INTRODUCTION
Forest community structure and composition may be largely determined during the early life-history stages of tree species (Hille Ris Lambers & Clark Reference HILLE RIS LAMBERS and CLARK2003). A leading explanation is the Janzen–Connell (J–C) hypothesis (Connell Reference CONNELL, Boer and Gradwell1971, Janzen Reference JANZEN1970), which states that host-specific predators or pathogens of seeds and seedlings strongly influence forest community composition and structure. Soil pathogens that attack seeds can restrict the recruitment of vulnerable tree species into suitable habitats (Dalling et al. Reference DALLING, SWAINE and GARWOOD1998, O'Hanlon-Manners & Kotanen Reference O'HANLON-MANNERS and KOTANEN2004). Studying the interactions between soil pathogens and host trees can therefore provide important insights into how the establishment potentials of tree species vary with environmental conditions.
Soil pathogens are ubiquitous in soils and cause seed mortality in seed-banking species in most terrestrial ecosystems (Agrios Reference AGRIOS2005, Blaney & Kotanen Reference BLANEY and KOTANEN2001). Experiments using fungicides in the field (Bell et al. Reference BELL, FRECKLETON and LEWIS2006, Gilbert Reference GILBERT, Burslem, Pinard and Hartley2005) and the laboratory (Liu et al. Reference LIU, YU, XIE and STAEHELIN2012a, b; Packer & Clay Reference PACKER and CLAY2000) indicate that the soil fungi that act as the main pathogens of a given plant are host specific (Kotanen Reference KOTANEN2007, Li et al. Reference LI, YU and WANG2009). Indeed, host-specific natural enemies influence plant community structure (Augspurger & Wilkinson Reference AUGSPURGER and WILKINSON2007, Freckleton & Lewis Reference FRECKLETON and LEWIS2006, Zhou & Hyde Reference ZHOU and HYDE2001), and the prevalence of host–pathogen interactions may be mediated by direct environmental effects on pathogen community composition and the abundance of natural enemies (Keesing et al. Reference KEESING, HOLT and OSTFELD2006, Malmstrom et al. Reference MALMSTROM, STONER, BRANDENBURG and NEWTON2006, Packer et al. Reference PACKER, HOLT, HUDSON, LAFFERTY and DOBSON2003, Power & Mitchell Reference POWER and MITCHELL2004). For example, soil pathogens inhibit seed germination in heterogeneous habitats (Gallery et al. Reference GALLERY, DALLING and ARNOLD2007, O'Hanlon-Manners & Kotanen Reference O'HANLON-MANNERS and KOTANEN2004, Reference O'HANLON-MANNERS and KOTANEN2006). However, few field experiments have focused on how environmental factors can affect the seed mortality induced by host-specific pathogens, thereby altering tree species composition under different environmental conditions.
We tested the hypothesis that environmental conditions affect the activity of host-specific seed and seedling pathogens in two types of tropical forest, thereby affecting the tree population structure and species composition in these different environments. First, we established two 1-ha permanent plots, one in tropical montane rain forest and the other in tropical cloud forest. Second, we selected two tree species present in both forest types: Cyclobalanopsis fleuryi and Cryptocarya chinensis. Third, we conducted a reciprocal field experiment to investigate whether soil pathogens differentially affect seed germination for these two tree species and whether host-specific pathogenicity varied with environmental conditions.
METHODS
Study sites and species
This experiment was conducted in the Bawangling Nature Reserve (BNR; 18°50′–19°05′N, 109°05′–109°25′E), located at the border between Changjiang and Baisha counties on Hainan, south China. The reserve covers approximately 72000 ha (Yu et al. Reference YU, ZANG and JIANG2001) and experiences three seasons (Zhang & Zang Reference ZHANG and ZANG2007): the cold season (November–February), the dry season (May–June), and the rainy season (July–October). Four types of natural forest vegetation are present in the reserve, spanning an altitudinal gradient: tropical lowland rain forest, tropical montane rain forest, tropical cloud forest and tropical dwarf forest.
Two sites at different altitudes were chosen for the reciprocal field experiment. Site 1 (950 m asl) was located in tropical montane rain forest (TMRF), which occurs in mountainous regions and receives high levels of rainfall. It is characterized by a closed canopy and high species diversity. Site 2 (1270 m asl) was located within tropical cloud forest (TCF), which occurs in humid regions that are frequently covered by clouds or mist. The forest canopy in TCF is dominated by various species of evergreen and deciduous trees. The mean daily air temperature declines from 22.2 °C to 21.8 °C, the soil temperature declines from 23.5 °C to 19.6 °C, and the mean daily relative humidity of the air increases from 85.7% to 88.4% as one moves from the montane rain forest to the cloud forest (Liu et al. Reference LIU, ZANG and DING2009, Zang et al. Reference ZANG, YANG and JIANG2001, Zhang & Zang Reference ZHANG and ZANG2007).
Two 1-ha permanent plots, one in tropical montane rain forest and one in tropical cloud forest, were established. All saplings and adult trees with a diameter at breast height (dbh) ≥ 1 cm were tagged, mapped, measured and identified. In total, 170 species from TMRF and 106 species from TCF were recorded.
Cryptocarya chinensis is the most common species in the TMRF of the BNR and is moderately abundant in the TCF. Cyclobalanopsis fleuryi is moderately abundant in both TMRF and TCF. However, the individual number of C. chinensis is greater in TMRF than in TCF, whereas the individual number of C. fleuryi varies only slightly between these two forest types. We statistically compared the importance value of each species between the two permanent plots to test whether altitude affects species composition (Table 1). Both species are shade tolerant (Yu et al. Reference YU, ZHANG and WANG1994) and have limited dispersal ability. Some biological features of the two species are compared in Table 2.
Table 1. Counts of individuals and importance value of two tree species in two 1-ha permanent plots in the Bawangling Nature Reserve on Hainan, south China.

Table 2. Biological characteristics of Cyclobalanopsis fleuryi and Cryptocarya chinensis.

Soil and seed collection
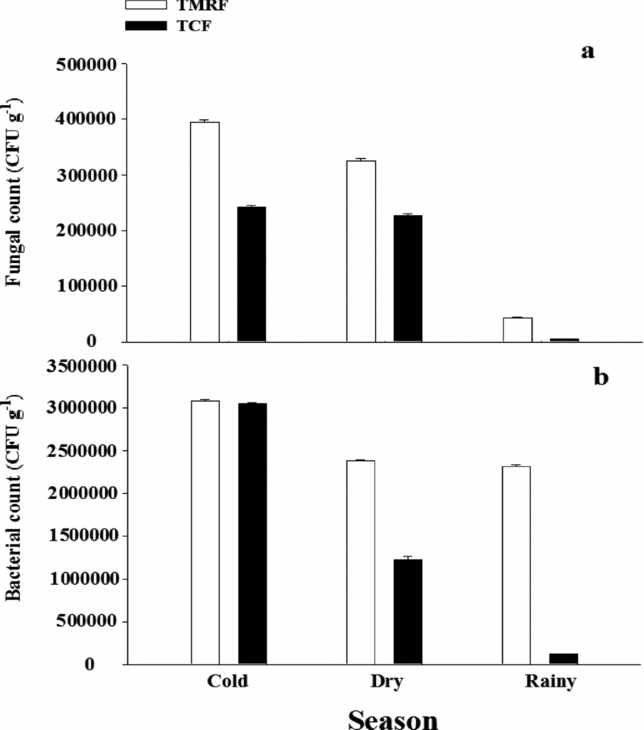
To obtain counts of soil fungi and bacteria in the two permanent plots, soil samples spanning 0 to 30 cm in depth were collected at random locations within each permanent plot during all three seasons. Counts of soil fungi and bacteria (Figure 1) were made using the dilution plate counting method (Lin Reference LIN1997, Warcup Reference WARCUP1955, Xu & Zheng Reference XU and ZHENG1986). To obtain experimental soils, we removed a soil core (10 cm in diameter, 30 cm deep) from within 1 m of the bole of eight randomly selected conspecific adults (average tree dbh ≥ 35 cm) in each plot. We therefore had four types of source soil: rhizospheric soil of C. fleuryi from TMRF (Cf-TMRF), rhizospheric soil of C. fleuryi from TCF (Cf-TCF), rhizospheric soil of C. chinensis from TMRF (Cc-TMRF) and rhizospheric soil of C. chinensis from TCF (Cc-TCF). Each type of source soil was divided into five treatments: parent soil (PS), fungicide-sterilized soil (FSS), autoclave-sterilized soil (ASS), Fusarium-added soil (F-AS) and Pythium-added soil (P-AS). Soils of each type were mixed and sterilized as described below.

Figure 1. Soil fungal (a) and bacterial (b) counts in each of three seasons (cold, dry and rainy) in 1-ha plots in tropical montane rain forest (TMRF) and tropical cloud forest (TCF) in the Bawangling Nature Reserve on Hainan, south China.
Seeds were collected from each study site. Prior to planting, seeds were surface sterilized with a 10% sodium hypochlorite solution for 10 min, thoroughly rinsed with distilled water, air-dried for 15 min, and weighed. The viability of all seeds was 100%. Tetrazolium chloride was used to determine the viability of ungerminated seeds recovered at the end of the study period.
Soil sterilization treatment
Fungicides were applied every 10 d according to the manufacturer's recommendations: 2 g mancozeb and 2 g fenaminosulf (Agricultural Service Ltd., Hebei, China) diluted to a concentration of 1:250 in water. The active ingredients in mancozeb and fenaminosulf were carbendazim and quintozene, respectively. Many systemic fungicides are specific to particular groups of fungi (Paul et al. Reference PAUL, AYRES and WYNESS1989), but this study required fungicides with broad-spectrum activities. Broad-spectrum fungicides can kill multiple types of fungi, such as Fusarium and Rhizoctonia. This fungicide mixture is frequently used to protect agricultural seeds and has been used successfully in similar ecological field studies (Hartill et al. Reference HARTILL, TOMPKINS and KLEINSMAN1983, Zhang et al. Reference ZHANG, WEI and ZHANG2005). We conducted laboratory trials to verify that mancozeb and fenaminosulf did not affect the germination of tree seeds in sterilized soil. The fungicide-sterilized soil treatment received 30 ml of fungicide solution applied to the soil surface of each pot, and the other soil treatments received an identical volume of water lacking fungicides.
For the autoclave-sterilized treatment, soil was autoclaved at 211 °C and 205.8 kPa for 1 h (Li et al. Reference LI, YU and WANG2009, Packer & Clay Reference PACKER and CLAY2000) to kill macro- and micro-invertebrates and most fungal and bacterial propagules (Kendrick Reference KENDRICK2000).
Soil microbial extraction
Soil micro-organisms (excluding arbuscular mycorrhizal fungi, AMF) were extracted from the rhizospheric soils of parent trees using a wet-sieving method adapted from Klironomos (Reference KLIRONOMOS2002). For each extraction, 30 g of parent-tree root soil was blended with 200 ml of tap water for 30 s. The liquid suspension was then washed through 250-, 45- and 20-μm analytical sieves with tap water, yielding 450 ml of extract (including washes). The sieves were cleaned ultrasonically for 5 min between each extraction (McCarthy-Neumann & Kobe Reference MCCARTHY-NEUMANN and KOBE2008). The extraction procedure was repeated until sufficient quantities of extract were obtained for the Fusarium-added soil treatment or Pythium-added soil treatment. The filtrate that passes through a 20-μm sieve consists primarily of pathogens and other non-mycorrhizal microbes (Yu Reference YU1979). Our further isolation work indicated that the filtrate of C. fleuryi soil contained Fusarium and that the filtrate of C. chinensis soil contained Pythium. In our laboratory pathogenicity tests, disease symptoms were observed in surface-sterilized C. fleuryi seeds inoculated with Fusarium and in surface-sterilized C. chinensis seeds inoculated with Pythium. To examine host specificity, the seeds of C. fleuryi were sown in Fusarium-added soil and Pythium-added soil, and the seeds of C. chinensis were sown in Fusarium-added soil and Pythium-added soil. For each tree species, two blocks of pathogen-addition treatments were included. In one treatment, 100 ml of filtrate of C. fleuryi soil containing Fusarium was added to autoclave-sterilized soil in each pot. In the other treatment, 100 ml of filtrate of C. chinensis soil containing Pythium was added to the autoclave-sterilized soil in each pot.
Experimental methods
The effects of soil-borne pathogens on seed germination of C. fleuryi and C. chinensis were studied using an experimental approach. Each species received 40 treatments, each consisting of one of four different soil sources (Cf-TMRF, Cc-TMRF, Cf-TCF or Cc-TCF) subjected to one of five different soil treatments (PS, FSS, ASS, F-AS or P-AS) at one of two forest types (TMRF or TCF). In the pot experiment, F-AS and P-AS treatments had only 40 pots each, and all the other treatments had 80 pots respectively, totally 2560 pots for each species.
Soil was packed into plastic pots (15 cm diameter). Seeds were then placed into the pots (two per pot for C. fleuryi, four per pot for C. chinensis). The experiments lasted from early November 2008 to late July 2009 in shade-houses that provided approximately 20% of full sunlight. The potted soil was watered regularly to maintain constant soil moisture. Seed germination was monitored daily.
Data analysis
For each experimental treatment, the proportion of seeds that germinated in each pot was calculated over the course of the experiment. The seed germination percentage (SGP) was averaged for each species in each forest type and analysed statistically. The basic analyses used a univariate analysis of variance in a general linear model (GLM). Proportions were arcsine-transformed prior to analysis, and tests for the effects of soil treatment and forest type on seed germination were conducted using one-way analysis of variance (ANOVA). Independent-sample t-tests were performed to compare seed germination proportions between the two forest types and between sterilized and non-sterilized soils. Averages ± 1 SE are reported. All data analyses were performed with SPSS for Windows (version 16.0; SPSS, Chicago, IL, USA).
RESULTS
Effect of soil pathogens on the SGP
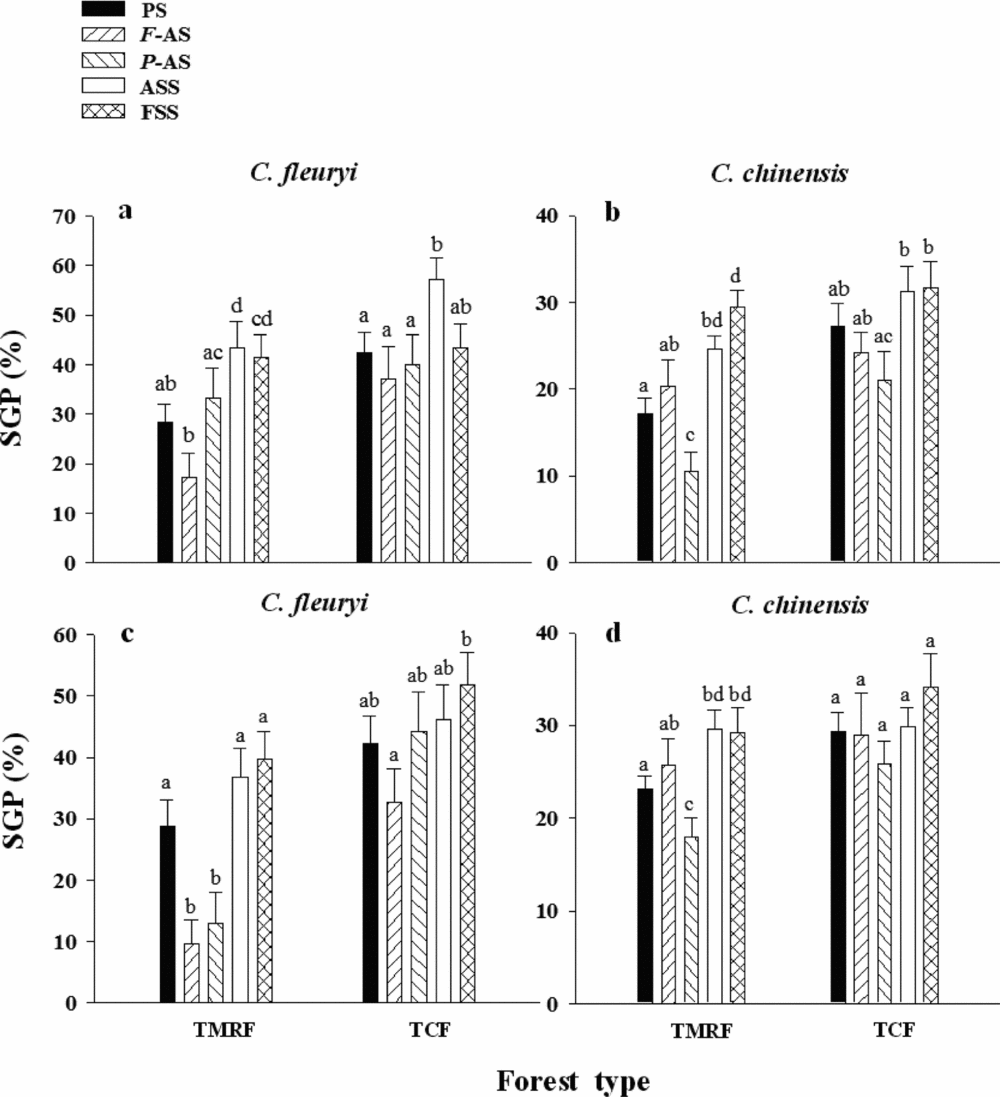
At the TMRF site, the SGP of C. fleuryi was significantly lower in PS than in either FSS (F = 0.1, P = 0.028, df = 159) or ASS (F = 0.5, P = 0.015, df = 159) in soils from TMRF (Figure 2a). Similarly, the SGP of C. chinensis was significantly lower in PS than in either FSS (F = 1.3, P < 0.001, df = 159) or ASS (F = 15.4, P = 0.004, df = 159) in soils from TMRF (Figure 2b). Fewer seeds of C. fleuryi germinated in PS than in either FSS or ASS in soils from TCF (Figure 2c), and the SGP of C. chinensis was significantly lower in PS than in either FSS (F = 13.7, P = 0.045, df = 159) or ASS (F = 9.9, P = 0.011, df = 159) in soils from TCF (Figure 2d).

Figure 2. Effects of host-specific pathogens on the germination of Cyclobalanopsis fleuryi and Cryptocarya chinensis seeds in tropical montane rain forest (TMRF) and tropical cloud forest (TCF) in the Bawangling Nature Reserve on Hainan, south China. The average seed germination percentages (SGPs) of C. fleuryi and C. chinensis seeds sown in pots with parent soil (PS), Fusarium-added soil (F-AS), Pythium-added soil (P-AS), autoclave-sterilized soil (ASS) and fungicide-sterilized soil (FSS) from TMRF (a, b) and TCF (c, d). Error bars represent ± 1 SE of the mean. Treatments sharing the same letter do not differ significantly (P > 0.05; ANOVA).
At the TCF site, seeds of C. fleuryi were significantly less likely to germinate in PS than in ASS (F = 3.4, P = 0.019, df = 159), but the seed germination of C. fleuryi was not significantly different between PS and FSS (Figure 2a) in soils from TMRF. Fewer seeds of C. chinensis germinated in PS than in either FSS or ASS (Figure 2b) in soils from TMRF. The SGPs for both tree species were not significantly different between PS and sterilized soil (FSS or ASS) in soils from TCF (Figure 2c, d).
Effect of pathogen host specificity on the SGP
At the TMRF site, significantly fewer C. fleuryi seeds germinated in F-AS than in either FSS (F = 1.0, P = 0.002, df = 119) or ASS (F = 1.4, P = 0.001, df = 119) for soil from TMRF (Figure 2a), and C. fleuryi seeds were significantly less likely to germinate in F-AS than in either FSS (F = 17.6, P < 0.001, df = 119) or ASS (F = 12.8, P < 0.001, df = 119) for soil from TCF (Figure 2c). Although the SGP of C. chinensis was lower in F-AS than in both types of sterilized soil (FSS and ASS), these differences were not significant. The SGP of C. fleuryi was significantly lower in P-AS than in either type of sterilized soil, although not as low as in F-AS. The SGP of C. chinensis was reduced to a greater extent in P-AS, with a significant difference relative to the SGPs in sterilized soil (FSS or ASS), and the degree of the reduction in the SGP of C. chinensis was greater than that of C. fleuryi in P-AS.
At the TCF site, in soils from TMRF (Figure 2a, b), significantly fewer C. fleuryi seeds germinated in F-AS than in ASS (F = 1.0, P = 0.011, df = 119; Figure 2a). Although the SGP of C. chinensis was also lower in F-AS, there was no significant difference between F-AS and either type of sterilized soil (FSS or ASS). The SGP of C. fleuryi was significantly lower in P-AS than in ASS, but the degree of the reduction was not significantly different between P-AS and F-AS. The SGP of C. chinensis was more significantly reduced in P-AS and was significantly lower in P-AS than in either FSS (F = 1.6, P = 0.039, df = 119) or ASS (F = 1.5, P = 0.035, df = 119) (Figure 2b). The degree of the reduction in the SGP of C. chinensis was greater than that of C. fleuryi in P-AS. In soil from TCF (Figure 2c, d), significantly fewer C. fleuryi seeds germinated in F-AS than in FSS (F = 0.3, P = 0.022, df = 119; Figure 2c); however, the SGP of C. chinensis did not differ significantly between F-AS and either FSS or ASS (Figure 2d). For both species in TCF soil, the SGP did not differ significantly between P-AS and either FSS or ASS.
Variation in the SGP with forest type, soil source and soil treatment
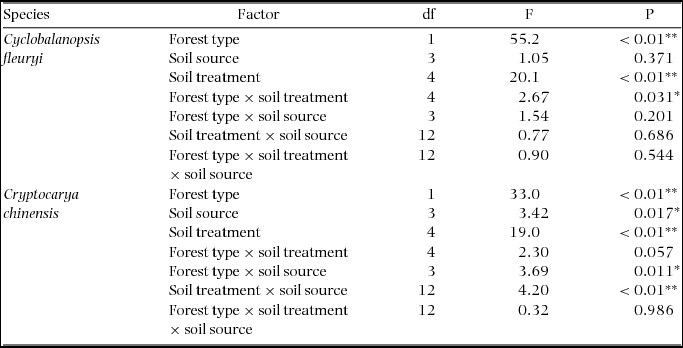
For both tree species, there were significant variations in the SGP between the two forest types and among the five soil treatments. The SGPs of C. fleuryi in PS (t = −2.56, P = 0.012, df = 158) and F-AS (t = −2.44, P = 0.018, df = 78) were significantly less in TMRF than in TCF, and there was a significant forest type × soil treatment interaction (Figure 2, Table 3). The SGPs of C. chinensis exhibited a similar pattern but with no significant forest type × soil treatment interaction (Table 3). The results also showed that the forest type × soil treatment × soil source interaction had no significant effect on the SGP of either C. fleuryi or C. chinensis; however, the SGPs of C. chinensis in soils from different sources was significantly influenced by the forest type and soil treatment (Table 3).
Table 3. The results of a general linear model (GLM) of the effects of multiple variables (forest type, soil source, soil treatment) on the seed germination percentages (SGPs) of Cyclobalanopsis fleuryi and Cryptocarya chinensis in the Bawangling Nature Reserve on Hainan, south China. Forest type: tropical montane rain forest (TMRF), tropical cloud forest (TCF). Soil source: rhizospheric soil of C. fleuryi from TMRF (Cf-TMRF), rhizospheric soil of C. fleuryi from TCF (Cf-TCF), rhizospheric soil of C. chinensis from TMRF (Cc-TMRF), rhizospheric soil of C. chinensis from TCF (Cc-TCF). Soil treatment: parent soil (PS), fungicide-sterilized soil (FSS), autoclave-sterilized soil (ASS), Fusarium-added soil (F-AS), Pythium-added soil (P-AS). The significant correlations indicated by asterisks: *P < 0.05, **P < 0.01.
DISCUSSION
Pathogen host specificity
Our data indicate that the soil-borne pathogens surrounding parent trees are highly host-specific. For both species in TMRF, germination was inhibited in non-sterilized soil but was significantly increased in sterilized and heterospecific soils. Additionally, the pathogen-addition treatments revealed that Fusarium obtained from Cyclobalanopsis fleuryi-associated soil had a stronger negative effect on the seed germination of Cyclobalanopsis fleuryi than Pythium from Cryptocarya chinensis-associated soil. In addition, the Pythium obtained from the soil beneath Cryptocarya chinensis adults had a more negative effect on the seed germination of Cryptocarya chinensis than the Fusarium obtained from the soil beneath Cyclobalanopsis fleuryi adults.
These host-specific effects may explain the findings of previous studies that identified soil pathogens as important factors affecting seed mortality in tropical forests. For example, Li et al. (Reference LI, YU and WANG2009) concluded that soil pathogens are an important source of seed mortality for Ormosia semicastrata. Similarly, Baskin & Baskin (Reference BASKIN and BASKIN1998) reported that the mortality of buried seeds in natural communities was caused by various microbial pathogens. The fungicide and autoclave treatments, which have been used in many studies, also indicate that fungal pathogens increase seed mortality (Blaney & Kotanen Reference BLANEY and KOTANEN2001, Lonsdale Reference LONSDALE1993). For example, the treatment of buried seeds of two common tropical pioneer tree species (Cecropia insignis and Miconia argentea) with fungicide increased survival by 40–45% in the forest understorey in Panama (Dalling et al. Reference DALLING, SWAINE and GARWOOD1998). O'Hanlon-Manners & Kotanen (Reference O'HANLON-MANNERS and KOTANEN2006) similarly found that Acer negundo seeds treated with fungicide exhibited a higher germination percentage than untreated seeds. However, only inoculation experiments with an isolated pathogen can definitively demonstrate whether a particular pathogen is host specific. A recent cross-inoculation study on the multi-host fungus Colletotrichum anthrisci showed that the virulence of specific isolates varies with the type of host plant (Konno et al. Reference KONNO, IWAMOTO and SEIWA2011, Packer & Clay Reference PACKER and CLAY2000). Liu et al. (Reference LIU, YU, XIE and STAEHELIN2012b) further demonstrated that this disease-inducing fungus was primarily distributed near the focal parent trees.
Effect of environmental factors on pathogen host specificity
The result of the univariate analysis of variance using a GLM showed that the forest type × soil treatment interaction was significant for C. fleuryi but not C. chinensis. These results indicate that the activity of Fusarium associated with C. fleuryi differed with forest type, whereas the activity of Pythium associated with C. chinensis did not. Our results indicate that environmental factors can directly impact the activity of soil-borne pathogens, but the effects were different for the host-specific fungi of two tree species. Differences in soil temperature and moisture between forest types may affect the physical characteristics of C. chinensis and C. fleuryi seeds differently, thus altering the effects of soil-borne pathogens on each tree species. It is also possible that the Fusarium associated with C. fleuryi is more susceptible to the differences in soil temperature and moisture between forest types. Previous studies have identified soil temperature and moisture as the two most important factors affecting the distributions and activity levels of host-specific pathogens; these factors regulate inoculum survival, spore germination, infection rates and sporulation. Tropical rain forests experience high levels of precipitation, resulting in high humidity and soil moisture, and warm temperatures, favouring pathogen growth and survival (Burdon Reference BURDON1987, Zang et al. Reference ZANG, TAO and LI2005). Our results are consistent with previous studies in other tropical forests that documented varying effects of soil pathogens on seed germination and seedling survival depending on environmental conditions (Bever Reference BEVER2002, Harms et al. Reference HARMS, WRIGHT, CALDERON, HERNANDEZ and HERRE2000, Peters Reference PETERS2003). Our findings are also consistent with the hypothesis that the effects of soil moisture on soil pathogen activity are weaker at low temperatures than at high temperatures (Kang et al. Reference KANG, KIM, LEE, LIM, JI, LEE and KIM2009). However, our study provides direct evidence that the effect of host-specific pathogens on different tree species vary with the environmental conditions.
Pathogen host specificity, environmental factors and plant communities
This study provides further evidence that different environmental conditions affect pathogen host specificity at the seed stage, potentially shaping tree population dynamics and species composition. Forest community structure and composition differ between TMRF and TCF (Yu et al. Reference YU, ZANG and JIANG2001, Zang et al. Reference ZANG, YANG and JIANG2001). Our counts of host-specific fungi and bacteria were lower in TCF than in TMRF, and the forest type had a greater effect on pathogen activity for C. fleuryi than for C. chinensis. These findings suggest that pathogen host specificity may alter the distributions of some common tree species and that this effect partially depends on the level of pathogen activity, which in turn may depend on environmental conditions. Previous studies have revealed that pathogen host specificity also acts at broader scales of community composition (Augspurger & Wilkinson Reference AUGSPURGER and WILKINSON2007, Bell et al. Reference BELL, FRECKLETON and LEWIS2006, Burdon & Chilvers Reference BURDON and CHILVERS1982, Gillett Reference GILLETT1962).
In our study, high seed mortality was largely attributable to pathogen host specificity. This result suggests that these soil pathogens may play a significant role in structuring natural plant communities. The effects of soil pathogens are not uniform and may vary significantly with environmental conditions, reflecting both host and soil pathogen activity. In particular, temperature and moisture are the most important environmental factors affecting disease distribution and the levels of pathogen activity (Burdon Reference BURDON1987). For disease to occur, a susceptible host and an infective pathogen must come into contact, and the environmental conditions must favour pathogen growth (O'Hanlon-Manners Reference O'HANLON-MANNERS2003). Thus, environmental factors may directly regulate soil pathogen communities and activity levels, which in turn are determined by pathogen host affinities and differences in host susceptibility to the soil microbial community.
Concluding remarks
This study confirms that pathogen host specificity affects the populations and species compositions of tree communities via differential effects on seed germination under different environmental conditions. In particular, this study provides new evidence that plant–pathogen interactions can influence the tree species composition of tropical rain and cloud forests.
ACKNOWLEDGEMENTS
We are grateful to Xiusen Yang, Changdong Xu and Qing Chen in Bawangling National Nature Reserve for their invaluable help with various aspects of this work. We thank Chunlan Kuang and Chunyan Wang from the Tropical Agricultural University of South China for their assistance in autoclaving soil. Comments from anonymous reviewers significantly improved the manuscript and are much appreciated. This study was financially supported by Key Projects of the National Natural Science of China (grant NO. 31230013 & 30730021).