Multipartite interactions in microbiomes of blood-feeding parasites
Due to their specialized diet and dependence on vertebrate hosts, blood-feeding animals serve as diverse ecological niches for beneficial, commensal and pathogenic microorganisms (Lehane, Reference Lehane2005; Rio et al. Reference Rio, Attardo and Weiss2016). In different blood-feeding lineages, distinct phylogenetic origin, feeding strategy and preference for vertebrate hosts have led to differences in microbiome composition and to the origin of species-specific symbioses adapted to particular hosts. Since blood-feeding arthropods are also the most prominent vectors of causative agents of diseases such as malaria, sleeping sickness, filariasis, dengue, typhus, and plague, their microbiome interactions are of great importance. For some blood-feeding lineages, stable beneficial endosymbioses are either hypothesized to be absent such as in some hard ticks (Ross et al. Reference Ross2017) or the host is known to be relying on only a few symbionts such as in tsetse flies (Rio et al. Reference Rio2012; Bing et al. Reference Bing2017). Host–symbiont–pathogen interactions in these parasitic lineages are thus relatively simple to study. On the contrary, numerous blood-feeding lineages such as mosquitoes rely on loosely associated gut symbionts, and fragmentary data on host–symbiont–pathogen interactions are available only for a handful of these species (Damiani et al. Reference Damiani2010; Capone et al. Reference Capone2013; Minard et al. Reference Minard, Mavingui and Moro2013; Coon et al. Reference Coon2014; Wang et al. Reference Wang2017).
Several decades of research on individual microorganisms of blood-feeding parasites has provided us with a wealth of species-specific experimental data (Ribeiro and Francischetti, Reference Ribeiro and Francischetti2003; Graça-Souza et al. Reference Graça-Souza2006), and recent developments in microbiome characterization methods will hopefully allow comprehensive comparative analyses proposed by the Parasite Microbiome Project (Dheilly et al. Reference Dheilly2017). First, the long history of experimental work shows that majority of blood-feeding parasites depend on beneficial symbionts for nutrition, particularly provision of B-vitamins or cofactors missing from the blood diet (Wigglesworth, Reference Wigglesworth1929, Reference Wigglesworth1936; Aschner, Reference Aschner1932; Brecher and Wigglesworth, Reference Brecher and Wigglesworth1944; Puchta, Reference Puchta1954, Reference Puchta1955; Michalkova et al. Reference Michalkova2014; Nikoh et al. Reference Nikoh2014; Manzano-Marin et al. Reference Manzano-Marin2015; Douglas, Reference Douglas2017), and some of these symbionts perhaps also contribute to blood digestion (Indergand and Graf, Reference Indergand and Graf2000; Pais et al. Reference Pais2008). Second, immature immune system of animal blood-feeding lineages such as larvae of tsetse flies was shown to be dependent on beneficial bacteria for maturation (Weiss et al. Reference Weiss, Wang and Aksoy2011, Reference Weiss, Maltz and Aksoy2012) and the innate immune system is highly modified for harbouring beneficial bacteria (Kim et al. Reference Kim2011; Wang and Aksoy, Reference Wang and Aksoy2012; Bing et al. Reference Bing2017). Microbiome composition also plays a clear role in vector competence (Weiss and Aksoy, Reference Weiss and Aksoy2011; Weiss et al. Reference Weiss2013) and many of microbiome interactions occurring in blood-feeding parasites seem to be antagonistic. Last for this review, but definitely not least, microbiome interactions in blood-feeding animals often result in all possible directions of gene exchange: (i) between two microorganisms coexisting in the same host (Richmond and Smith, Reference Richmond and Smith2007; Nikoh et al. Reference Nikoh2014), (ii) from a microorganism to its animal host (Brelsfoard et al. Reference Brelsfoard2014) or (iii) from an animal host to its microorganism (Klasson et al. Reference Klasson2009; Woolfit et al. Reference Woolfit2009).
All of these interactions outlined above and discussed throughout this review are of medical and veterinary importance since they can be potentially leveraged for the elimination of diseases transmitted by blood-feeding vectors (reviewed by Berasategui et al. Reference Berasategui2015). A fascinating aspect in the biology of blood-feeding parasites is also the interactions with the vertebrate host the haematophagous parasite feeds on. However, these interactions are out of scope of this review and were already thoroughly discussed elsewhere (Schoeler and Wikel, Reference Schoeler and Wikel2001; Fontaine et al. Reference Fontaine2011). Here, I focus on nutrition, immune cross-talk and gene exchange and review these interactions for microbiome members of blood-feeding parasites with particular attention being paid to the interactions among the parasitic host, its obligate symbionts and other facultative/pathogenic bacteria and eukaryotes in the microbiome.
Multiple independent origins of blood-feeding in animals
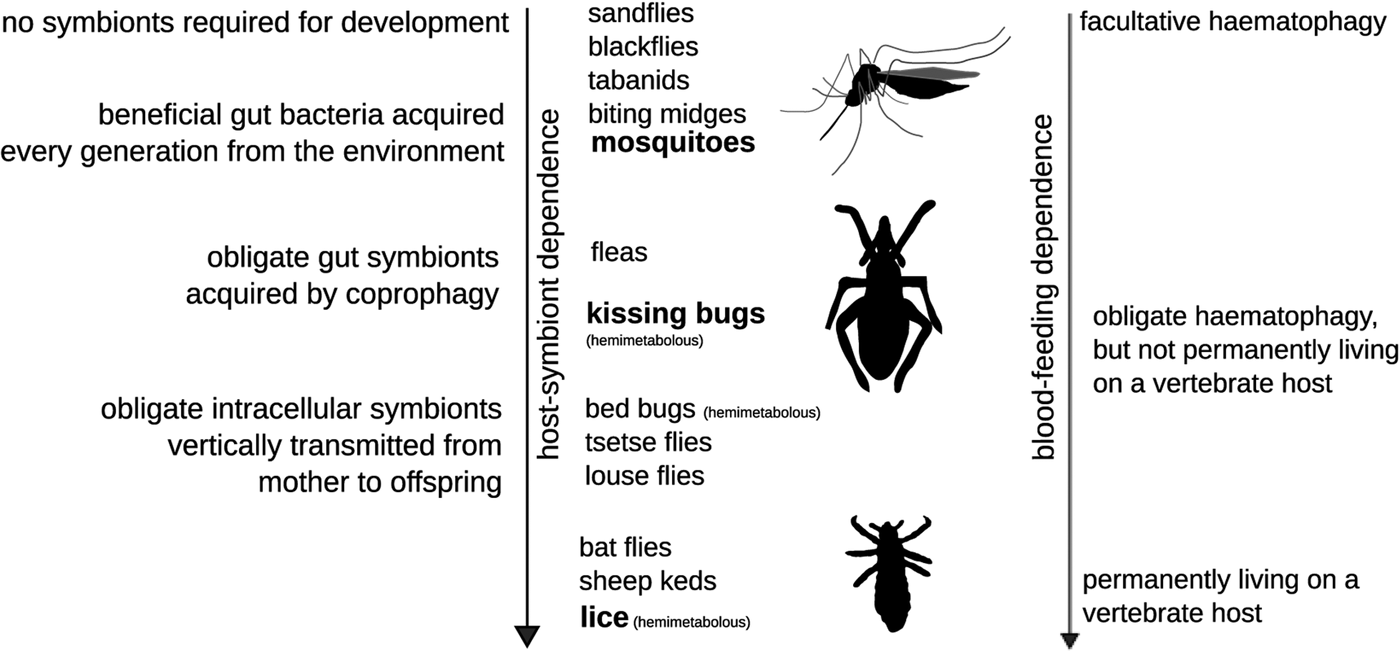
Blood-feeding has originated multiple times independently as a feeding strategy in animals as diverse as arthropods, nematodes, platyhelminths, annelids and vertebrates (Table 1). Vertebrates that at least partially feed on blood include parasitic lampreys and other fishes (Tetlock et al. Reference Tetlock2012), some bird species such as vampire ground finches (Schluter and Grant, Reference Schluter and Grant1984) and mammals such as vampire bats (Carrillo-Araujo et al. Reference Carrillo-Araujo2015). Haematophagy is, however, mostly a domain of arthropods (insects, ticks and mites) and other invertebrates (e.g. leeches, nematodes and Schistosoma spp.; Table 1). The most species-rich blood-feeding animals are insects with estimated 14 000 blood-feeding species (Adams, Reference Adams1999) of mosquitoes, black flies, sand flies, biting midges, tabanids, tsetse flies, bat flies, louse flies, lice, fleas, kissing bugs and bed bugs (Table 1). Consequently, different animal lineages greatly differ in the level of dependence on blood (Mans and Neitz, Reference Mans and Neitz2004; Lehane, Reference Lehane2005) – either being their main (obligatory haematophagy) or partial food source (facultative haematophagy) (Fig. 1). Facultative haematophages feed also on other alternative diets and they are thus in most cases not fully dependent on microorganisms to provide them with nutrients such as B-vitamins and cofactors. Facultative haematophagy is, for example, known from the vampire ground finch Geospiza septentrionalis (Schluter and Grant, Reference Schluter and Grant1984) or males of vampire moths Calyptra spp. (Bänziger, Reference Bänziger1975). What is the effect of this episodic blood-feeding on microbiome composition was never studied in detail.

Fig. 1. Dependence of the parasitic host on blood-feeding likely influences its relationship with symbiotic bacteria. I note that extracellular gut symbionts acquired every generation from the environment are more common in blood-feeding parasites that are not intimately associated with their hosts or also feed on other diets than blood at least during their larval development. Blood-feeding lineages outlined are holometabolous unless stated otherwise (kissing bugs, bed bugs and lice are hemimetabolous). Intracellular symbioses heritable through ovaries (or secretions of milk glands in viviparous Hippoboscoidea) are more common in parasites that spend their life cycle tightly associated with their host and do not feed on other diets than blood.
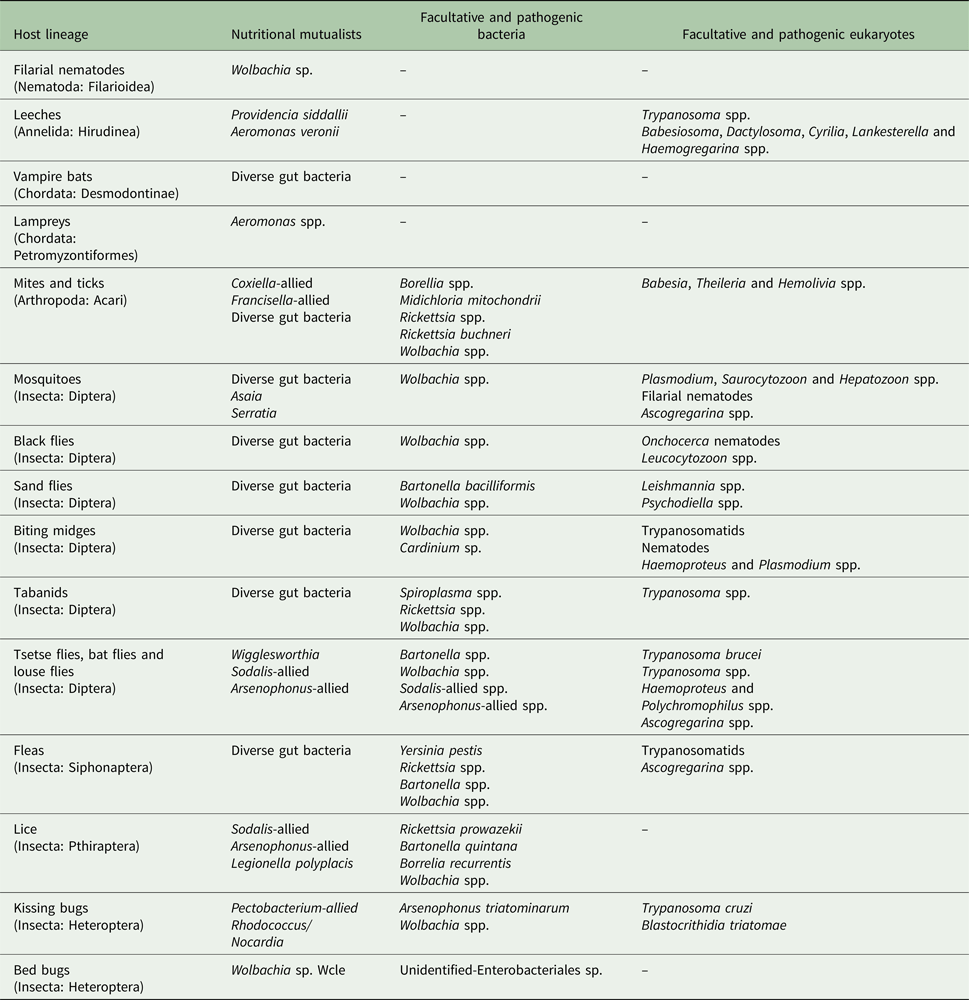
Table 1. Selected blood-feeding parasites and their microbiomes
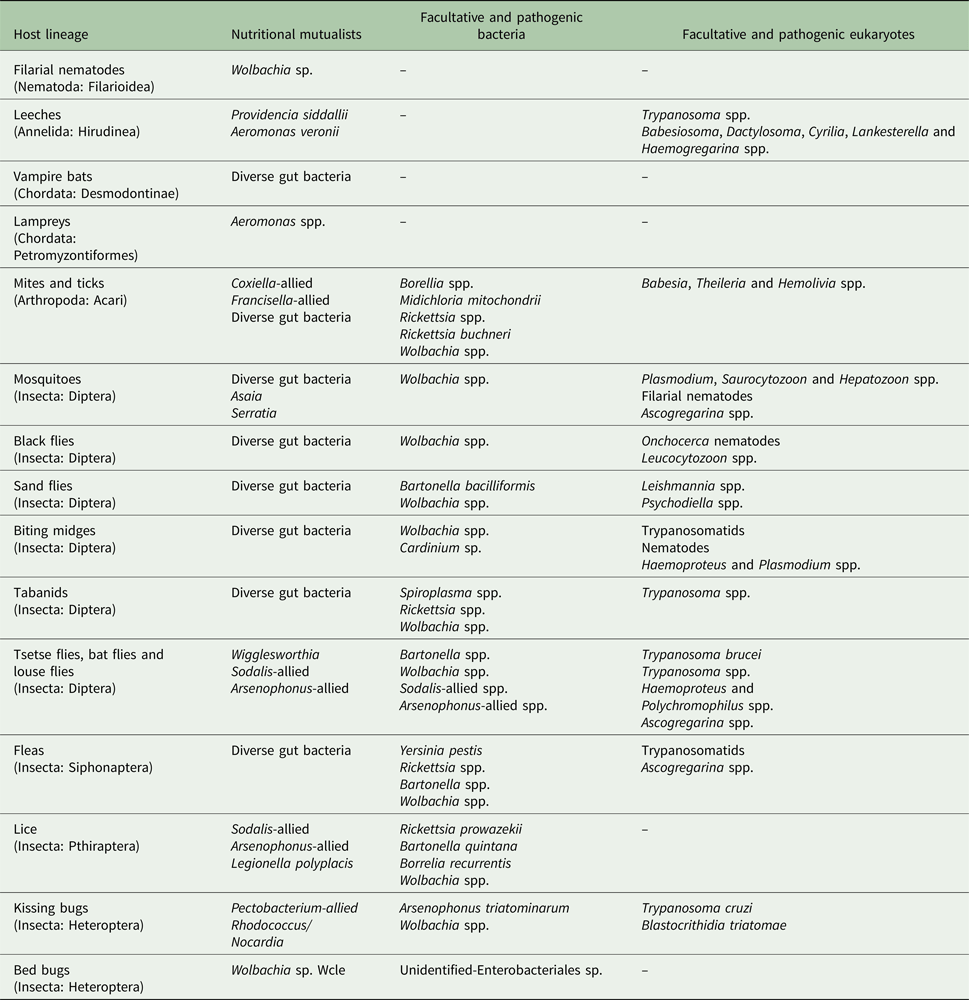
Viruses are not shown here since most of the arthropod species can transmit a diversity of arboviruses. I note that the table is not exhaustive and only shows major microbiome members reported to date. Blood-feeding lineages with no bacterial symbionts detected so far such as hookworms (Nematoda: Strongylida), barber's pole worms (Nematoda: Filarioidea) and Schistosoma blood flukes (Platyhelminthes: Trematoda) were omitted from this table for simplicity.
In other blood-feeding parasites such as mosquitoes, blood-feeding is only used by adults. Both sexes feed on plant juices and nectar, but only adult females feed on blood (Takken and Verhulst, Reference Takken and Verhulst2013). Interestingly, a gradient of dependence on a blood meal occurs in mosquitoes. It can be either not required for successful reproduction (autogenous species), required only for the second clutch of eggs (partially anautogenous), or absolutely crucial for reproduction (anautogenous species) (Lehane, Reference Lehane2005). Pre-existing energy/nutrient reserves play an important role during the first gonotrophic cycle of female mosquitos (Zhou et al. Reference Zhou, Pennington and Wells2004) and larval microbiome composition can be responsible for either providing these reserves or initiating other processes essential for mosquito development. Recently, aerobic respiration by bacteria in larvae was identified as a crucial factor that triggers growth and ecdysone-induced molting of mosquitoes (Coon et al. Reference Coon2017). In contrast to facultative haematophages, obligate haematophages such as lice, bed bugs or kissing bugs cannot survive on other diets than blood and their blood dependence (Table 1) is usually reflected by obligate nutritional bacteria (Beard et al. Reference Beard, Cordon-Rosales and Durvasula2002; Kirkness et al. Reference Kirkness2010; Nikoh et al. Reference Nikoh2014). Most blood-feeding insects undergo complete metamorphosis (i.e. are holometabolous such as fleas and all dipterans). Hemimetabolous parasites comprise only true bugs (bed bugs and kissing bugs) and lice. Interestingly, the only strictly haematophagous holometabolous insects that also house obligate intracellular bacteria are Hippoboscoidea flies (tsetse flies, louse flies and bat flies). These dipterans develop by the so-called adenotrophic viviparity – larvae are retained within the female's body, nourished through secretions of ‘milk glands’ (also used for symbiont transfer), and pupate immediately after birth (Lehane, Reference Lehane2005).
Remarkable diversity of mutualistic, commensal and pathogenic microorganisms in parasites feeding on blood
Similarly to beneficial symbioses of other animals, symbioses of blood-feeding invertebrates can be roughly divided into two groups based on their cellular localization: extracellular and intracellular (Moran et al. Reference Moran, McCutcheon and Nakabachi2008; Engel and Moran, Reference Engel and Moran2013). Numerous blood-feeding animals only house extracellularly localized gut symbionts that have to be acquired de novo every generation from the environment. Such extracellular symbioses seem to be more common in facultatively blood-feeding dipterans, but they are also found in some obligatory blood-feeding arthropods, for instance kissing bugs (Heteroptera: Reduviidae: Triatominae). Unlike to social insects, stinkbugs or some beetles (Kikuchi et al. Reference Kikuchi2009; Kwong and Moran, Reference Kwong and Moran2016; Salem et al. Reference Salem2017), none of the gut symbionts reported from blood-feeding arthropods have been convincingly shown to have relatively direct trans-generational transmission (e.g. by egg smearing or individual-to-individual transfer) and have to be acquired every generation from their environment, for example, by coprophagy of actinomycetes Rhodococcus rhodnii by Rhodnius prolixus kissing bugs (Beard et al. Reference Beard, Cordon-Rosales and Durvasula2002; Eichler and Schaub, Reference Eichler and Schaub2002). This acquisition of microbiota from the environment inevitably leads to much higher dynamicity in microbiome composition (e.g. symbiont losses, multiple origins and replacements) and in some lineages, such as in Ixodes scapularis ticks, a stable microbiome is probably absent and the importance of microbiota for the host reproduction and development should be thoroughly tested (Ross et al. Reference Ross2017).
The second group of blood-feeding animals, exemplified by lice or bed bugs, houses intracellular bacteria in specialized cells (bacteriocytes) sometimes even forming organs (bacteriomes) and these bacteria are heritable through oocyte transfer or in a unique case of viviparous Hippoboscoidea (tsetse flies, louse flies and bat flies) through secretions of ‘milk glands’ from the mother to larvae (Hosokawa et al. Reference Hosokawa2012; Balmand et al. Reference Balmand2013; Nováková et al. Reference Nováková2015). In a similar manner to other heritable symbiotic bacteria, genomes of these symbionts undergo genome reduction (Table 2) and many other changes well known for intracellular symbioses (McCutcheon and Moran, Reference McCutcheon and Moran2011). Enlarged host bacteriocytes housing symbionts are in many cases somehow connected to the gut, either being directly a portion of midgut in tsetse flies and louse flies (Balmand et al. Reference Balmand2013; Nováková et al. Reference Nováková2015) or localized in proximity of the digestive track and reproductive tissues in many lice species, bat flies or bed bugs (Ries, Reference Ries1931; Buchner, Reference Buchner1965; Sasaki-Fukatsu et al. Reference Sasaki-Fukatsu2006; Hosokawa et al. Reference Hosokawa2010, Reference Hosokawa2012). Surprisingly, intracellular symbionts of blood-feeding animals are localized freely in the cytoplasm and retain at least some components of bacterial cell envelope, namely peptidoglycan matrix and outer membrane proteins (Akman et al. Reference Akman2002; Kirkness et al. Reference Kirkness2010). The intracytoplasmic localization is in stark contrast to symbionts of plant-feeding insects that are surrounded by a host-derived symbiosomal membrane (McCutcheon and Moran, Reference McCutcheon and Moran2011). These cellular features are likely responsible for less severe genome reduction (>500 kbp) of symbionts in blood-feeding animals when compared with symbionts of plant-sap-feeding insects that are more integrated in the host cell (McCutcheon and Moran, Reference McCutcheon and Moran2011; Moran and Bennett, Reference Moran and Bennett2014).
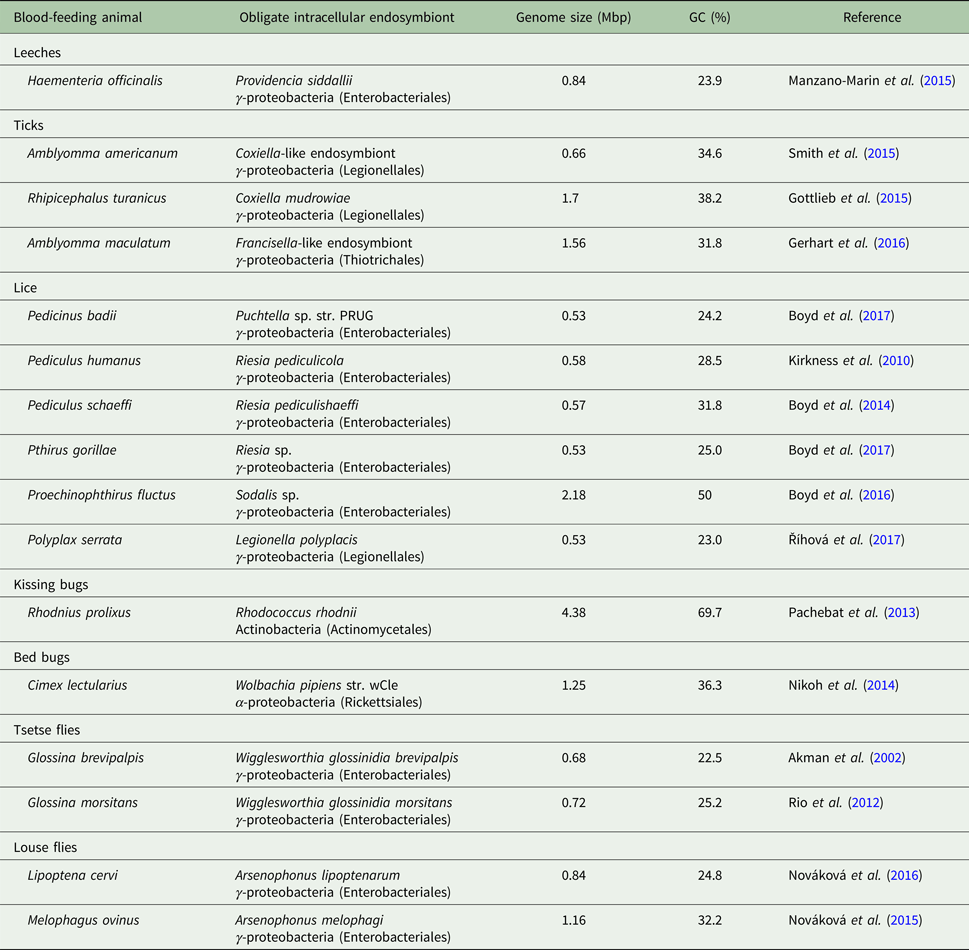
Table 2. Genome properties of obligate nutritional symbionts of blood-feeding parasites
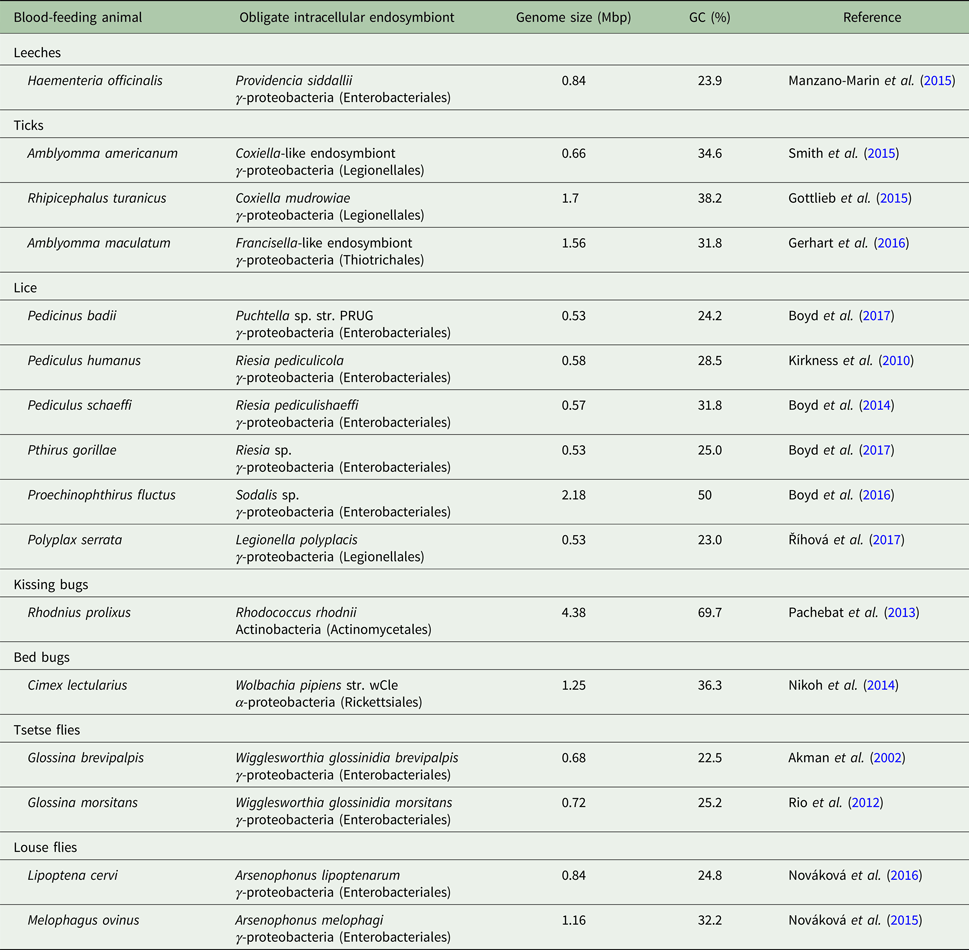
Candidatus status of uncultured symbiont species was omitted for simplicity. The only symbiont that is extracellularly localized is Rhodococcus rhodnii from Rhodnius kissing bugs.
An additional factor that likely contributes to this less severe genome reduction is the symbiosis age. Blood-feeding parasites of warm-blooded animals radiated together with their hosts, birds and mammals, relatively recently (<100 mya). Whether blood-feeding parasites of reptiles and dinosaurs had bacterial symbionts remains a matter of debates. In comparison, symbioses of sap-feeding insects can be up to several hundred million years old (e.g. 280 mya for Sulcia-Auchenorrhyncha symbioses). The intracellular localization, although resulting in tighter host–symbiont integration, does not prevent recurrent symbiont replacements that are frequently observed in blood-feeding animals (Morse et al. Reference Morse2013; Duron et al. Reference Duron2017; Šochová et al. Reference Šochová2017). One question has been pervasive in the literature about blood-feeding parasites for decades. What were ‘free-living’ ancestors of obligate symbionts in these parasites? Research progress of the last few years seems to have answered this question. Majority of obligate symbionts in blood-feeding parasites originate from facultative and pathogenic ancestors such as Wolbachia wCle in bed bugs, Arsenophonus/Riesia in louse flies and lice, Legionella polyplacis and Sodalis-allied symbionts in lice, Coxiella and Francisella-allied symbionts in ticks, and Providencia siddallii in leeches (Table 2).
Diversity of facultative bacteria in blood-feeding parasites is still relatively under-explored, although common facultative bacteria from several genera (Wolbachia, Cardinium, Rickettsia, Arsenophonus and Sodalis) were found in a number of hosts (Table 2) (Palavesam et al. Reference Palavesam2012; Lawrence et al. Reference Lawrence2015; Kelly et al. Reference Kelly2017). Even less explored is the diversity of unicellular eukaryotes. This is particularly striking because many insect pathogens and commensals, such as apicomplexans, trypanosomatids, amoebae, ciliates and microsporidia (Becnel et al. Reference Becnel, White and Shapiro2005; Morrison, Reference Morrison2009; Maslov et al. Reference Maslov2013; Vávra and Lukeš, Reference Vávra and Lukeš2013; Geiger et al. Reference Geiger2016), are due to their life cycle present in the gut lumen, along gut microvilli, in salivary glands, near to bacteriocytes, or even inside oocytes of blood-feeding animals. Such co-occurrences likely result in more interactions with beneficial symbionts than currently anticipated. Possible interactions could include scavenging of nutrients synthesized by obligate bacteria or hiding from the host immune system in the symbiotic tissue.
Nutritional interactions between blood-sucking parasites and their obligate symbionts
Genome and transcriptome sequencing has revolutionized the study of interactions between symbiotic bacteria and their animal hosts (McCutcheon and Moran, Reference McCutcheon and Moran2011). It is now rarely questioned that obligate and co-obligate symbionts provide B-vitamins and cofactors to blood-sucking hosts (Douglas, Reference Douglas2017). Interestingly, there are at least two groups of obligately blood-sucking arthropods, kissing bugs and some tick lineages, that do not depend on obligate intracellular symbionts for acquisition of B-vitamins (da Mota et al. Reference da Mota2012; Ross et al. Reference Ross2017). Therefore, these compounds remain to be either acquired from blood or provided by environmentally acquired extracellular gut symbionts. What is generally not clear is which particular B-vitamins and co-factors are truly needed by different blood-feeding species and which are needed only by their symbiotic bacteria. Additional nutritional co-operations between blood-feeding hosts could likely also involve amino acid and nitrogen metabolism or participation on blood digestion.
So far, there are paired host–symbiont genomes available from only three obligately blood-sucking arthropods – Wigglesworthia glossinidia from tsetse flies, Riesia pediculicola from human lice and Wolbachia sp. Cle from bed bugs (Akman et al. Reference Akman2002; Kirkness et al. Reference Kirkness2010; International Glossina Genome Initiative, 2014; Nikoh et al. Reference Nikoh2014; Benoit et al. Reference Benoit2016; Rosenfeld et al. Reference Rosenfeld2016). This lack of data hinders drawing any strong conclusions about nutritional interactions in the blood-sucking systems because it is not certain which co-factors are needed by host-encoded enzymes. Based only on genomic data, Wigglesworthia, Riesia and Wolbachia sp. Cle should be capable of synthesizing biotin, riboflavin, folate and pyridoxine (Fig. 2). Obligate symbionts in other blood-feeding systems appear to be also capable of providing nicotinamide, pantothenate/coenzyme A and thiamine (Fig. 2). Thiamine provision is perhaps the most controversial since this cofactor is clearly acquired from the blood diet and imported into bacterial cells by a thiamine ABC transporter (Fig. 2) in hominid lice, tsetse flies and louse flies (Kirkness et al. Reference Kirkness2010; Rio et al. Reference Rio2012; Nováková et al. Reference Nováková2015).

Fig. 2. B-vitamin and co-factor biosynthetic pathways encoded in the genomes of endosymbionts in blood-feeding parasites. Only species harbouring intracellular symbionts are shown for simplicity. Genome sequences available for the human louse, tsetse fly and bed bug do not suggest that host-derived enzymes of blood-feeding parasites complement partial biosynthetic pathways of their intracellular symbionts.
Contrary to plant-feeding insects where the host cell expression complements amino acid biosynthesis carried out by symbionts (Hansen and Moran, Reference Hansen and Moran2011), the host role in biosynthesis of symbiont-provided B-vitamins is basically absent in blood-feeding arthropods. For example, it is in tsetse flies limited only to the expression of a multi-vitamin transporter to distribute B-vitamins from bacteriocytes to other tissues (Bing et al. Reference Bing2017). However, RNA-seq (or quantitative proteomics) studies inspecting blood-feeding parasites are rarely including data for both the host and its microbiome, so further research is needed to inspect possible roles of bacterial symbionts in other key physiological processes such as blood digestion and haeme detoxification (Williamson et al. Reference Williamson2003; Sojka et al. Reference Sojka2013).
The importance of symbiotic bacteria for amino acid and nitrogen metabolism in blood-sucking animals is usually considered to be of lower importance than co-factor provision, although several pathways producing amino acids are sometimes retained (Rio et al. Reference Rio2012; Pachebat et al. Reference Pachebat2013; Nováková et al. Reference Nováková2015; Boyd et al. Reference Boyd2016). These pathways can be of biological importance, for example, the shikimate pathway is retained in the genome of W. glossinidia from Glossina morsitans but absent in the genome of Glossina brevipalpis (Rio et al. Reference Rio2012). Chorismate, a shikimate pathway product, can be used for the synthesis of phenylalanine and folate, and might thus increase vector competency of G. morsitans for African trypanosomes (Trypanosoma brucei brucei). Trypanosomes cannot synthesize these compounds but are known to encode transporters to scavenge them from the environment (Rio et al. Reference Rio2012).
Immune cross-talk: distinguishing between pathogenic and beneficial microorganisms
Host control and immunity maintenance of vertically transmitted obligate symbionts have been mainly studied in symbiotic animals that feed on other diets than blood, for example, in Sitophilus weevils (Login et al. Reference Login2011). Several ancient and well-established hereditary symbionts in Hemiptera have been shown to be missing bacterial cell envelope structures recognized by the insect immune system – peptidoglycan and lipopolysaccharides (McCutcheon and Moran, Reference McCutcheon and Moran2011). However, as discussed above, even the most extremely reduced symbiont genomes from blood-sucking parasites still retain some of the structures recognized as of bacterial origin by the host peptidoglycan-recognition proteins (PGRPs) or Gram-negative binding proteins.
Interestingly, two insect groups with complete genomes for both the host and its obligate symbiont available (aphids and lice) have jettisoned PGRPs, genes from the immunodeficiency signalling (IMD) pathway and many antimicrobial peptides (Gerardo et al. Reference Gerardo2010; Kirkness et al. Reference Kirkness2010). Additional genome data imply that if the PGRPs are present, as shown, for example, in tsetse flies, one of the PGRPs retains an amidase activity. By recycling peptidoglycan in bacteriocytes and milk glands of female tsetse flies, the activity shields symbionts from recognition by other PGRPs and expression of lineage-specific antimicrobial peptides mediated by the IMD pathway (Wang et al. Reference Wang2009).
Living both extracellularly and intracellularly in different insect tissues (Fig. 3), facultative symbionts and pathogens need to hide their cells from the host immune system and/or to be resistant to its antimicrobial peptides. Outer membrane proteins are generally hypothesized to be responsible for hiding bacterial cells from the host immunity and therefore allowing widespread persistence of facultative symbionts in insects (Weiss et al. Reference Weiss2008). Even when recognized, cells of facultative symbionts were shown to be much more resistant to antimicrobial peptides of their hosts than bacteria from different hosts such as Escherichia coli. For example, Sodalis glossinidius forms biofilms in the host tissue that reduce the effect of antimicrobial peptides (Maltz et al. Reference Maltz2012). Since Sodalis gene expression can be modulated in accordance with the bacterial cell density by quorum sensing (Pontes et al. Reference Pontes2008; Enomoto et al. Reference Enomoto2017), it can rapidly adapt when targeted by the host immune system to either become less or more virulent depending on its host. Understanding these density-dependent interactions with the host or other microorganisms will be essential to fully take advantage of facultative symbionts such as Sodalis (De Vooght et al. Reference De Vooght2014) or Wolbachia (Hoffmann et al. Reference Hoffmann2011) for the elimination of causative agents of sleeping sickness, malaria and dengue or other viruses.
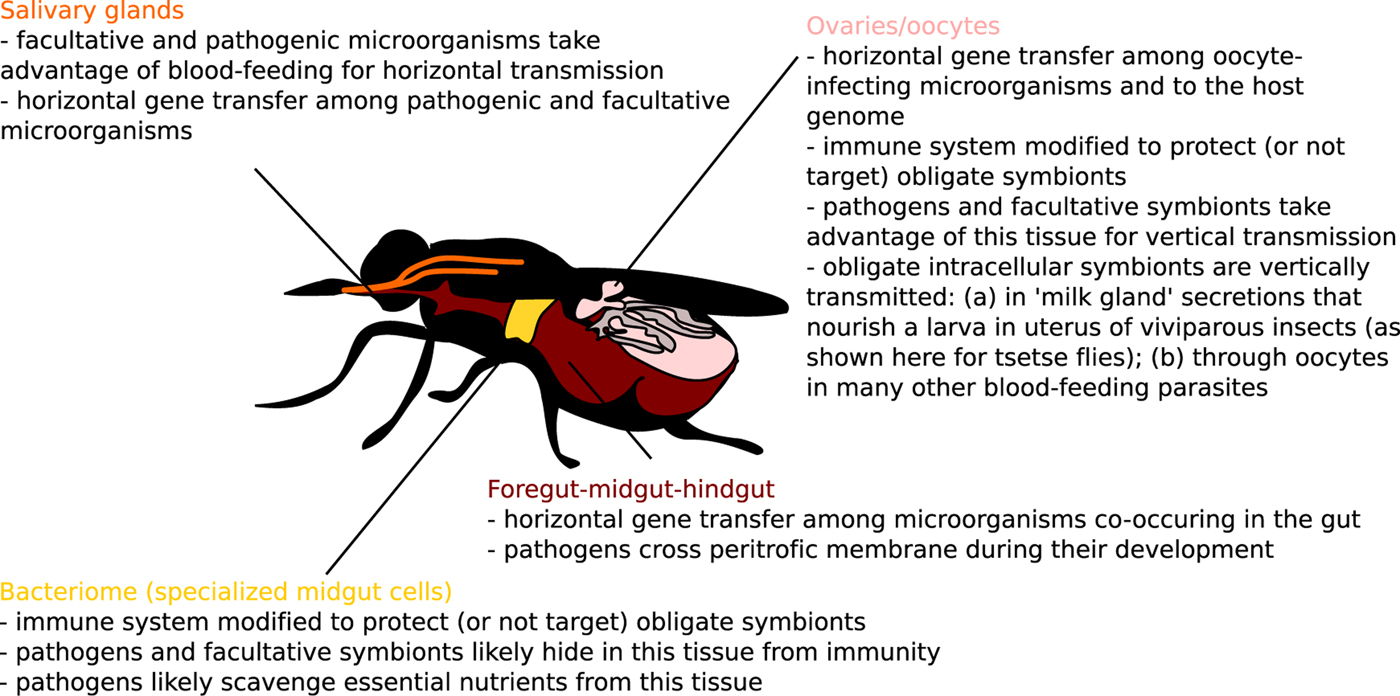
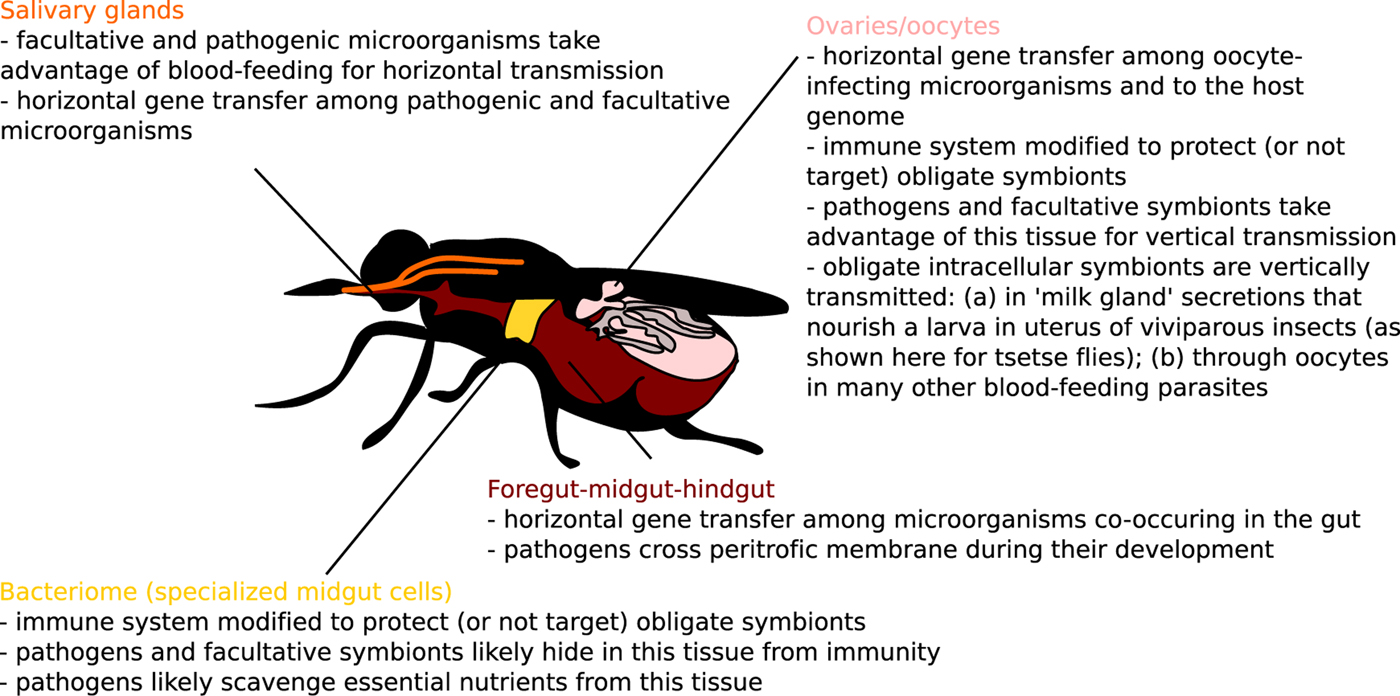
Fig. 3. Host–microbiome gene exchange and immune cross-talk hot spots in blood-feeding parasites (melting pots and intracellular arenas of evolution) highlighted for one model blood-feeding species, Glossina sp.
Blood-feeding arthropods form a peritrophic matrix in their gut to separate the blood meal from their gut tissue. This non-cellular membrane is composed of chitin and many diverse proteins and proteoglycans (Shao et al. Reference Shao, Devenport and Jacobs-Lorena2001). The matrix likely has several functions from digestion improvement to mechanical, chemical and pathogen protection (Lehane, Reference Lehane1997; Shao et al. Reference Shao, Devenport and Jacobs-Lorena2001). Interestingly, reducing the permeability of this matrix was shown to reduce immune response to bacteria in some blood-feeding animals. For example, Anopheles gambiae mosquitoes form a dityrosine network in a mucus layer under the peritrophic matrix and this mucus prevents activation of immunity by bacteria ingested with a blood meal (Kumar et al. Reference Kumar2010). Whether this or similar mechanisms blocking access from the gut lumen to epithelial tissue are common in blood-feeding animals is currently unknown. What is certain is that the matrix is a constant battle field where many microbes such as Plasmodium sp. or S. glossinidius use chitinases to penetrate the membrane during their development (Langer and Vinetz, Reference Langer and Vinetz2001; Rose et al. Reference Rose2014).
Horizontal gene transfer in microbiomes of blood-feeding parasites
A concept of ‘melting pots of evolution’ was originally raised to highlight environments with much increased opportunity for horizontal gene transfer (HGT) among organisms living in such environments (e.g. bacteria and viruses co-infecting vacuoles of amoebae) (Moliner et al. Reference Moliner, Fournier and Raoult2010). Very similar concept was described for oocytes of multicellular eukaryotes as ‘intracellular arenas’ (Bordenstein and Wernegreen, Reference Bordenstein and Wernegreen2004). Incidentally, oocytes (or any segregated germline cells) represent so-called ‘weak links’ allowing vertical inheritance of foreign genes in multicellular organisms (Huang, Reference Huang2013), and it is probably not a coincidence that such environments in which primarily prokaryotes exchange genes, simply by chance, also seem to support higher frequency of prokaryote-to-eukaryote HGT (Husnik and McCutcheon, Reference Husnik and McCutcheon2018). In terms of melting pots of HGT in blood-feeding parasites, there are at least three tissues (Fig. 3) that serve as microbiome meeting points: salivary glands, digestive tracts and reproductive tissues (such as oocytes or ‘milk glands’ in tsetse flies).
Oocytes are germline cells that are analogous to amoebal cells in a way that they are quite often shared by several different microorganisms that take advantage of oocytes for vertical transmission (Husnik and McCutcheon, Reference Husnik and McCutcheon2018). For example, genomes of obligate Wolbachia and Legionella symbionts in bed bugs and Polyplax lice contain a biotin operon acquired horizontally from either Cardinium, Wolbachia or Rickettsia (Gerth and Bleidorn, Reference Gerth and Bleidorn2016). This operon likely assisted these Wolbachia and Legionella species when becoming nutritional symbionts (Nikoh et al. Reference Nikoh2014; Říhová et al. Reference Říhová2017). These genes were also found in mealybug and whitefly genomes (Luan et al. Reference Luan2015; Husnik and McCutcheon, Reference Husnik and McCutcheon2016) suggesting that animal genomes not only acquire genes from bacteria (Husnik and McCutcheon, Reference Husnik and McCutcheon2018), but also that evolutionary history of some of these gene transfer events can be difficult to reliably infer (and resembling pangenomes). For example, mosquitoes and Wolbachia share two genes that were likely acquired by Wolbachia from the mosquito genome (Klasson et al. Reference Klasson2009; Woolfit et al. Reference Woolfit2009), but taxon sampling for these genes is too poor to confidently name the specific gene donor and acceptor.
Perhaps the best understood blood-feeding animals in terms of HGT are arthropods that are well known to primarily acquire genes from oocyte-infecting microorganisms such as reproductive manipulators shifting sex ratio of the host population or facultative symbionts capable of jumping among hosts (Sloan et al. Reference Sloan2014; Luan et al. Reference Luan2015; Husnik and McCutcheon, Reference Husnik and McCutcheon2016). The only animal tissue that can mediate heritable HGT not only among microbiome members, but also to the host genome are germline cells. HGTs from Wolbachia and other bacteria are fairly common in genomes of blood-feeding animals such as Glossina spp. (Brelsfoard et al. Reference Brelsfoard2014), R. prolixus (Mesquita et al. Reference Mesquita2015) and hookworms Ancylostoma ceylanicum and Necator americanus (Schwarz et al. Reference Schwarz2015). Potentially the most HGT-rich genome of a blood-feeding animal is the bed bug genome, but unfortunately the two published bed bug genomes greatly differ in HGT analysis (Benoit et al. Reference Benoit2016; Rosenfeld et al. Reference Rosenfeld2016). Functional role of gene transfer events in blood-feeding parasites mirrors frequently acquired genes in other eukaryotes, particularly genes involved in protection, nutrition and adaptations to extreme environments (Husnik and McCutcheon, Reference Husnik and McCutcheon2018). A fascinating example of blood-feeding arthropods that use a gene of bacterial origin for protection is known from I. scapularis ticks that are likely using an amidase transferred from a bacterium to protect themselves from bacterial pathogens such as Borrelia (Chou et al. Reference Chou2014). Nutritional gene transfer was described from Brugia malayi filarial nematodes that acquired a bacterial gene for a ferrochelatase responsible for the terminal step in haeme biosynthesis (Wu et al. Reference Wu2013). Since it is an essential gene, this ferrochelatase – or any other HGTs from different blood-feeding parasites – could be used as potential drug targets as suggested from other parasites, for instance cryptosporidia, microsporidia or Blastocystis spp. (Alexander et al. Reference Alexander2016; Sateriale and Striepen, Reference Sateriale and Striepen2016; Eme et al. Reference Eme2017). HGT is not equally common for all animals, and there are, of course, parasites that seem not to be frequently involved in gene acquisition from bacteria. One of such lineages is the human louse that was suggested to contain no genes of recognizable recent bacterial origin in its genome (Kirkness et al. Reference Kirkness2010).
Other environments of blood-feeding parasites that house a dynamic community of tightly interacting viruses, prokaryotes and eukaryotes are tissues specialized for blood-feeding, particularly the digestive tract and salivary glands. HGT of pathogenicity-related genes between facultative or pathogenic microorganisms transmitted by blood-feeding parasites likely takes place in these tissues (Fig. 3). For example, genomes of mosquito-associated Spiroplasma spp. contain multiple gene acquisitions from the Mycoides–Entomoplasmataceae clade of ruminant pathogens (Lo and Kuo, Reference Lo and Kuo2017). HGT can also occur between a facultative bacterial symbiont and a protist pathogen. A phospholipase of bacterial origin was likely transferred from the S. glossinidius genome to the T. brucei genome in the gut environment of their tsetse fly vector (Richmond and Smith, Reference Richmond and Smith2007). Genomes of bacterial pathogens such as Bartonella, Rickettsia, Borrelia, Coxiella, Francisella or Yersinia that are transmitted by blood-feeding vectors are notoriously known to be replete with pathogenicity regions of HGT origin (Gillespie et al. Reference Gillespie2012; Guy et al. Reference Guy2013; Eggers et al. Reference Eggers2016; Moses et al. Reference Moses2017). Since proximity is essential to increase opportunity of gene transfer, it seems plausible that successful gene transfer events more likely take place when bacterial pathogens co-occur in, for example, midgut or salivary glands of their blood-feeding host rather than when co-infecting vertebrate hosts.
Conclusions
The research of blood-feeding animals has a long history due to the role some of these parasites play as vectors in the transmission of viruses, pathogenic bacteria, protists or even other animals such as filarial nematodes. This long history of research on medically important model species leads to a paradoxical situation in which some model species with relatively species-poor, but stable microbiomes (e.g. tsetse flies or lice) have well-studied microbiomes, but other model species with more species-rich and less stable microbiomes (e.g. many dipterans) have less-studied microbiomes. This review highlights the importance of microorganisms for some blood-feeding parasites and advocates for taxonomic breadth in parasite microbiome research, particularly to understand microbiomes of vector species with richer communities of loosely associated (and sometimes larvae-specific) microorganisms.
Financial Support
F.H. was supported by a postdoctoral research fellowship from the European Molecular Biology Organization (EMBO; ALTF 1260-2016).
Conflicts of Interest
None.
Ethical Standards
Not applicable.