Introduction
Thorium, uranium and rare-earth-element (REE) minerals are of interest as possible prototypes of nuclear waste forms (Lumpkin et al., Reference Lumpkin, Gao, Giere, Williams, Mariano and Geisler2014). In particular, synthetic materials chemically and structurally related to zirconolite are considered as potential components of advanced materials which can be used for durable containment of high-level radioactive wastes, including trivalent and tetravalent actinides, such as unrecyclable plutonium, as well as neptunium, curium and americium (Ringwood et al., Reference Ringwood and Kelly1986; Donald et al., Reference Donald, Metcalfe and Taylor1997; Laverov et al., Reference Laverov, Yudintsev, Stefanovsky, Omel'yanenko and Nikonov2006; Barinova et al., Reference Barinova, Borovinskaya, Ratnikov and Ignat'eva2008; Zhang et al., Reference Zhang, Gregg, Kong, Jovanovich and Triani2017). As a rule, in nature zirconolite-group minerals contain uranium and thorium, the total content of which can reach 15–20 wt.% (Williams and Gieré, Reference Williams and Gieré1996; Hurai et al., Reference Hurai, Huraiová, Gajdošová, Konečný, Slobodník and Siegfried2018).
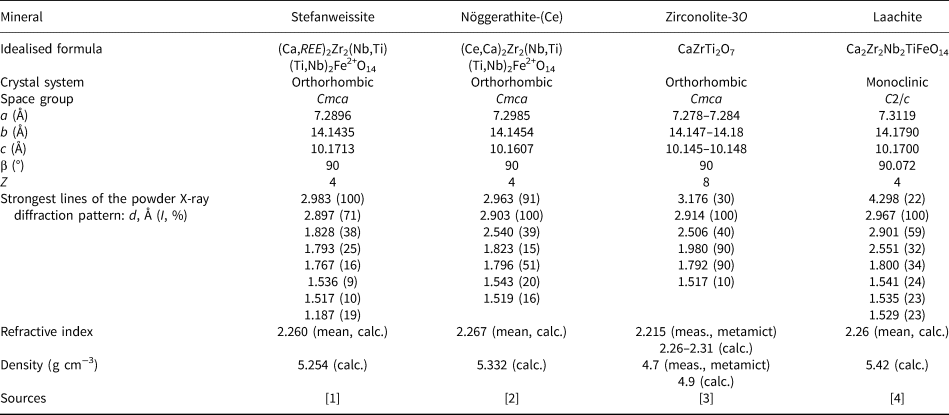
Zirconolite-type minerals exhibit significant structural and chemical diversity. Currently, there are four minerals (one with three polytypes) belonging to this family: zirconolite-3O (formerly polymignite) (Ca,REE)2Zr2(Ti,Nb)3FeO14, zirconolite-3T (Ca,REE)2Zr2(Ti,Nb)3FeO14, zirconolite-2M (Ca,REE)2Zr2(Ti,Nb)3FeO14, laachite Ca2Zr2Nb2TiFeO14, nöggerathite-(Ce) (Ce,Ca)2Zr2(Nb,Ti)(Ti,Nb)2Fe2+O14, and described in this paper stefanweissite (Ca,REE)2Zr2(Nb,Ti)(Ti,Nb)2Fe2+O14. Zirconolite-3O (Ca,REE)2Zr2(Ti,Nb)3FeO14 and zirconolite-3T are the most common zirconolite-type species. Laachite, nöggerathite-(Ce) and stefanweissite discovered in sanidinites of the Laach Lake area are rare. Unlike most other samples of zirconolite-type minerals, they are non-metamict being crystallised not earlier than a few dozens of million years ago. (Litt et al., Reference Litt, Brauer, Goslar, Merk, Balaga, Mueller, Ralska-Jasiewiczowa, Stebich and Negendank2001; Schmitt et al., Reference Schmitt, Wetzel, Cooper, Zou and Wörner2010).
The new mineral species stefanweissite is named in honour of PhD Stefan Weiss (b. 1955), German geologist, mineralogist and petrologist, and editor of the LAPIS magazine since May 1993, effectively promoting mineralogy among collectors and amateurs. From 1979 to 2017, Stefan Weiss has published more than 180 articles in LAPIS on general and descriptive mineralogy, classic mineral localities, regional mineralogy and new finds in the Alps, Cornwall and Saxony. He regularly edits the LAPIS magazine series ‘Neue Mineralien’ which has presented, since 1992, more than 1550 abstracts on new mineral species. Stefan Weiss is also an author of several books including those about German mineral localities.
The new mineral stefanweissite and its name were approved by the International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) (|IMA2018-020, Chukanov et al., Reference Chukanov, Zubkova, Pekov, Vigasina, Polekhovsky, Ternes, Schüller, Britvin and Pushcharovsky2018c). The type specimen is deposited in the collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia, with the registration number 5191/1.
Occurrence, general appearance and mechanical properties
Stefanweissite occurs in the In den Dellen (Zieglowski) pumice quarry (50°23′40″N, 7°17′12″E), 1.5 km NE of Mendig, Laach Lake (Laacher See) volcano, Eifel region, Rhineland-Palatinate, Germany. It forms isolated flattened long-prismatic crystals up to 0.03 mm × 0.07 mm × 1.0 mm and acicular crystals up to 2 mm long and 0.02 mm thick typically combined in radiated aggregates (Fig 1) in cavities inside sanidinite volcanic ejecta. Usually, the dominant crystal form is {001} and other forms are {011}, {010}, {111), as well as occasionally {100}. Associated minerals are sanidine, nosean, biotite, augite, titanite, ferriallanite-(La), magnetite, baddeleyite and a pyrochlore-group mineral.

Fig. 1. Aggregates of brown lath- and needle-shaped crystals of stefanweissite on sanidine, in association with biotite (a, c) and magnetite (b). Photographer: Stefan Wolfsried. Field of view widths: (a) 1 mm; (b) 1.5 mm; (c) 0.8 mm; and (d) 2 mm.
Crystals of stefanweissite are translucent to transparent. The colour is brown, reddish-brown to very dark brownish-red, with red–brown internal reflections; some thin needles are yellowish-brown. The lustre is adamantine. The streak is light brown to yellow. No cleavage is observed; the fracture is uneven. Density could not be measured due to the absence of heavy liquids with D > 5 g cm−3 and insufficient amount of material to measure density by hydrostatic weighing or volumetric methods. Density calculated using the empirical formula is 5.254 g cm−3.
Optical properties
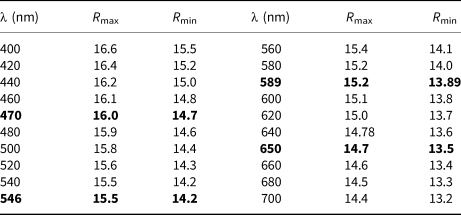
Reflectance values for stefanweissite have been measured in air using a MSF-21 micro-spectrophotometer (LOMO company, St. Petersburg, Russia) with the monochromator slit width of 0.4 mm and beam diameter of 0.1 mm. SiC (Reflection standard 474251, No. 545, Germany) was used as a standard. The reflectance values (R max/R min) are given in Table 1.
Table 1. Reflectance values (R max/R min) for stefanweissite.

Reflectance values for four wavelengths recommended by the IMA Commission on Ore Microscopy are given in bold type.
In reflected light, stefanweissite is anisotropic, with ∆R 589 = 1.27%. The mineral is light grey, with brown internal reflections.
The mean n value, calculated from the Gladstone–Dale equation using the empirical formula and the calculated density, is 2.260.
Raman spectroscopy
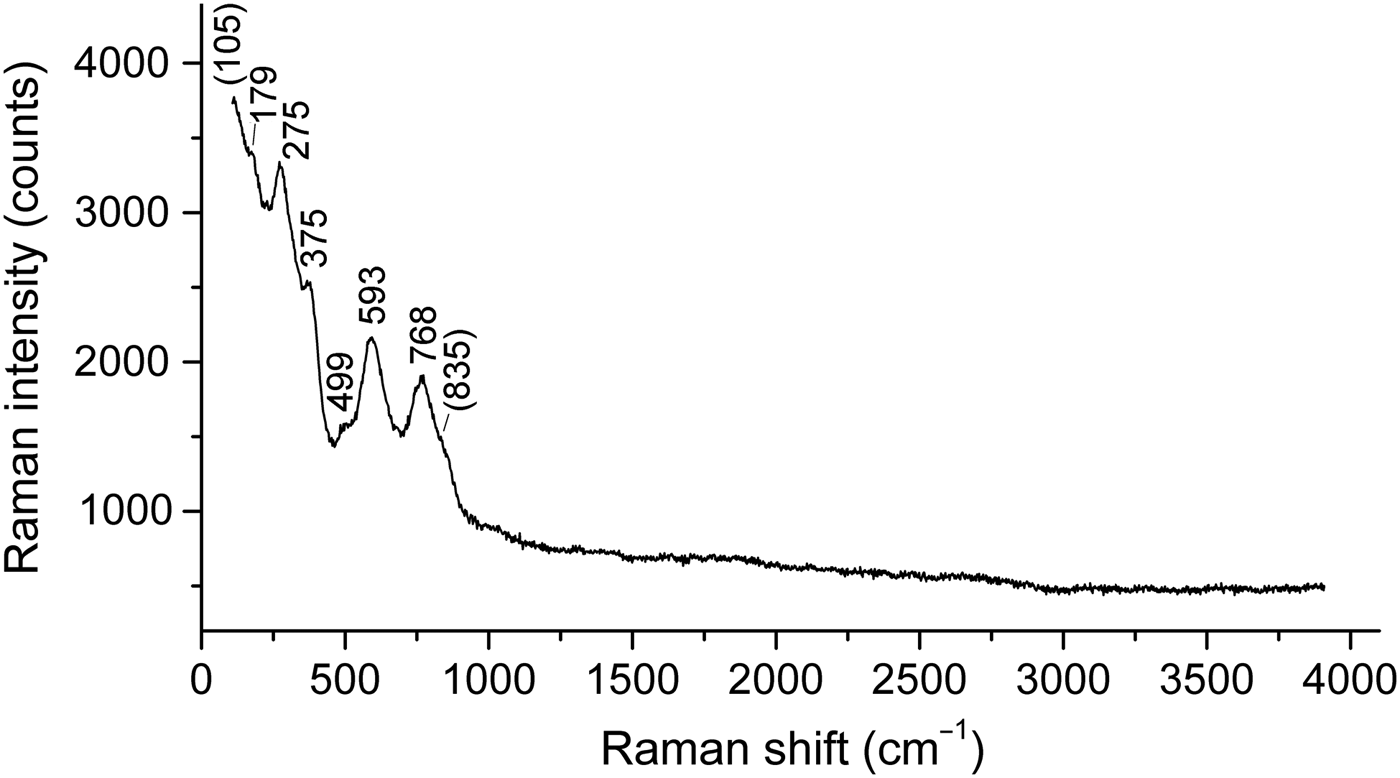
The Raman spectrum of a randomly oriented stefanweissite crystal (Fig. 2) was obtained using an EnSpectr R532 spectrometer based on an OLYMPUS CX 41 microscope coupled with a diode laser (λ = 532 nm) at room temperature. The spectrum was recorded in the range from 100 to 4000 cm−1 with a diffraction grating (1800 gr mm−1) and spectral resolution ~6 cm−1. The output power of the laser beam was ~9 mW. The diameter of the focal spot on the sample was <10 µm. The back-scattered Raman signal was collected with 40× objective; signal acquisition time for a single scan of the spectral range was 2 s and the signal was averaged over 100 scans.
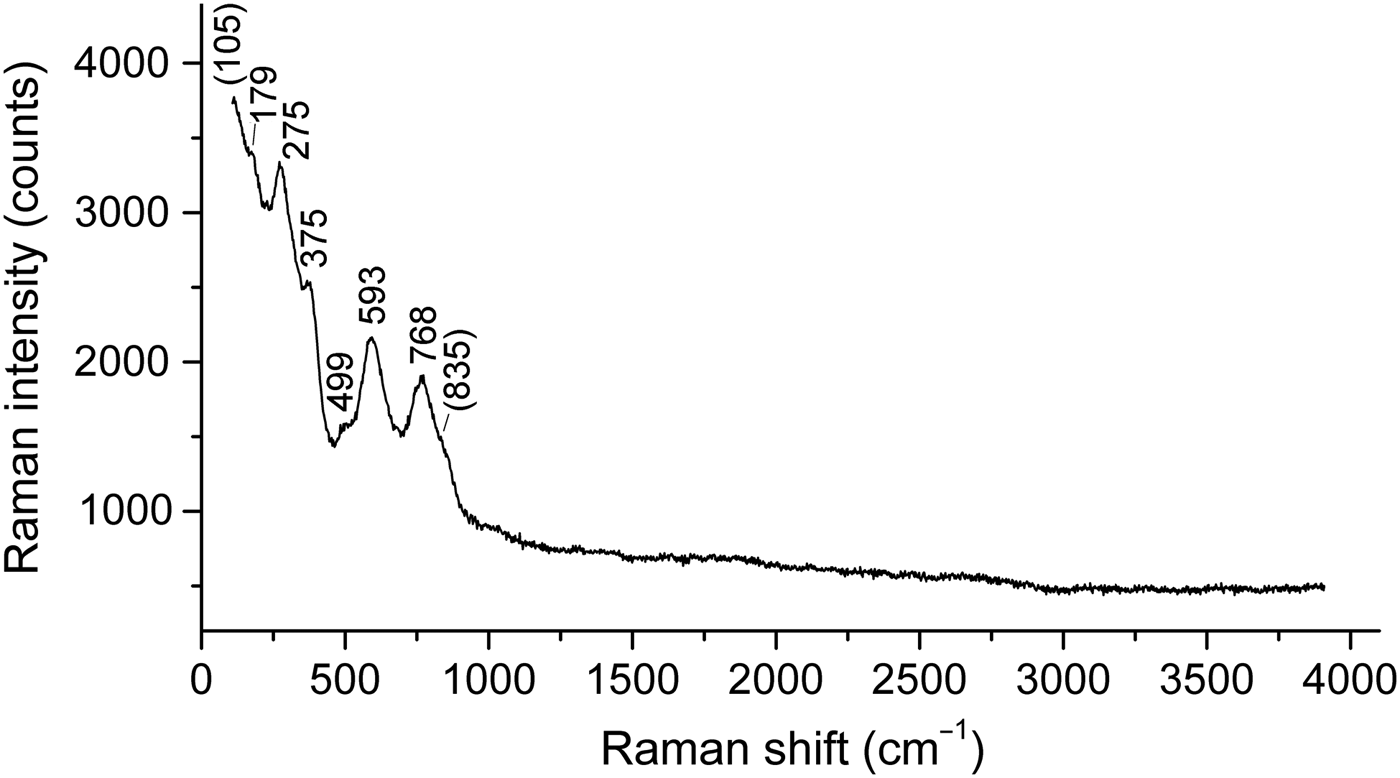
Fig. 2. Raman spectrum of stefanweissite.
The Raman spectrum of stefanweissite shows the absence of bands of H2O molecules (in the ranges 2500–3800 and 1500– 700 cm−1), OH groups (2500–3800 cm−1) and CO32− anions (1040–1100 cm−1). According to Salamat et al. (Reference Salamat, McMillan, Firth, Woodhead, Hector, Garbarino, Stennett and Hyatt2013), the band at 768 cm−1 with the shoulder at 835 cm−1 is to be assigned to symmetric stretching vibrations of TiO6 groups in the zirconolite-type structure. By analogy with monoclinic ZrO2 (Geisler et al., Reference Geisler, Zhang and Salje2003), the band at 593 cm−1 can be assigned to Zr–O-stretching vibrations. The bands below 400 cm−1 correspond to lattice modes involving (Ca,REE)–O-stretching and O–(Ti,Nb,Zr)–O bending vibrations.
Chemical data
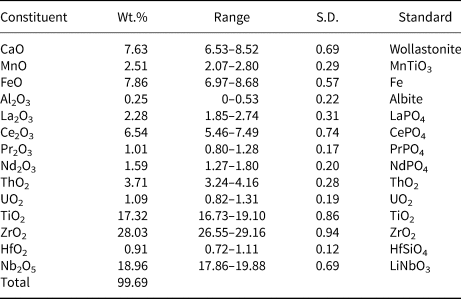
The chemical composition of the new mineral was determined using an Oxford INCA Wave 700 electron microprobe (wavelength-dispersive spectroscopy mode, 20 kV, 600 pA and 300 nm beam diameter). Analytical data (average of seven spot analyses) are given in Table 2. Contents of other elements with atomic numbers >8 are below detection limits. On the basis of structural data (see below) and by analogy with the related mineral laachite (Chukanov et al., Reference Chukanov, Krivovichev, Pakhomova, Pekov, Schäfer, Vigasina and Van2014), iron and manganese are considered as Fe2+ and Mn2+, respectively.
Table 2. Chemical composition of stefanweissite.

S.D. – Standard deviation
The empirical formula based on 14 O atoms per formula unit (apfu) (in accordance with structural data, Z = 4) is Ca1.13(Ce0.33La0.12Nd0.08Pr0.05)Σ0.58Th0.12U0.03Mn0.29Fe0.91Al0.04Zr1.89Hf0.04Ti1.80Nb1.19O14. The simplified formula is (Ca,REE)2Zr2(Nb,Ti)(Ti,Nb)2Fe2+O14.
X-ray diffraction and crystal structure
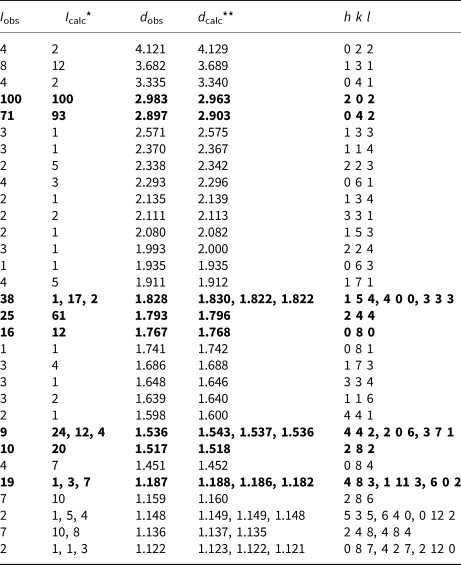
Powder X-ray diffraction data were collected using a Rigaku RAXIS Rapid II diffractometer with a curved image plate detector, rotating anode, and in Debye–Scherrer geometry. The accelerating voltage = 40 kV, current = 15 mA and exposure = 15 min. The distance between sample and detector was 127.4 mm. Data (in Å for CoKα) are listed in Table 3.
Table 3. Powder X-ray diffraction data for stefanweissite.
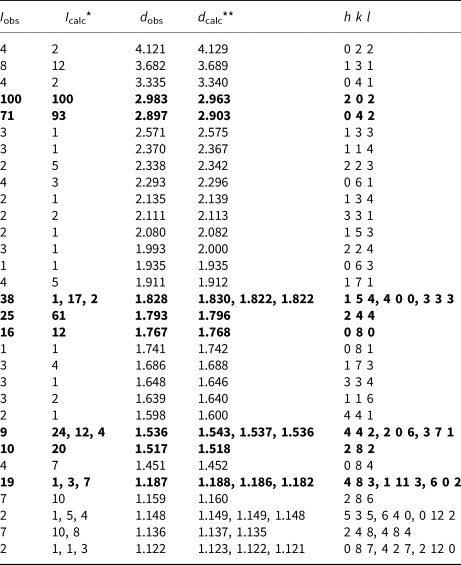
*For the calculated pattern, only reflections with intensities ≥1 are given; **For the unit-cell parameters calculated from single-crystal data.
The strongest lines are given in bold.
Diffraction peaks are indexed agreeably in the orthorhombic unit cell, space group Cmca. The unit-cell parameters calculated from the powder data are: a = 7.285(2) Å, b = 14.141(3) Å, c = 10.166(2) Å and V = 1047(1) Å3.
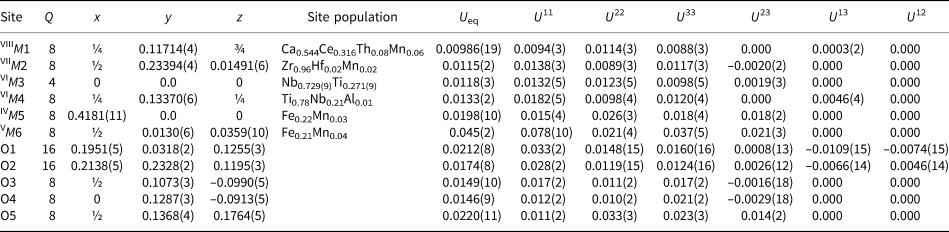
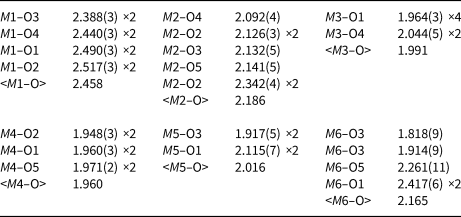
Single-crystal X-ray studies were carried out using an XCaliburS CCD diffractometer, using MoKα radiation (λ = 0.71073 Å). For crystal data, data collection information and structure refinement details see Table 4. Site coordinates, equivalent thermal displacement parameters, site populations and site multiplicities are given in Table 5; selected interatomic distances are listed in Table 6. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Crystal data, data collection information and structure refinement details.

w = 1/[σ2(Fo2) + (aP)2+ bP] where P is [2Fc2+ Max(Fo2,0)]/3.
Table 5. Coordinates, site multiplicities (Q), site populations, and equivalent and anisotropic thermal displacement parameters (U eq, in Å2), for stefanweissite.
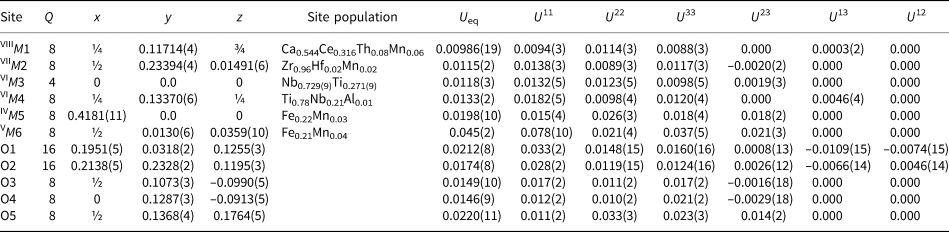
Table 6. Selected interatomic distances (Å) in the structure of stefanweissite.
Like other minerals related to zirconolite-3O, the crystal structure of stefanweissite demonstrates alternation of two types of bent polyhedral layers, namely an octahedral layer (Fig. 3a), and a layer of cations having seven- and eight-fold coordinations: edge-sharing Ca-dominant distorted cubes [the M1 site] and Zr-dominant mono-capped octahedra [the M2 site] (Fig. 3b). The octahedral layer is built by vertex-sharing M3O6 and M4O6 octahedra forming three- and six-membered rings (hexagonal-tungsten-bronze type arrays). The adjacent M5 and M6 sites have the coordination numbers 4 and 5, respectively. These sites are located in the centres of six-membered rings and are statistically occupied with Fe2+ as the major cation (Fig. 3c). The M1-centred polyhedron shares edges with neighbouring M1-centred cubes to form rows along the a axis. Similar rows are formed by seven-fold M2-centred polyhedra (mono-capped octahedra). Adjacent rows of eight- and seven-fold polyhedra are linked with each other via common edges forming a dense layer. A general view of the crystal structure of stefanweissite is shown in Fig. 4.
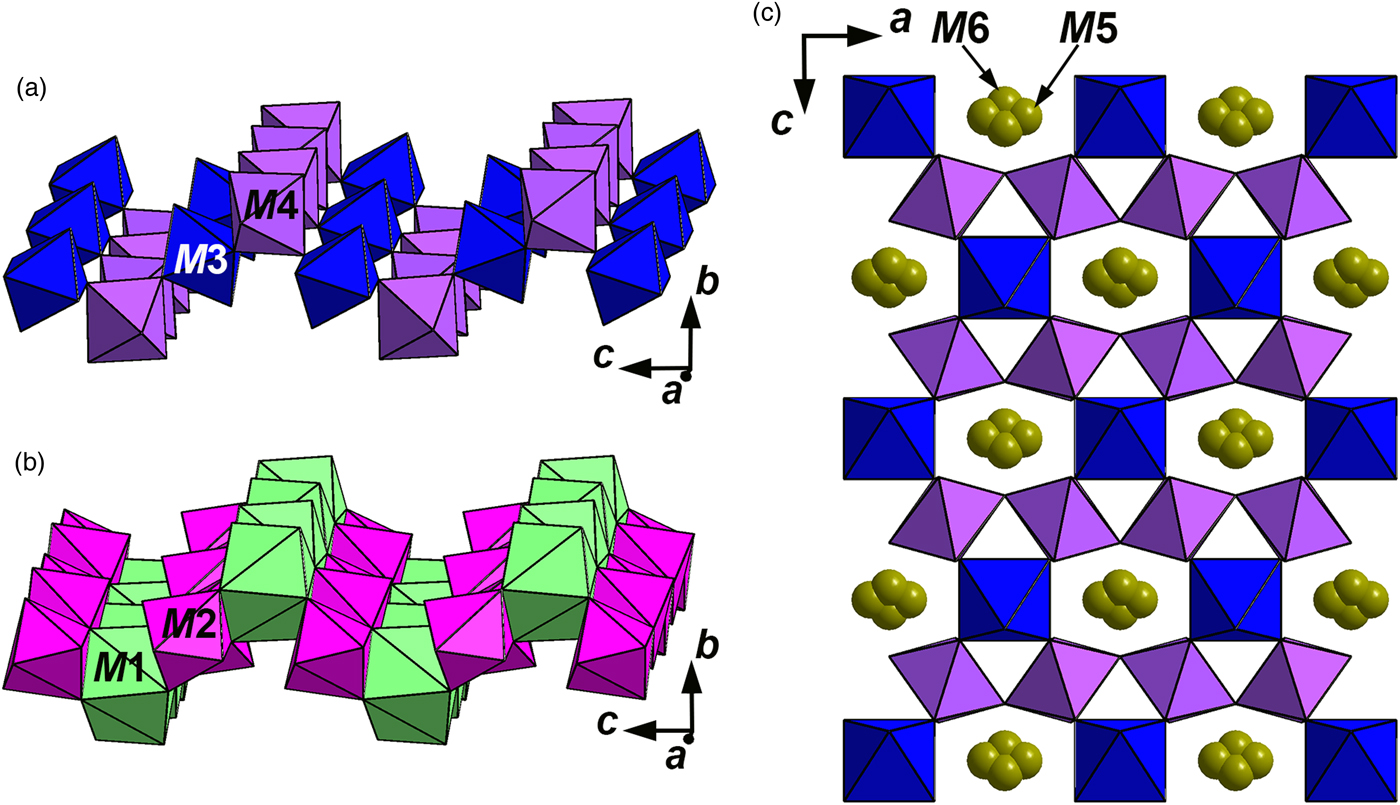
Fig. 3. Octahedral (a) and large-cation (b) layers and arrangement of Fe-dominant sites M5 and M6 inside the octahedral layer (c) in the structure of stefanweissite.
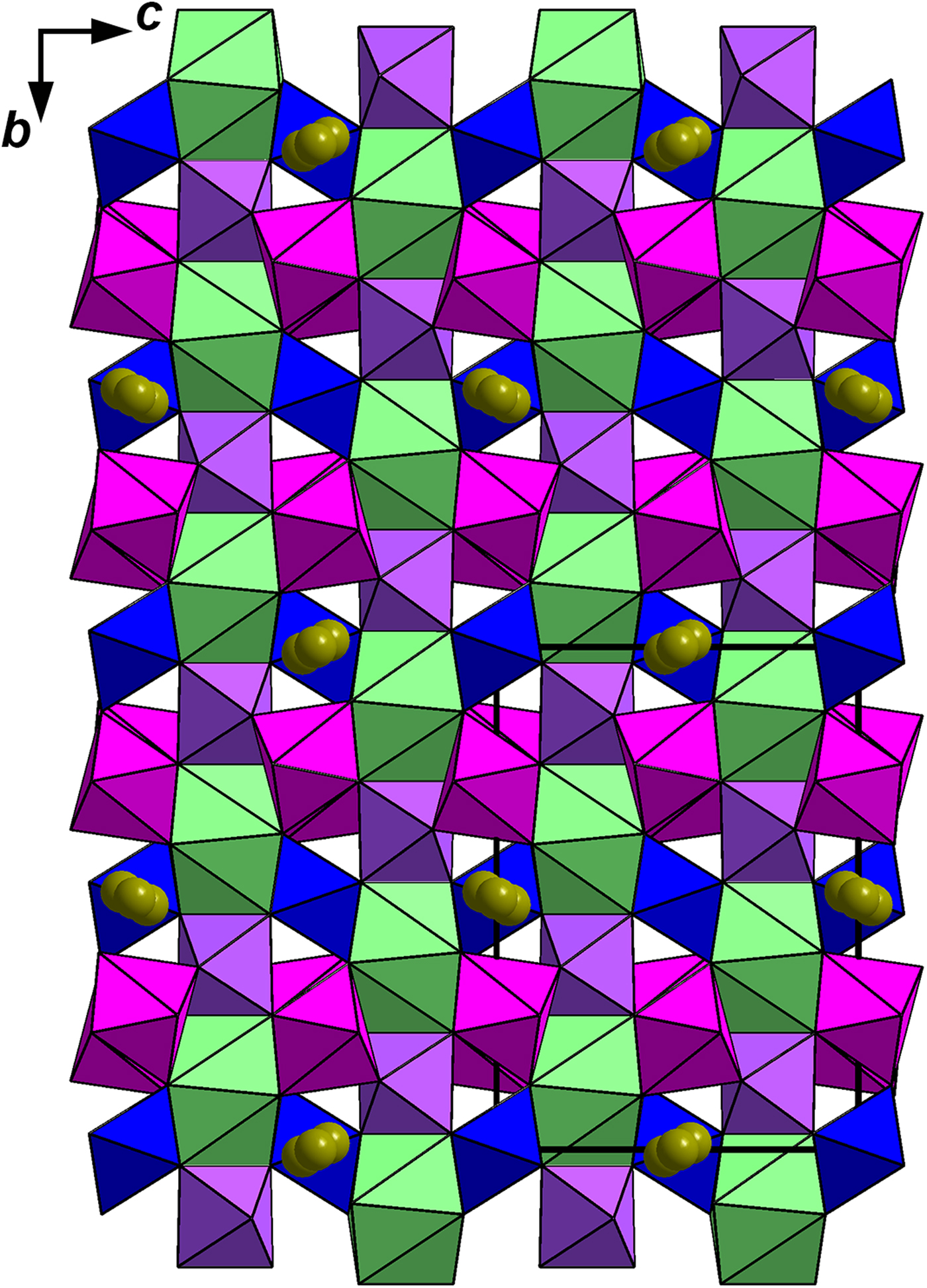
Fig. 4. The crystal structure of stefanweissite: general view. The unit cell is outlined. For legend see Fig. 3.
The refined crystal-chemical formula of stefanweissite is (Z = 4, REE are modelled by Ce, coordination numbers of cations are indicated with Roman numerals): VIII(Ca0.544REE 0.316Th0.08Mn0.06)2VII(Zr0.96Hf0.02Mn0.02)2VI(Nb0.729(9)Ti0.271(9))VI(Ti0.78Nb0.21Al0.01)2 IV(Fe0.22Mn0.03)2V(Fe0.21Mn0.04)2O14.
Discussion
Stefanweissite is isostructural with zirconolite-3O and nöggerathite-(Ce) (IMA2017-107 Chukanov et al., Reference Chukanov, Zubkova, Britvin, Pekov, Vigasina, Schäfer, Ternes, Schüller, Ermolaeva and Pushcharovsky2018b) (see Tables 6 and 7). Zirconolite-3O was originally described as ‘polymignite’ (Berzelius, Reference Berzelius1824; Brøgger, Reference Brøgger1890) and later redefined and renamed (Bayliss et al., Reference Bayliss, Mazzi, Munno and White1989; Mazzi and Munno, Reference Mazzi and Munno1983; Chukhrov and Bonshtedt-Kupletskaya, Reference Chukhrov and Bonshtedt-Kupletskaya1967; Pudovkina et al., Reference Pudovkina, Chernitzova and Pyatenko1969). ‘Polymignite’ is usually metamict and its powder X-ray diffraction pattern can be obtained only after heating. However, all zirconolite-related minerals from sanidinites of the Laach Lake volcano, including laachite, zirconolite-3T (Zubkova et al., Reference Zubkova, Chukanov, Pekov, Ternes, Schüller and Pushcharovsky2018), nöggerathite-(Ce), and stefanweissite are non-metamict because of their young geological age: the last eruption of the Laach Lake volcano was ~13,000 years ago.
Table 7. Comparative data for stefanweissite and related minerals.
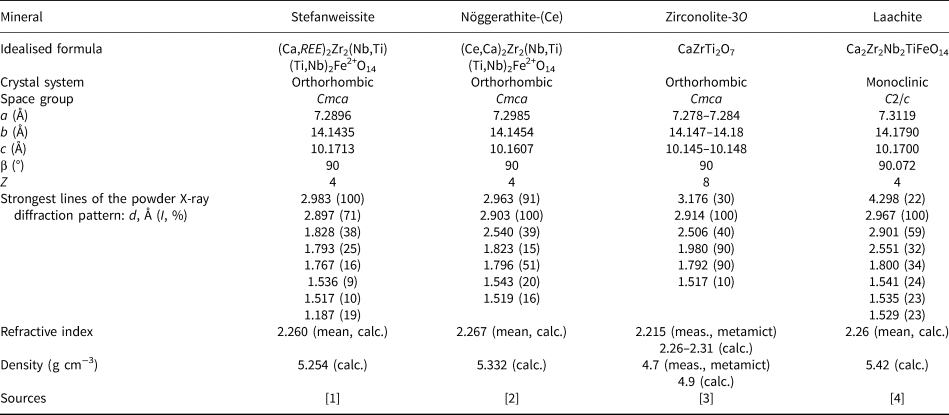
Sources: [1] This work. [2] Chukanov et al. (Reference Chukanov, Zubkova, Britvin, Pekov, Vigasina, Schäfer, Ternes, Schüller, Ermolaeva and Pushcharovsky2018a). [3] Borodin et al. (1956); Chukhrov and Bonshtedt-Kupletskaya (Reference Chukhrov and Bonshtedt-Kupletskaya1967); Pudovkina et al. (Reference Pudovkina, Chernitzova and Pyatenko1969); Sinclair and Eggleton (1982); Mazzi and Munno (Reference Mazzi and Munno1983); Bayliss et al. (Reference Bayliss, Mazzi, Munno and White1989); Della Ventura et al. (2000). [4] Chukanov et al. (Reference Chukanov, Krivovichev, Pakhomova, Pekov, Schäfer, Vigasina and Van2014).
The filling of the M5/M6 split-site in stefanweissite and noggerathite-(Ce) is similar, but different from that in zirconolite-3O and laachite. The resulting U eq value for M6 in stefanweissite is significantly higher than that for M5 (Table 5) which could be considered as an indication of overestimation of the reported site scattering. On the other hand, the occupation of the M6 site in stefanweissite is in accordance with the chemical data and is similar to that in isostructural nöggerathite-(Ce). The high value of U eq in our case may be a result of the further disorder of the M6 site along the a axis: the analysis of anisotropic values shows a higher value of U 11 in comparison with U 22 and U 33 (Table 5). However we prefer to give the averaged site position because the problem of localisation of the corresponding sub-sites does not have a unique solution.
Other crystalline minerals related to zirconolite-3O and occurring in sanidinite of the Laach Lake volcano are nöggerathite-(Ce) (Ce,Ca)2Zr2(Nb,Ti)(Ti,Nb)2Fe2+O14 and laachite Ca2Zr2Nb2TiFeO14. Laachite is a monoclinic (pseudo-orthorhombic) analogue of zirconolite-3O with Nb prevailing over Ti in two octahedral sites (Chukanov et al., Reference Chukanov, Krivovichev, Pakhomova, Pekov, Schäfer, Vigasina and Van2014).
Nöggerathite-(Ce) (Chukanov et al., Reference Chukanov, Zubkova, Britvin, Pekov, Vigasina, Schäfer, Ternes, Schüller, Ermolaeva and Pushcharovsky2018a) is isostructural with stefanweissite and differs from the latter by the predominance of REE over Ca in sites having 8-fold coordination. However the status of these minerals requires a separate discussion.
There are three different rules that are now used in the nomenclature of minerals, including the Levinson rule of combining REE, the rule of a dominant component at a site – the dominant-valency rule, and the rule of the main charge-balancing component. Sometimes these rules contradict each other, and we have to decide, what rule has a priority in a given case.
Chemical compositions of 23 samples of zirconolite-type minerals from the Laach Lake volcano have been determined by us. Most of them correspond to stefanweissite with Ca > ΣREE.
In the first description of nöggerathite-(Ce) (Chukanov et al., Reference Chukanov, Zubkova, Britvin, Pekov, Vigasina, Schäfer, Ternes, Schüller, Ermolaeva and Pushcharovsky2018a) data on two samples are given. One of them (Sample 1) for which chemical data were obtained and crystal structure was solved, has the crystal-chemical formula VIII(REE 0.88Ca0.80Mn0.24Th0.08)VII(Zr1.88Mn0.12)VI(Nb1.22Ti0.78)VI(Ti1.48Nb0.48Al0.04)IV(Fe0.48Mn0.08)V(Fe0.40Mn0.04)2O14 and the following refined unit-cell parameters: a = 7.2985(3), b = 14.1454(4), c = 10.1607(4) Å and V = 1048.99(7) Å3. Another one (Sample 2) was characterised by chemical, optical and powder X-ray diffraction data, as well as Raman spectroscopy. Its crystal-chemical formula is (Ce0.57La0.19Nd0.12Pr0.05Y0.06)Σ0.99Ca0.79Th0.06Mn0.49Fe0.77Al0.10Zr1.89Ti1.96Nb1.00O14 and the refined unit-cell parameters are: a = 7.296(1), b = 14.147(2), c = 10.161(1) Å and V = 1048.9(2) Å3.
X-ray diffraction data of both samples correspond to the space group Cmca, and obviously they are isostructural. In the sample of nöggerathite-(Ce) ΣREE prevails over Ca at the M1 site, and on the basis of the Levinson rule this mineral should be considered as Ce-dominant (REE prevail over Ca in total and Ce prevails over other REE). In addition, in Sample 1 REE are the main charge-balancing component (and, consequently, can be considered as a species-defining component in the nöggerathite-(Ce) like Fe3+ in ferrihollandite, V3+ in mannardite, Sn4+ in nigerite-group minerals, K in barytolamprophyllite [for the site occupied as (Ba,Sr,K,Na)], Na in nabalamprophyllite [for the site occupied as (Ba,Na,Sr,K)] etc. On the other hand, in Sample 1 the sum of bivalent cations (Ca + Mn) prevails over ΣREE at the M1 site. Consequently, in terms of the dominant-valency rule (Hatert and Burke, Reference Hatert and Burke2008) Sample 1 should be considered as a Ca-dominant mineral.
Sample 2 of nöggerathite-(Ce) is REE-dominant in terms of all nomenclature rules (i.e. dominant-valency rule, dominant-constituent rule, Levinson rule and charge-balancing component rule).
The dominant-valency rule was applied to some minerals with heterovalent substitutions at two sites, e.g. in the nomenclature systems for minerals of the amphibole, arrojadite and epidote groups (Hawthorne and Oberti Reference Hawthorne and Oberti2006; Cámara et al. Reference Cámara, Oberti, Chopin and Medenbach2006; Chopin et al. Reference Chopin, Oberti and Cámara2006; Armbruster et al. Reference Armbruster, Bonazzi, Akasaka, Bermanec, Chopin, Gieré, Heuss- Assbichler, Liebscher, Menchetti, Pan and Pasero2006). However in minerals belonging to the nöggerathite-(Ce)–stefanweissite series coupled heterovalent–homovalent substitutions at four sites takes place: (REE,Ca,Mn)2(Zr,Mn)2(Nb,Ti)(Ti,Nb)2Fe2+O14. As a result, the end-member formula Ca2Zr2NbTi2Fe2+O14 written following the dominant-valency rule for a sample with REE > Ca but REE < Ca + Mn is not charge-balanced, and this is not the only case of a mineral that does not have an adequate end-member formula with one component at each site. This raises the question of how formulae of such minerals should be written. A way to solve this problem would be to write an end-member formula with two cations in the same site, as was done for the minerals of the lamprophyllite group: for example, the formula of lamprophyllite given in the IMA list of minerals (Pasero, Reference Pasero2018) updated in November 2018 is (SrNa)Ti2Na3Ti(Si2O7)2O2(OH)2. This formula was accepted based on the fact that Na is the main charge-balancing univalent cation at the A site despite the fact that the Sr content prevails significantly over the Na content at this site. On the basis of this precedent, the end-member formulae of stefanweissite and nöggerathite-(Ce) could be written as Ca2Zr2Nb(TiNb)Fe2+O14 and (CaCe)Zr2NbTi2Fe2+O14, respectively.
The rules for writing end-member formulae in such complicated cases are not fully worked out, and appropriate guidelines made by the IMA CNMNC would be useful. This issue concerns not only zirconolite-related minerals. Such situations are numerous. For example, the end-member formula for alumoåkermanite (Ca,Na)2(Al,Mg,Fe2+)(Si2O7) with one cation at each site, i.e. Ca2Al(Si2O7), is not charge-balanced. The charge-balanced formula would be (CaNa)Al(Si2O7). The other well-known examples are loparite-(Ce) and melanostibite with the idealised formulae (NaREE)Ti2O6 and Mn2+(Sb5+,Fe)O3 [ = Mn2+(Sb5+0.5Fe3+0.5)O3 i.e., with Sb and Fe in the same site], respectively. This is only a small number of examples to illustrate this nomenclature problem.
It is important to note that nöggerathite-(Ce) (the richest in REE), was actually described by Della Ventura et al. (2000) before the approval of this mineral. It is orthorhombic [with a = 7.284(5), b = 14.18(8) and c = 10.145(8) Å], with the empirical formula (ranges): Ca0.312–0.337Y0.079–0.093La0.069–0.086Ce0.269–0.296Pr0.020–0.028Nd0.052–0.062Sm0.004–0.006Gd0.005–0.006Dy0.003–0.006Er0.003–0.005Th0.014–0.073U0.008–0.022Mn0.345–0.397Mg0.003–0.005Al0.020–0.025Fe0.301–0.339Zr0.888–0.910Hf0.006–0.009Ti0.840–0.888Nb0.575–0.613Ta0.007–0.009Si0.000–0.012O7.000. In particular, the empirical formula corresponding to spot analysis number 1 from the paper by Della Ventura et al. (2000), calculated on 14 O apfu is (REE 1.14Ca0.63Mn0.17Th0.04U0.02)Σ2[(Fe0.65Mn0.55Al0.05Mg0.01)Σ1.26(Zr1.79Hf0.01)Σ1.8(Ti1.71Nb1.19Ta0.02)Σ2.92Si0.02]Σ6O14. These data confirm that the existence of nöggerathite-(Ce) as a REE-dominant mineral and its distinction from stefanweissite are undeniable.
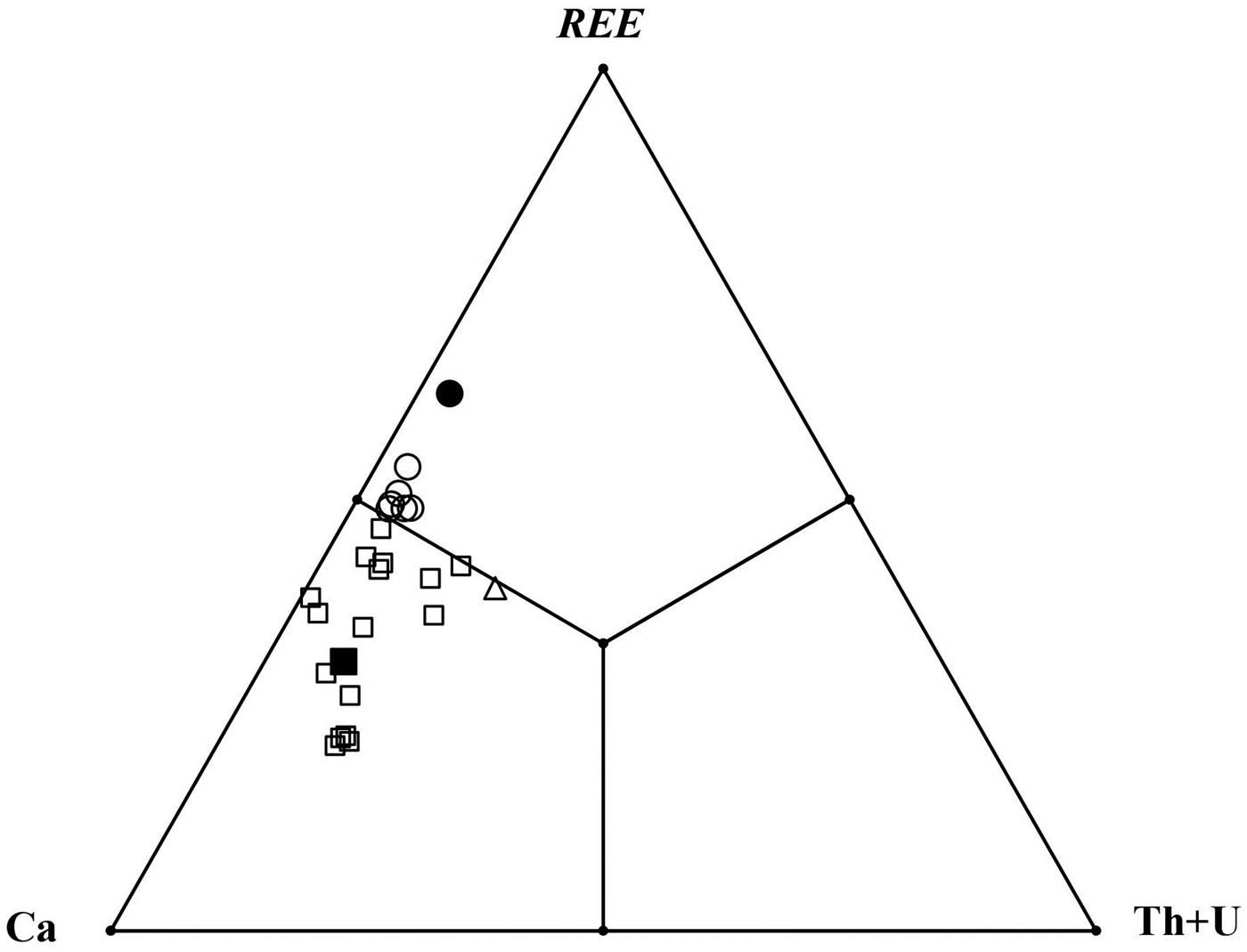
Comparative data for stefanweissite, nöggerathite-(Ce), zirconolite-3O and laachite are given in Table 7. A spread of compositions between stefanweissite and nöggerathite-(Ce) is shown in Fig. 5.
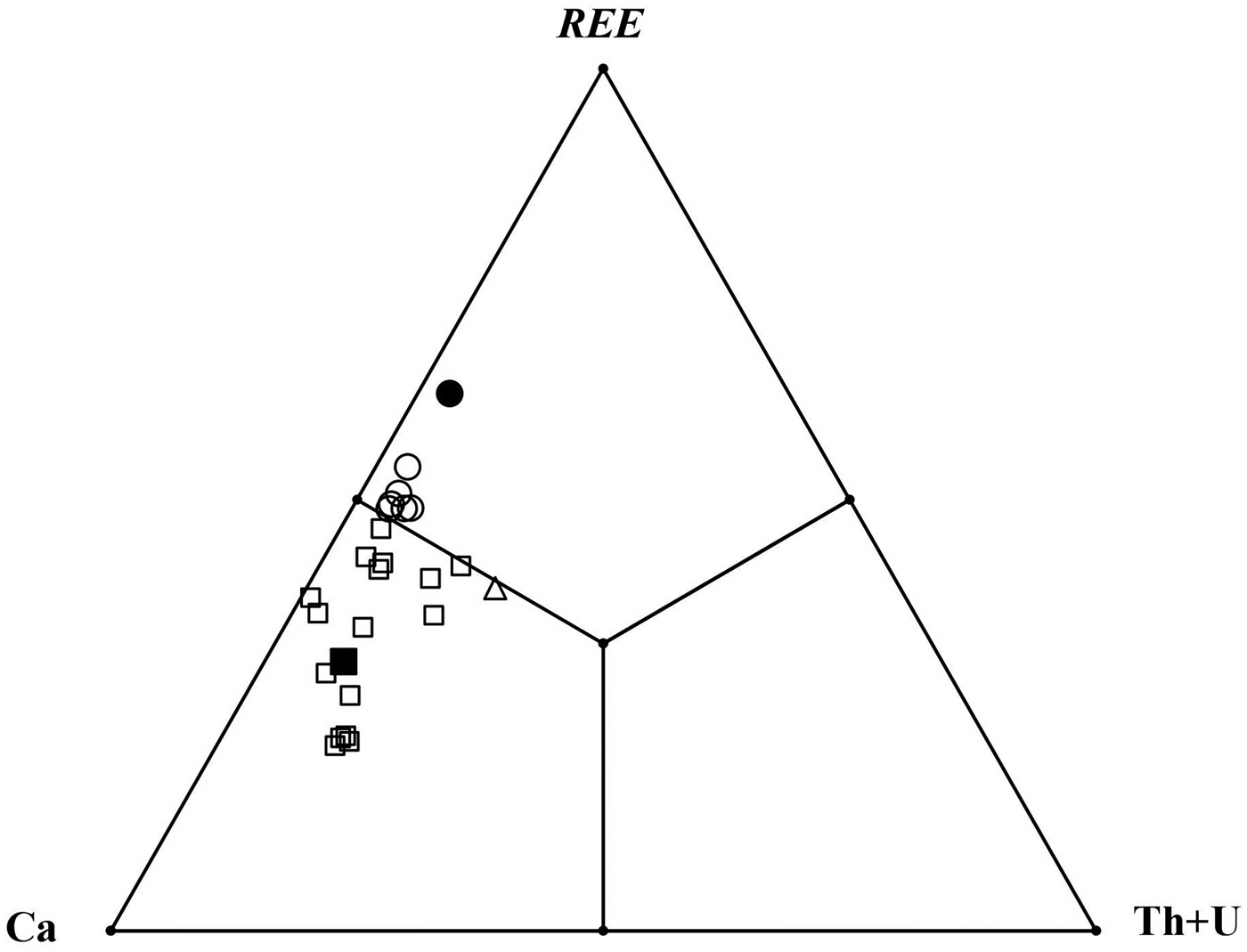
Fig. 5. Ratios of large VIIIM1 cations in orthorhombic zirconolite-type minerals from the Laach Lake volcano: the holotype stefanweissite (black square); other stefanweissite samples (open squares); and nöggerathite-(Ce) (open circles – our data; black circle – analysis 1 from Della Ventura et al., Reference Della Ventura, Bellatreccia and Williams2000).
Among the analyses of ~300 zirconolite samples compiled by Williams and Gieré (Reference Williams and Gieré1996), there are only a minor number where the content of Nb2O5 exceeds 10 wt.%. Unlike zirconolite, stefanweissite, laachite and nöggerathite-(Ce) contain niobium as a species-defining component. The composition of a mineral with Nb:Ti ≈ 43:57 from Vuoriyarvi, Northern Karelia, Russia described by Borodin et al. (Reference Borodin, Bykova, Kapitonova and Pyatenko1960) as ‘niobozirconolite’ corresponds to stefanweissite.
Acknowledgements
This work was supported by the Russian Science Foundation, grant no. 14-17-00048 (in part for the chemical study of stefanweissite and other zirconolite-type minerals) and Russian Foundation for Basic Research, grant no. 18-29-12007 (in parts for mineralogical study, Raman spectroscopy and X-ray structural analysis). The authors thank the X-ray Diffraction Centre of Saint-Petersburg State University for instrumental and computational resources.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.171.