbidity and mortality associated with a lower respiratory illness, due to infection by the respiratory syncytial virus than infants without heart disease.1–3 This is particularly apparent when patients with congenital heart disease with a history of recent bronchiolitis due to the respiratory syncytial virus undergo corrective surgery.4 The combination of the underlying cardiac physiology, the immature characteristics of the lung with limited reserve, complex cardiopulmonary interactions, and presence of heart failure, lays the foundation for a “perfect storm” brought about by the acute effects of respiratory syncytial viral bronchiolitis. The cumulative effects of direct cytopathology produced by the bronchiolitis, along with secondary inflammatory changes in the lung, compromise the patient with congenital heart disease, often resulting in an inability to return to baseline. The clinician may frequently face the need to intervene surgically on behalf of the child under less than ideal conditions, and at greater risk.
There are approximately 20 million alveoluses in the newborn lung, this number increasing to about 300 million by the age of 8 years, and between 200 and 600 million by adulthood.5–7 Due to their small diameter, the airways of newborns have greater resistance compared with the older child.4 This necessitates an increased workload for ventilation, a phenomenon that can increase dramatically with even minor luminal compromise.8 Increased peripheral resistance affects ventilatory distribution, and makes newborns more vulnerable to hypoxaemia. Obviously, any prematurity of the lung will further compound these abnormalities.
Depending upon the type of cardiac defect present, the lung of the infant may be over-circulated, as for example in the setting of ventricular septal defect, or under-circulated as with tetralogy of Fallot. Pulmonary over-circulation associated with left-to-right shunting may result in mucosal oedema, and luminal embarrassment, as well as vascular and/or cardiac compression of the large bronchuses. In the under-circulated lung, as seen with right-to-left shunting, lung volume may be decreased, and airways may in turn be globally small.9
Infants also lack effective collateral ventilation, so that the plugging by mucus of a single airway during an infection of the lower respiratory tract can easily result in atelectasis and abnormal exchange of gases. The cellular debris and oedema often associated with infection-related inflammation can also produce relatively greater obstruction in the small airways of infants.
Efficient exchange of gases in the lung is dependent upon maintaining a dry interface between the alveoluses and the capillaries. Simply stated, the amount of fluid produced in the lung must be less than or equal to the amount of fluid that is eliminated. Otherwise, accumulation of fluid, and oedema, will result over time. In the mature lung, multiple safety factors assure this favourable balance. In a series of experiments in dogs, in which the left atrial pressure was sequentially increased, Guyton and Lindsey10 demonstrated that, despite increases in the microvascular hydrostatic pressure, the principal driving pressure for fluid filtration in the lung, balance was maintained over a wide range of left atrial pressures.10 The safety factors include, but are not limited to, vascular recruitability, integrity of the interstitial matrix, low endothelial and epithelial oncotic and hydrostatic conductance, active sodium uptake from the alveoluses, and effective lymphatic drainage. In the newborn, there are maturational differences that lessen the capacity of the safety factors. Likewise, the changes associated with congestive heart failure may further limit the ability of the newborn lung to compensate during respiratory syncytial viral bronchiolitis.
Postnatal pulmonary vascular and lymphatic development parallel growth of the airways.5 As alveoluses are added while the lung matures, vascular and lymphatic surface area increases, so that pulmonary vascular resistance and the ability to drain fluid from the lung increases with age. At birth, the pulmonary vascular bed of the newborn lung is nearly fully recruited.11 This is vastly different, however, from the adult pulmonary vascular bed, which is primarily recruited in the base and middle lobes. In the adult, when flow of blood to the lungs increases, for example, as a result of exercise, an increase in left atrial pressure or stress causes additional pulmonary vascular channels to be recruited. Thus, despite an increase in pulmonary flow, the microvascular hydrostatic pressure does not increase significantly, thanks to a decrease in pulmonary vascular resistance. Furthermore, not only are vascular channels recruited, but also additional lymphatics that can drain interstitial fluid.
In contrast, because the pulmonary resistance is relatively fixed in the newborn, further increases in flow of blood necessitate an increase in microvascular hydrostatic pressure. This results in increased transvascular fluid filtration. Likewise, no additional lymphatic channels are recruited to drain the interstitial fluid.
The two primary classes of molecules of the interstitial matrix include collagen, principally type IV, and proteoglycans, which are polysaccharide-protein conjugates.12, 13 The collagen is responsible for maintaining alveolar-capillary integrity. While rendering some structural support, the proteoglycans serve a significant role in lung fluid maintenance.13
The proteoglycans are hydrophilic molecules. With an increase in pulmonary microvascular hydrostatic pressure, transvascular filtration increases. Interstitial water is then bound to the proteoglycans, being held in reserve until it can be drained by the lymphatic system. This maintains a dry interface between the alveoluses and the capillaries. These molecules, coupled with the capacitance of the interstitium, are likely to account for the lack of development of pulmonary oedema during acute increases in pulmonary microvascular hydrostatic pressure.14 There are no data to suggest that the newborn lung does not share similar safety factors against oedema as does the mature lung. In the presence of heart failure, however, transvascular fluid filtration increases, and the interstitium can become distended. With this, the proteoglycans are stretched, and can lose their ability to bind water, which can then enter the alveolar space. In the presence of high microvascular hydrostatic pressure, it is possible that capillary endothelium, and possibly the alveolar epithelium, lose their sieving properties, allowing proteins and solutes to leak into the interstitium and alveoluses. In cases of extreme pressure in animal models, there is interruption of the endothelium and alveoluses, a condition referred to as “stress failure”.12, 15 This can lead to interstitial and alveolar haemorrhage. Fu et al.16 demonstrated that pressure-induced microfractures of the capillary endothelium and alveolar epithelium occur at much lower microvascular hydrostatic pressures in newborn animals than in adults.
The chronic effects of heart failure can increase the alveolar capillary interface with basal laminar thickening brought about by added matrix deposition.17 This thickening can add resistance to gas exchange across the alveolar capillary interface, and make the patient more vulnerable to hypoxaemic complications.
Endothelial dysfunction in patients with heart failure may alter pulmonary haemodynamics and affect fluid filtration in the lung.18 Patients who have high pulmonary flow have elevated levels of endothelin in the serum.19 A powerful vasoconstrictor, endothelin can cause an increase in microvascular hydrostatic pressure. Likewise, decreased local production of nitric oxide may also contribute to altered pulmonary vascular tone and increased microvascular hydrostatic pressure.
Recently, another important mechanism has been recognised for the elimination of excess fluid from the airways, namely the active uptake of alveolar sodium.20, 21 The type II bronchial epithelial cells are asymmetrical, with the apical portion facing the airway and the basolateral portion facing the pulmonary interstitium. Sodium is actively pumped from the alveolar space by active epithelial sodium channels along the apical pole. Sodium is then pumped from the bronchial epithelial cell into the interstitium by basolateral sodium-potassium ATPase pumps. The osmotic gradient created by this ion shift draws water out of the alveolar space. The activity of these sodium pumps is enhanced by catecholamines, steroids, thyroid hormones and inhibitors of acetylcholine esterase, but is reduced by various factors that are present in congenital heart disease and congestive heart failure, including atrial natriuretic peptide, cytokines and the presence of alveolar hypoxia.21, 22
The majority of pulmonary lymphatic channels eventually drain into the thoracic duct, which in turn drains into the central venous system. Drake et al.23 have demonstrated that increasing the central venous pressure can affect drainage through the thoracic duct. In the fetal and newborn lamb, Johnson et al.24 demonstrated that the flow of lymph through the lungs ceases at much lower central venous pressures than it does in the adult sheep. This may have significant clinical implications for patients with congenital heart disease and heart failure. This relative sensitivity to central venous hypertension may explain, at least in part, altered pulmonary mechanics in conditions such as chronic lung disease, for example, bronchopulmonary dysplasia, those with an unstable early bidirectional Glenn anastomosis, and those with right heart failure from congenital cardiac disease.25
Little is known regarding the control of lymphatic vascular smooth muscle. In some lymphatic beds, such as that in the mesentery, an intrinsic pulsatility of the lymphatic mural smooth muscle occurs, which presumably helps drive lymph toward the central venous system. Inhibitors of this pulsatility include nitric oxide, and nitrogen-bearing vasodilators such as sodium nitroprusside.26 Although unproven, this inhibition, if it exists, in the pulmonary lymphatic bed may have clinical implications for the patient with congenital heart disease or congestive heart failure in whom vasodilators are being used.
The factors outlined above, which may be compounded by congenital heart disease, increase the susceptibility of the infant lung to further respiratory complications, for example those arising from infection by the respiratory syncytial virus.
For the child with congenital heart disease, development of respiratory syncytial viral bronchiolitis can be the final component of the “perfect storm” that leads to a disastrous outcome. Not only is the patient at risk of serious morbidity and mortality associated with the acute disease, but the effects of illness can confound future scheduled surgical management.4
So, what are the characteristics of acute respiratory syncytial viral bronchiolitis? Certainly bronchial epithelial sloughing, impaired surfactant production, and airway obstruction, can lead to segmental atelectasis and alveolar hyperinflation – evident on the typical chest radiograph in respiratory syncytial viral bronchiolitis.27 There is strong evidence that the pathophysiology goes well beyond these direct cytopathological effects. Carpenter et al.28 demonstrated that pulmonary oedema occurs in animals with respiratory syncytial viral bronchiolitis, especially in the presence of hypoxia. The fact that respiratory syncytial viral bronchiolitis results in significant perivascular infiltrates strongly suggests that an inflammatory component exists in these patients.29 This inflammatory response, in turn, leads to altered vascular tone and permeability changes of the alveolar–capillary interface (Table).
Table. Aetiologies of pulmonary oedema: risk factors for development of pulmonary oedema are outlined as a function of immaturity of the lung, events occurring during heart failure and during acute respiratory syncytial virus lower respiratory tract infection. Columns indicate whether risk results in increased lung fluid formation or elimination.

Infected bronchial epithelial cells release proinflammatory cytokines and chemokines, for example, interleukin-1, interleukin-6, interleukin-8, and tumour necrosis factor-alpha, which in turn recruit inflammatory cells, including T lymphocytes, into the lung.30 T lymphocytes then produce additional cytokines which are patterned by either a T helper cells of either type 1 or 2 profile. An antiviral response of the type 1 helper cells, which is typical of a mature response, is characterised by cytokines that include interleukin-12 and interferon activity. A response by the type 2 helper cells, characterised by production of interleukin-4, and interleukin-5, promotes eosinophilia, and may enhance an inflammatory state. The airway secretions of infants with respiratory syncytial viral bronchiolitis reflect a response patterned by the type 2 helper cells.31–33 As a result, cytokines with vasoactive effects linger in the lung of the patient infected by the respiratory syncytial virus.34
The permeability characteristics of the capillary endothelium may be altered by cytokines. Vascular permeability factor has been found in increased amounts in the airways of children with respiratory syncytial viral bronchiolitis. Due to the increased oncotic and hydraulic conductance of the microvascular bed, there is increased transcapillary filtration, overloading an already taxed ability to drain the interstitium of the patient with congenital heart disease. Less well known are the effects of these cytokines on the alveolar epithelial cells that are normally almost impervious to water and solutes.
The vasoactive effects of the perivascular inflammatory response in respiratory syncytial viral bronchiolitis may also contribute to formation of oedema. Cytokine-induced production of endothelin in the lung results in increased pulmonary vascular tone, increasing pulmonary microvascular hydrostatic pressure, which promotes free water filtration.29 This hypothesis, compounded by a change in oncotic conductance as described earlier is strengthened by a series of experiments by Carpenter and Stenmark,35 in which they demonstrated a reduction in formation of pulmonary oedema and extravasation of protein in animals with hypoxic respiratory syncytial viral bronchiolitis pre-treated with endothelin receptor blockers.35
Respiratory syncytial viral bronchiolitis may also directly alter the expression and activity of sodium channels, important for elimination of excess fluid from the airways. This has been shown to occur during infection with other viral pathogens, such as the influenza virus and adenovirus.36, 37 In addition, hypoxia is thought to impair alveolar liquid clearance by inhibiting epithelial sodium transport.29, 38, 39 Thus, the respiratory syncytial virus may directly and indirectly, via associated regional hypoxia, impair fluid clearance mechanisms.
The development of pulmonary oedema due to infection by the respiratory syncytial virus, therefore, involves the interplay of multiple factors. In overview, raised hydrostatic forces act to increase leakage of fluid into the lung interstitium, accentuated under conditions where there is direct injury to the capillaries. Together with concurrent impairments of the protective mechanisms for the alveolar clearance of liquids, alveolar flooding can result (Fig. 1).29

Figure 1. Potential aetiology of pulmonary oedema in respiratory syncytial viral (RSV) infection.29 Adapted, with permission, from Carpenter TC, Stenmark KR. Predisposition of infants with chronic lung disease to respiratory syncytial virus-induced respiratory failure: a vascular hypothesis. Pediatr Infect Dis J 2004; 23 (1 Suppl): S33–S40.
The effects of infection by the respiratory syncytial virus are not only confined to acute morbidity; there may also be long-term sequels. This has been clearly illustrated by a number of studies linking infection to the incidence of subsequent reactive disease of the airways or asthma.40–42
While the majority of clinical studies in this area are retrospective, perhaps the most convincing clinical evidence to date comes from a prospective controlled study by Sigurs et al.,43, 44 in which previously healthy children have now been followed up for 7.5 years. At this time, the cumulative prevalence of asthma was significantly higher in those infected by the respiratory syncytial virus group than in the control group (p < 0.0001, 95% confidence interval of 2.79–30.55). Furthermore, the cumulative prevalence of “any wheezing” was also significantly different between the two groups (p < 0.001, 95% confidence interval of 1.40–2.79). Similar patterns were seen up to 7.5 years of age in the occurrence of current asthma and current “any wheeze” (Fig. 2).

Figure 2. The occurrence of current asthma and “any wheezing” in 47 children hospitalised with respiratory syncytial virus (RSV) in infancy, compared with that in 93 matched controls, at 1 year, 3 years and 7.5 years of age.43,44 “Current” is defined as being during the preceding year. *p 0.003; **p < 0.001; ***p < 0.0001; [dagger]p < 0.004 Reproduced, with permission, from Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161: 1501–1507.
The authors concluded that the results of this long-term prospective study, together with data from a number of other studies of index children and comparison groups,40 provide strong evidence that respiratory syncytial viral bronchiolitis in infancy is associated with a higher risk of developing subsequent bronchial obstructive disease. This supports the theory that the respiratory syncytial virus plays a significant role in influencing the mechanisms involved in the development of asthma and allergy at least in some children.
A number of theories have been proposed to explain the potential cellular and molecular mechanisms underlying the association between respiratory syncytial viral infection and subsequent reactive airways disease and asthma. The virus may predispose to reactive disease or asthma by altering airway immune responses, and/or by dysregulating neural control of the airways.42, 45–48
Infection may lead to a loss of chemoreceptors and breakdown of the normal anatomical barriers to antigens, via epithelial and cilial damage.46 The virus is also known to induce significant local inflammation, with profuse release of cytokines,42, 49 and it is possible that these inflammatory mediators may alter the local immune memory in the lung. Studies in animal models suggest that respiratory syncytial virus infection at an early age shifts the subsequent immune response pattern, from a protective immunity mediated by Type 1 helper cells to an allergic or atopic response mediated by the type 2 helper cells. These studies suggest that it is the timing of the initial infection that determines any potential subsequent disruption of the normal balance of the helper cells.42, 50, 51 A strategy of delaying respiratory syncytial viral infection beyond infancy by prophylaxis may therefore have the potential to reduce respiratory morbidity in later childhood by interrupting the processes intrinsic in alteration of airway immune responses.
Another mechanism that may have implications for the management of vulnerable infants and young children, such as those with congenital heart disease, is neurogenic-mediated pulmonary inflammation41, 48, 52, 53 caused by respiratory syncytial virus-induced damage to sensory nerve fibres in the lung. This damage results in the release of substance P, a very potent inflammatory mediator, which leads to recruitment of leucocytes, mast cells and eosinophils, and an increase in levels of inflammatory cytokines. The respiratory syncytial virus may also act to increase the expression of nerve growth factor, and neurotrophin receptors, thereby triggering other inflammatory and neuronal pathways that contribute to inflammation and hyper-reactivity of the airways.48
It is our belief that, in reality, the mechanisms outlined above may be linked, and that a combined neuroimmune response may underlie the proposed connection between respiratory syncytial viral infection in infancy and development of reactive airways disease and asthma in later life.
Asthma is a major clinical problem, affecting as many as 300 million individuals worldwide. With such an extensive prevalence, the global economic and personal burden resulting from this disease is enormous.54
Considering the increasing body of evidence reporting a link between respiratory syncytial viral infection in infancy and development of subsequent reactive airways disease and asthma in later life, it follows that there may be long-term health and economic benefits of preventing infection by the respiratory syncytial virus. Prophylaxis in infancy, therefore, provides an exciting prospect as a strategy for abrogating subsequent development of persistent wheezing and asthma-like symptoms in later childhood.
This rationale is supported by animal studies which show that the monoclonal antibody palivizumab (Synagis®), can inhibit respiratory syncytial virus-induced neurogenic-mediated inflammation in rat airways (Fig. 3).55 Palivizumab was effective in inhibiting neurogenic inflammation when given 24 hours before, or 72 hours after, intranasal respiratory syncytial virus inoculation. As no direct effect was observed in pathogen-free rats, it was concluded that the anti-inflammatory effect of palivizumab results from inhibition of viral entry into the airway epithelium. It is also proposed that palivizumab may have therapeutic activity when administered early after upper respiratory tract infection, prior to the establishment of widespread infection in the lungs. This suggests that administration of palivizumab not only prevents respiratory syncytial viral infection but, if infection does occur, it may limit the severity of the acute airway inflammation, thereby potentially protecting against subsequent reactive airways disease or asthma.

Figure 3. The effect of palivizumab on respiratory syncytial virus (RSV)-induced neurogenic inflammation in rats.55 ***p < 0.001. Reproduced, with permission, from Piedimonte G, King KA, Holmgren NL, Bertrand PJ, Rodriguez MM, Hirsch RL. A humanized monoclonal antibody against respiratory syncytial virus (palivizumab) inhibits RSV-induced neurogenic-mediated inflammation in rat airways. Pediatr Res 2000; 47: 351–356.
To extrapolate these findings to the clinical situation, a multinational, prospective, case-controlled observational study is being conducted to determine whether prophylaxis leads to a decreased incidence and severity of subsequent reactive airways disease in children. The study, which was initiated in 2001, has enrolled high-risk preterm infants, less than or equal to 35 weeks gestation, without chronic lung disease from centres based in Germany, Spain, The Netherlands, Sweden, Poland and Canada. These infants will be followed up for a total of three years. The primary endpoints include incidence of asthma, defined as three episodes of physician-documented wheeze, or recurrent wheezing reported by caregivers. The secondary endpoints include hospitalisation for respiratory illness and use of medications for reactive airways disease. Interim findings were presented at the meeting of the European Respiratory Society in Glasgow.56 It was demonstrated that prophylaxis with palivizumab in preterm infants reduced recurrent wheezing and asthma in the subsequent 1 and 1.5–2 years compared with control preterm infants who had not been thus treated.
Results from this study are eagerly awaited, as clinical evidence of an effective therapeutic strategy that protects against development of reactive airways disease or asthma would have healthcare and economic benefits on a global scale.
It is extremely important to protect vulnerable infants and young children with congenital heart disease from the additional acute pulmonary morbidity that results from severe respiratory syncytial viral infection. It is also becoming increasingly apparent that infection by the respiratory syncytial virus may have long-term effects on the lung, manifested as an increase in the incidence of subsequent reactive disease of the airways and asthma. Such long-term effects may have widespread implications, and impose additional burdens on health and quality of life, particularly for those with congenital heart disease.
The potential for prophylaxis in young children against the respiratory syncytial virus in order to prevent the potential subsequent recurrence of reactive airways disease or asthma in later life is an exciting therapeutic prospect, and one which could have widespread clinical impact. Future developments in this area are awaited with anticipation.
We are grateful to Thomson ACUMED® for some editorial assistance in the development of this paper. Sources of financial support: This paper is based on presentations given at a meeting funded by an unrestricted educational grant from Abbott Laboratories.

Table.
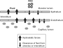
Potential aetiology of pulmonary oedema in respiratory syncytial viral (RSV) infection.29 Adapted, with permission, from Carpenter TC, Stenmark KR. Predisposition of infants with chronic lung disease to respiratory syncytial virus-induced respiratory failure: a vascular hypothesis. Pediatr Infect Dis J 2004; 23 (1 Suppl): S33–S40.

The occurrence of current asthma and “any wheezing” in 47 children hospitalised with respiratory syncytial virus (RSV) in infancy, compared with that in 93 matched controls, at 1 year, 3 years and 7.5 years of age.43,44 “Current” is defined as being during the preceding year. *p 0.003; **p < 0.001; ***p < 0.0001; [dagger]p < 0.004 Reproduced, with permission, from Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161: 1501–1507.

The effect of palivizumab on respiratory syncytial virus (RSV)-induced neurogenic inflammation in rats.55 ***p < 0.001. Reproduced, with permission, from Piedimonte G, King KA, Holmgren NL, Bertrand PJ, Rodriguez MM, Hirsch RL. A humanized monoclonal antibody against respiratory syncytial virus (palivizumab) inhibits RSV-induced neurogenic-mediated inflammation in rat airways. Pediatr Res 2000; 47: 351–356.