Introduction
Familial/genetic factors contribute meaningfully to the liability to irrational fears and phobias when examined one at a time (e.g. Torgersen, Reference Torgersen1979; Phillips et al. Reference Phillips, Fulker and Rose1987; Fyer et al. Reference Fyer, Mannuzza, Gallops, Martin, Aaronson, Gorman, Liebowitz and Klein1990; Stevenson et al. Reference Stevenson, Batten and Cherner1992; Page & Martin, Reference Page and Martin1998; Skre et al. Reference Skre, Onstad, Torgersen, Philos, Lygren and Kringlen2000). When multiple fears or phobias have been examined jointly in genetically informative samples of children (Lichtenstein & Annas, Reference Lichtenstein and Annas2000), adults (Kendler et al. Reference Kendler, Neale, Kessler, Heath and Eaves1992, Reference Kendler, Myers, Prescott and Neale2001; Hettema et al. Reference Hettema, Prescott, Myers, Neale and Kendler2005) or adults and their parents (Phillips et al. Reference Phillips, Fulker and Rose1987), genetic risk has been shown to result partly from factors shared in common across fears and partly from genetic factors specific to individual fears.
Genetic susceptibilities to fears are likely to have arisen because they promote survival (Seligman, Reference Seligman1971; Marks & Nesse, Reference Marks and Nesse1994). However, human developmental studies show a typical progression of fears – what Marks termed an ‘ontogenetic parade’ (Marks, Reference Marks1987, p. 109). This pattern may have emerged in human evolution because the adaptiveness of fears varied over development. That is, fears that are protective for a young child will differ from those that would promote survival for a late adolescent. If evolution has shaped this ontogenetic parade, genetic risk factors for fears should prove to be developmentally dynamic.
To our knowledge, no study has tracked the development of fears through time in a genetically informative sample so as to examine the degree of stability of genetic and environmental risk factors both for a general fear factor and for individual fears. In this report we present such an analysis of self-report situational, animal, blood-injury and social fears obtained at the ages of 13–14, 16–17 and 19–20 in twins from the Swedish Twin Study of Child and Adolescent Development.
Method
Sample
The sample was obtained from the population-based Swedish Twin Registry, which contains information on all twins born in Sweden since 1886 (Lichtenstein et al. Reference Lichtenstein, de Faire, Floderus, Svartengren, Svedberg and Pedersen2002). As detailed elsewhere, this sample – called the Swedish Twin Study of Child and Adolescent Development (T-CHAD; Lichtenstein et al. Reference Lichtenstein, Tuvblad, Larsson and Carlstrom2007) – began with all twin pairs born in Sweden between May 1985 and December 1986 where both twins were alive and residing in Sweden in 1994. To date, this sample has been assessed four times from ages 8 to 20 years. In this report, we will examine the three assessments completed by the twins themselves as the fear data collected at age 8–9 was solely via parental report. The number (and response rates) obtained at these three waves were: age 13–14 (n=2263, 78%), age 16–17 (n=2369, 82%), and age 19–20 (n=1705, 59%).
Each of the questionnaires was approved by the Ethics Committee of the Karolinska Institute, Stockholm, Sweden. No specific informed consent was required as response to the questionnaire is seen in Sweden as constituting consent. Two prior reports have examined this fear data from the T-CHAD study. One examined results only at age 8–9 (Lichtenstein & Annas, Reference Lichtenstein and Annas2000) and a second examined the developmental patterns of situational, animal and blood-injury fears examined one at a time in univariate models that combined reports from twins and parents across all four ages (Kendler et al. Reference Kendler, Gardner, Annas, Neale, Eaves and Lichtensteinin press).
Zygosity determination
Zygosity determination was based on well validated questions to both twins and parents chosen by a discriminant analysis of 106 same-sex pairs from this sample that had their zygosity determined by typing 16 polymorphic DNA markers (Lichtenstein et al. Reference Lichtenstein, Tuvblad, Larsson and Carlstrom2007). Those with uncertain scores were classified as zygosity unknown.
Measures
We used a fear questionnaire developed by Fredrikson et al. (Reference Fredrikson, Annas, Fischer and Wik1996) that asked the twins to rate the intensity of their fear for specific objects or situations on a scale ranging from 0 (no fear) to 10 (maximal fear). An English translation of these items is given in Table 1.
Table 1. Factor loadings of individual fears at age 16–17

Bold indicates the factor to which the individual fear was assigned.
Statistical methods
For these analyses of fears, we began with 2717 twin individuals of whom 199 had unknown zygosity and 114 were completely missing data on self-report fears. The remaining 2404 individuals came from 1182 complete pairs and 40 single twins. Of these pairs, 242 were female monozygotic (MZ), 182 were female dizygotic (DZ), 240 were male MZ, 168 were male DZ, and 390 were opposite-sex DZ pairs.
The model used in these analyses is presented in Fig. 1 which illustrates the additive genetic component. As in other twin analyses, we decompose the sources of variance in fears into three components: additive genetic effects (A), shared environment (C), and individual-specific environment (E). The model has three major features. First, it contains one general fear factor for each assessment age (13–14, 16–17 and 19–20 years), here called, for simplicity, times 1, 2 and 3. This general (or common) factor captures those influences that impact on all four specific fears: animal, blood-injury, situational, and social. These general fear factors are in turn influenced by genetic and environmental factors parameterized as a trivariate Cholesky decomposition. The first additive genetic factor (AGF1) influences general fear across all three time periods. The second and third additive genetic factors (AGF2 and AGF3) influence general fear across, respectively, the second and third time periods, and only the third time period. If genetic risk factors for the general level of fearfulness were highly stable over time, the paths from AGF1 to the fear factors (paths AGF11, AGF12 and AGF13 in Fig. 1) would be large and the paths from AGF2 (paths AGF22 and AGF23) and AGF3 (path AGF33) would approach zero. If the genetic risk factors for general fearfulness were developmentally dynamic, then we would expect substantial estimates of the paths from AGF2 and AGF3 representing newly arising genetic influences. The structure of shared and unique environmental influences on the general fear factor would be similarly parameterized. Second, the model contains paths from the general fear factors at each age to the individual fears. These paths are permitted to vary across the three times of measurement. The magnitude of these paths reflects the degree to which individual variation in the specific fears result from differences in the level of general fearfulness. Third, the model contains genetic and environmental influences unique to each fear. These factors are also modeled as trivariate Cholesky decompositions. Thus, we have for additive genetic influences unique to animal fears, one factor (AAni1) that influences animal fears across all three time periods (via paths AAni11, AAni12, and AAni13 in Fig. 1), a second factor (AAni2) that uniquely influences animal fears at the second and third time periods (via paths AAni22 and AAni23), and AAni3 that specifically influences only animal fears and only at the third time period (via path AAni33). As with the general factor, if the genetic factors that impact specifically on fears of animals are highly stable over time, we would expect paths from AAni1 to be much larger than those seen with AAni2 or AAni3. Developmentally dynamic genetic influences specific to animal fears would be seen as substantial loadings on paths from AAni2 or AAni3. The structure of shared and unique environmental influences on specific fears would be similarly parameterized.

Fig. 1. Genetic components of the full model fitted to self-report measures of animal (Ani), blood-injury (Bld), situational (Sit) and social (Soc) fears at ages 13–14 (time 1), 16–17 (time 2) and 19–20 (time 3). For full details see text. The model includes both a general fear factor and specific effects on each individual fear at each time-point. Genetic and environment effects on the general and specific fears are modeled as a trivariate Cholesky decomposition with a first factor accounting for effects over the three waves of assessment, a second factor accounting for effects at times 2 and 3 and a third factor that impacts only at time 3. For the factor and path names, the first number refers to the factor. For the path names only, the second number refers to the time period on which it is acting. In the path and factor name ‘G’ stands for general (as in ‘general’ or common factor) and ‘A’ stands for additive genetic. So, AGF1 is the first additive general factor. Path AGF13 is the path from that first additive general factor that acts at the third time period, i.e. age 19–20 and path AAni12 is the first additive factor specific to animal phobias at the second time period (age 16–17).
A model of this complexity (with five Cholesky decompositions, one for the common latent factor overlying the measurement model and one for each of four specific fears) has a very large number of potential submodels. We decided, a priori, to only test for ‘qualitative’ and ‘quantitative’ sex effects, and for the influences of genetic and environmental factors on the general fear factors, and globally for all effects specific to each fear. Qualitative sex effects permit genetic factors that influence a trait to be at least partially distinct in males and females. In this case it was implemented by allowing the genetic correlation in opposite-sex twins to be estimated for each of the five Cholesky decompositions rather than fixing them at 0.5. Quantitative sex effects permit the same genetic factors to impact to different degrees in the two sexes. This was implemented by allowing all path coefficients to be estimated separately by sex. This is not identical to the scalar sex limitation model described by Neale et al. (Reference Neale, Roysamb and Jacobson2006) as it does not constrain the genetic correlation matrices to be the same in males and females. We did not fit a model setting E specifics to zero since this would assume, unrealistically, that we measured the level of individual fears without error.
Twin analyses were performed using the Mx software package (Neale et al. Reference Neale, Boker, Xie and Maes2003). Levels of fear were treated as a continuous trait. Because the sum scores for the items identified by factor analyses had a Poisson-like distribution, we did a square-root transformation followed by standardization separately for each sex, age and zygosity group. As a typical example, for animal fears, the percentage of the sample with reports at 0, 1, 2 and 3 time-points was, respectively, 0.3, 12.0, 34.4 and 53.3%.
Due to the large sample sizes and the large parameter spaces involved in these models, the Bayesian Information Criterion (BIC; Schwarz, Reference Schwarz1978) was used for model comparison as it is less prone to overfitting than is Akaike's Information Criterion (AIC) in these circumstances (Markon & Krueger, Reference Markon and Krueger2004). The lower the BIC value, the better the balance of explanatory power and parsimony.
Results
Factor structure and within- and cross-age phenotypic correlations
Varimax factor loadings for the individual fears as reported at age 16–17 are seen in Fig. 1 and a very similar pattern was seen at the other ages. Four factors were easily identified which we labeled, respectively, situational, social, animal and blood-injury fears. All items loaded clearly on one factor with the exception of fear of dogs which was not included in further analyses.
The correlations in levels of self-report animal, blood-injury, situational and social fears both within and across ages are seen in Table 2. As might be expected, within-age cross-fear and within-fear cross-age correlations were consistently higher than cross-fear cross-age correlations. In addition, the correlations between individual fears within each age tended to decline with increasing age.
Table 2. Within- and cross-age phenotypic correlations for animal (Ani), blood-injury (Bld), situational (Sit) and social (Soc) fears

Twin correlations
The within-occasion twin correlations for all four fears across the three times of assessment are given in Table 3. Four trends are noteworthy. First, with a single exception (social fear in male–male twins at age 19–20), correlations in MZ twin pairs exceeded those observed in DZ pairs suggesting a genetic effect on fears. Second, there was no consistent trend for correlations to be greater in male–male versus female–female pairs. Third, correlations in opposite-sex DZ pairs tended to be lower than those observed in same-sex pairs, although this was consistently seen only in animal fears. Fourth, correlations declined with advancing age, somewhat more consistently in female–female than in male–male pairs.
Table 3. Within occasion twin correlations for animal, blood-injury, situational and social fears at ages 13–14, 16–17, and 19–20

MZ, Monozygotic; DZ, dizygotic.
Model fitting
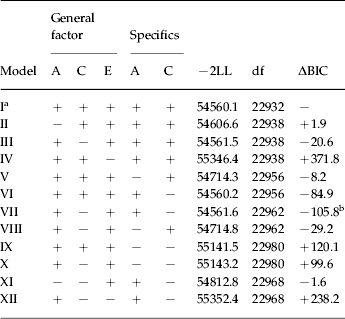
We began with a full model with no quantitative or qualitative sex effects that contained A, C and E impacting on the general fear factors and A, C and E acting on the specific fears [−2 log likelihood (−2LL)=54560.1, df=22932, BIC=−54223.1]. This model fit modestly better by BIC than the same model that included qualitative sex effects (−2LL=54528.4, df=22927, ΔBIC=+1.9) and much better than the same model with either quantitative sex effects alone (−2LL=54374.3, df=22833, ΔBIC=+259.0) or both qualitative and quantitative sex effects (−2LL=54370.8, df=22828, ΔBIC=+275.0).
We proceeded to more specific model fitting (as outlined in Table 3) comparing results to those obtained for the full model without sex effects (model I). In models II–VI, we systematically set to zero each of the A, C and E components (either for the general factor or the specific fears) one at a time. By BIC, the model fit disimproved when we set the A or E influences on the general factor to zero, improved modestly when the A specifics were set to zero and improved substantially when either the C general factor or the C specifics were constrained to zero.
In models VII–IX, we took the three components that when individually set to zero improved the model fit and set them to zero two at a time. As seen in Table 4, by far the best fit was obtained when both the C general factor and C specifics were constrained to zero (model VII). We then tried to improve on model VII by additionally setting to zero the A specifics (model X), the A general factor (model XI) and the E general factor (model XII). None of these approached model VII in their BIC value, thus rendering this our best-fit model.
Table 4. Multivariate model fitting results for animal, blood-injury, situational and social fears assessed at ages 13–14, 16–17, and 19–20
−2LL, −2 log likelihood; df, degrees of freedom; ΔBIC, change in the Bayesian Information Criterion.
Results in this table do not always sum to unity because of rounding errors.
a Absolute BIC=−54223.1.
b Best-fit model.
Parameter estimates of best-fit model
The parameter estimates for additive genetic and individual-specific environmental effects in our best-fit model VII are seen in Fig. 2 (a and b), respectively. Focusing first on the general factors, four observations are worthy of comment. First, genetic influences on general fear decline over time, with heritability estimated to be 64% at age 13–14, 56% at age 16–17 and 47% at age 19–20. Second, genetic effects on the general fear factor demonstrated evidence both for stable and developmentally dynamic risk. The first common genetic factor (AGF1) accounts for 60% of the genetic effect at age 16–17 (34% out of a total of 56%) and 56% at age 19–20 (27% out of a total of 47%). Conversely, important new risk factors for general fear, not evidenced at age 13–14, were seen at both ages 16–17 and 19–20. Third, the loading of the four individual fears on the general factor was relatively stable over time. At each time-point, the loading was highest on situational fear, lowest on social fear and intermediate on blood and animal fears. Fourth, the general fear factor accounted for progressively less of the variance of the individual fears over time with loadings declining consistently for animal, blood-injury and social fears but not situational fears.

Fig. 2. (a) Parameter estimates for additive genetic effects derived from the best-fit model VII in Table 3. (b) Parameter estimates for individual-specific environmental effects derived from the best-fit model VII in Table 3. In the path name ‘E’ stands for individual-specific environment so EGF1 stands for the first general individual-specific environmental factor and Ebld1 stands for the first individual-specific environmental factor specific to blood-injury fears. (See Fig. 1 legend for abbreviations used.)
The total heritability estimates for each of the four specific fears as well as the sources of those genetic effects are shown in Table 5. For example, for animal fears, the total heritability at age 13–14 was 56%. This came from two sources – 26% from genetic effects specific to animal fears at age 13–14 and 30% from the common factor. At age 16–17, the results are a bit more complex. The total heritability declined modestly to 53%. A total of 29% comes from specific effects and 24% from the common factor. However, the 29% from specific effects itself has two sources – 22% from genetic factors that began at age 13–14 and are having a continued effect up to age 16–17, and 7% from genetic factors that are new at age 16–17. At age 19–20, the results are yet more complicated. Total heritability for animal fears has declined further to 45%, of which 29% comes from specific genetic effects and only 16% from the common genetic fear factor. The genetic effects of 29% from specific effects now has three sources – 19% from genetic factors that began at age 13–14 and are having a continued effect at age 19–20, 8% from genetic factors that began at age 16–17 and are impacting at age 19–20, and only 2% from genetic effects specific to animal fears at age 19–20.
Table 5. Estimates of and sources for additive genetic effects on animal, blood-injury, situational and social fears

T1, Contribution from time 1 (age 13–14); T2, contribution from time 2 (age 16–17); T3, contribution from time 3 (age 19–20).
Results in this table do not always sum to unity because of rounding errors.
Three patterns of findings in Table 5 are worth emphasizing. First, as seen with the general fear factor, heritabilities for all four specific fears declined with advancing age. Second, for animal, blood and social fears, genetic effects shared with other fears declined more rapidly with age than did genetic effects specific to the individual fears. For example, for animal fears at age 13–14, 30/56=54% of the genetic effects came from the general fear factor. The comparable figure at age 19–20 was 16/45=36%. However, this was not the case with situational fear where the proportion of genetic effects resulting from the common fear factor was slightly higher at age 19–20 (71%) than age 13–14 (67%). Third, the degree of cross-time continuity in the fear-specific genetic effects differed widely across the four specific fears. Animal fears demonstrated the highest level of continuity in genetic risk – 66% of genetic effects specific to this fear in early adulthood resulted from genetic influences evident at age 13–14. Social fears had the lowest degree of continuity over time. Only 30% of the specific genetic effects at age 19–20 resulted from genetic influences that began to be evident at age 13–14. Particularly striking was the large set of new genetic influences specific to social fear that ‘came on-line’ at age 19–20. The degree of continuity in the specific genetic risk factors for situational and blood-injury fears fell in between these two extremes.
Estimates of the individual-specific environmental effects in the best-fitting model VII are shown in Table 6. Two patterns of findings are noteworthy. First, compared to the genetic effects seen in Table 5, a higher proportion of unique environmental effects are specific to the individual fears. However, our results indicate that some environmental experiences unique to individual twins influence the general level of fearfulness and these experiences impact most strongly on situational and least strongly on social fears. Second, environmental effects specific to individual fears demonstrate less temporal continuity than the specific genetic effects. Although modest, unique environmental experiences specific to the individual fears are most temporally stable for situational fears and least for social fears.
Table 6. Estimates of and sources for unique environmental effects on animal, blood-injury, situational and social fears

T1, Contribution from time 1 (age 13–14); T2, contribution from time 2 (age 16–17); T3, contribution from time 3 (age 19–20).
Discussion
These analyses sought to clarify the pattern of genetic and environmental influences on the development, from early adolescence to young adulthood, of factors unique to and shared among the most commonly experienced classes of fears. Of the many particular results of our analyses, we would emphasize six. First, no strong statistical evidence emerged for quantitative or qualitative sex effects or shared environmental effects on these fears. However, it is worth nothing that the level of statistical evidence against qualitative sex effects is modest. Second, we found a robust common factor that had a relatively stable structure over time with highest loadings on situational and lowest loadings on social fears. Third, although both the common and specific genetic influences on fears declined in importance with increasing age, for animal, blood-injury and social fears the common genetic influences declined more rapidly. That is, with increasing age, genetic influences on fears tended to become more specific in their effect. Fourth, the cross-time continuity in the specific genetic effects differed widely across the fears being highest for animal and lowest for social fears. Social fears had the largest ‘burst’ of specific genetic effects coming in the transition from late adolescence to early adulthood. Fifth, environmental factors unique to individuals impacted both on the general fear factor as well as on the specific fears. That is, our modeling gives the sensible clinical expectation that some traumatic experiences in children will result in a general state of heightened fear for most stimuli while other experiences produce elevations in only specific classes of fears. Compared to the genetic effects, the impact of the unique environment was more time specific. This would be expected because the unique environmental effects specific to individual fears are confounded with errors of measurement which would, by definition, be time-specific in their impact. Nonetheless, we did find unequivocal evidence for enduring specific environmental effects both at the level of the general fear factor and at the level of the specific fears.
Our findings can be usefully viewed in the context of three prior cross-sectional multivariate studies of fears and phobias. Lichtenstein and Annas examined fears as reported by parents in this same sample when they were aged 8–9 years (Lichtenstein & Annas, Reference Lichtenstein and Annas2000). Data were only available at that age for animal, situational and mutilation fears (equivalent to our blood-injury fears). Consistent with our findings, a trivariate analysis revealed that situational fears loaded most strongly on the common fear factor. However, unlike our results, they found substantial evidence for shared environmental factors. Loadings on our common factor are not very similar to those found for clinically defined phobias assessed at personal interview in adult twins from Virginia. In both females (Kendler et al. Reference Kendler, Neale, Kessler, Heath and Eaves1992) and males (Kendler et al. Reference Kendler, Myers, Prescott and Neale2001), animal rather than situational phobia loaded most strongly on the common genetic factor (although in males, as in our sample, social phobia had the lowest genetic common factor loading; Kendler et al. Reference Kendler, Myers, Prescott and Neale2001). As in the present study, no evidence was found for shared environmental effects for clinical phobias in the female sample (Kendler et al. Reference Kendler, Neale, Kessler, Heath and Eaves1992). Shared environmental effects were detected on the general phobia factor in males, but the loadings were quite modest on animal, blood-injury, situational and social phobias (Kendler et al. Reference Kendler, Myers, Prescott and Neale2001). A strength of the present study is its longitudinal design which suggests that shared environmental effects are at most of modest importance for the development of fears in early adulthood. Nevertheless, the available studies differ in ages, assessment methods and informants, and we would like to be cautious in drawing too definitive judgment regarding absence of shared environmental effects in adult fears and phobias
The total heritability of our fear measures (ranging from 49% to 56% at age 13–14 and 34–45% at age 19–20) are somewhat higher than those found for clinically diagnosed phobias in the general population Virginia Twin Studies (Kendler et al. Reference Kendler, Neale, Kessler, Heath and Eaves1992, Reference Kendler, Myers, Prescott and Neale2001) and for self-report fears in a study of twins and their families (Phillips et al. Reference Phillips, Fulker and Rose1987), but broadly similar in magnitude to those found for self-report levels of fears in adult twin sample from Norway (Skre et al. Reference Skre, Onstad, Torgersen, Philos, Lygren and Kringlen2000) and Australia (Page & Martin, Reference Page and Martin1998) and for parent-rated fears in 12-year-old British twins (Stevenson et al. Reference Stevenson, Batten and Cherner1992).
Our results can also be usefully compared to our prior analyses examining one fear at a time incorporating both parental and self-reports (Kendler et al. Reference Kendler, Gardner, Annas, Neale, Eaves and Lichtensteinin press). These analyses – which were restricted to situational, animal and blood-injury fears because information on social fears was lacking from the age 8–9 assessment – also showed convincing evidence for a developmentally dynamic genome although we were unable to discriminate those aspects that related to the common versus fear-specific factors. These analyses did find evidence for qualitative sex effects for animal and blood-injury but not for situational fears as well as shared environmental effects – although the latter declined rapidly with age. We did not detect the sex effects with our single more complete model and our inability to find shared environmental effects likely results from our exclusion of the first time-point (age 8–9) for which we had only parental ratings.
Our finding of declining levels of heritability of fears from early adolescence to early adulthood was unexpected given evidence from a recent meta-analysis that in this age range heritability typically increases for symptoms of internalizing and externalizing disorders (Bergen et al. Reference Bergen, Gardner and Kendler2007). However, this pattern of results might emerge because fears have a different developmental trajectory than other internalizing symptoms. For example, while rates of depression and anxiety typically increase from early adolescence into early adulthood (and then slowly decline with further aging) (Jorm, Reference Jorm2000), mean rates of fears tend to decline over adolescence (Marks, Reference Marks1987) and this is the pattern seen for all the fears in this data.
Declining heritability for fears over time could arise from two major processes: declining genetic variance or increasing environmental variance. A preliminary examination of the within-time unstandardized variances in fear scores, decomposed into their genetic and environmental components, supports the first of these hypotheses. The raw genetic variance for all four specific fears declined over time while the individual-specific environmental variances were stable (for blood-injury, situational and social fears) or increased modestly (for animal fears).
We found two developmental patterns of the genetic risk factors for fear to be particularly intriguing. First, as individuals aged, genetic effects of fears became slowly more specific in their effect with the general fear factor accounting for a declining amount of the total genetic variance. It remains to be seen what neurobiological or psychological processes might be responsible for the increasing ‘specialization’ of these genetic systems that subserve fear. Our results may reflect a general trend, observed in a recent long-term follow-up study, for increasing specificity amongst the anxiety disorders with aging (Gregory et al. Reference Gregory, Caspi, Moffitt, Koenen, Eley and Poulton2007).
Second, our analytic methods permitted us to examine the continuity and discontinuity of genetic effects unique to individual fears. It is particularly interesting to contrast the pattern seen in Table 5 for animal versus social fears. At both ages 16–17 and 19–20, the large proportion of genetic effects for animal fears was that present at age 13 with little evidence for new genetic effects. For social fears, the exact opposite pattern is seen. Most of the specific genetic effects at these two ages are new and were not phenotypically manifest in early adolescence.
Animal fears and phobias typically arise considerably earlier than do social fears (Marks, Reference Marks1987; Kendler et al. Reference Kendler, Neale, Kessler, Heath and Eaves1992). Our results would be consistent with the following hypothesis. The developmental period during which mild levels of animal fears become maximally adaptive is in childhood or early adolescence while for social fears, this period is delayed until later in adolescence. If this were true, evolution might, by the mechanism of stabilizing selection (Hartl, Reference Hartl1980), have shaped genetic effects for social fears to become manifest years after those seen for genetic effects on animal fears.
Situational fears had the highest loading on the general fear factor at all three times of assessment. Furthermore, unlike animal, blood-injury and social fears, the proportion of genetic effects due to the general factor did not decline over time. From early adolescence to early adulthood, situational fears appear to index, better than any other kind of fear, the general level of fearfulness. Furthermore, this pattern was largely a result of genetic factors.
Psychiatric genetics in general and gene-finding efforts in particularly have largely assumed a static genome – that genetic effects on psychiatrically relevant traits are temporally stable. The findings of this study, consistent with results for a range of phenotypes including antisocial behavior (Jacobson et al. Reference Jacobson, Prescott and Kendler2002; Silberg et al. Reference Silberg, Rutter, Tracy, Maes and Eaves2007), cognitive abilities (Cardon et al. Reference Cardon, Fulker, DeFries and Plomin1992), disordered eating (Klump et al. Reference Klump, Burt, McGue and Iacono2007), plasma lipids (Nance et al. Reference Nance, Bodurtha, Eaves, Hewitt, Maes, Segrest, Meyer, Neale and Schieken1998; Middelberg et al. Reference Middelberg, Martin and Whitfield2006) and weight (Fischbein et al. Reference Fischbein, Molenaar and Boomsma1990; Fabsitz et al. Reference Fabsitz, Carmelli and Hewitt1992), suggest that the assumption of a static genome may not be a safe one. That is, genes influencing a trait at one age need not be the same as those which impact on the same trait at a later time period.
Limitations
These results should be considered in the context of four potentially important methodological limitations. First, results from this sample – a single birth cohort from Sweden – might not extrapolate to other ethnic groups. Second, our sample was not sufficiently large to give us a great deal of power to detect sex effects (Prescott & Gottesman, Reference Prescott and Gottesman1993) or to detect modest shared environmental influences in the setting of significant genetic effects (Martin et al. Reference Martin, Eaves, Kearsey and Davies1978). Our inability to detect sex or shared environmental influences should not be regarded as strong evidence for their absence. Third, we have self-report measures of fear only over a relatively limited age period – age 13–20. This age range is surely insufficient to detect all the important developmental processes in the formation of fears in particular missing the very important period of early childhood (Marks, Reference Marks1987). Fourth, our model was too complex to permit confidence intervals to be estimated in realistic run times. Therefore, our assessments of increasing and decreasing parameter estimates were necessarily qualitative and may or may not represent statistically significant changes.
Declaration of Interest
None.
Acknowledgements
This study was supported in part by NIH grants MH068643, MH-65322, and the Swedish Council for Working Life and Social Research (project 2004-0383) and the Swedish Research Council (2004-1415).