Introduction
Cryptosporidium parvum is an enteric protozoan that causes diarrhoea in both immunocompetent and immunocompromised hosts. Cryptosporidiosis is a potentially life-threatening disease in deeply immunocompromised patients, contributes significantly to morbidity among children in developing countries especially if they are malnourished and is now recognized as an important global health concern (Checkley et al., Reference Checkley, White, Jaganath, Arrowood, Chalmers, Chen, Fayer, Griffiths, Guerrant, Hedstrom, Huston, Kotloff, Kang, Mead, Miller, Petri, Priest, Roos, Striepen, Thompson, Ward, Van Voorhis, Xiao, Zhu and Houpt2015). Cryptosporidium can extend to mucosal epithelial surfaces of other organs than intestine in approximately 20% of immunocompromised patients (Chen et al., Reference Chen, Keithly, Paya and LaRusso2002; Hunter and Nichols, Reference Hunter and Nichols2002; Checkley et al., Reference Checkley, White, Jaganath, Arrowood, Chalmers, Chen, Fayer, Griffiths, Guerrant, Hedstrom, Huston, Kotloff, Kang, Mead, Miller, Petri, Priest, Roos, Striepen, Thompson, Ward, Van Voorhis, Xiao, Zhu and Houpt2015) and respiratory cryptosporidiosis has been documented in up to a third of children presenting with diarrhoea (Mor et al., Reference Mor, Tumwine, Ndeezi, Srinivasan, Kaddu-Mulindwa, Tzipori and Griffiths2010; Sponseller et al., Reference Sponseller, Griffiths and Tzipori2014). These clinical situations justify the use of a parenteral anticryptosporidial agent. Efforts have been initiated to find drugs with anticryptosporidial activity that are both safe and effective in all clinical situations (Chavez and White, Reference Chavez and White2018), but currently nitazoxanide (NTZ), first-in class thiazolides, remains the only drug approved for the treatment of diarrhoea due to cryptosporidiosis in children and adults in the USA and in many countries of Latin-America, the Middle East, Africa and Asia. NTZ is a synthetic nitrothiazolyl salicylamide which is deacetylated in the gastrointestinal tract into tizoxanide (TIZ) its effective circulating drug in vivo (Broekhuysen et al., Reference Broekhuysen, Stockis, Lins, De Graeve and Rossignol2000; Rossignol et al., Reference Rossignol, Ayoub and Ayers2001). NTZ is recognized for its record of safety (The Medical Letter, 2013), but has limited effectiveness in malnourished children and in deeply immunocompromised individuals although the reasons for NTZ failure are not fully understood (Sparks et al., Reference Sparks, Nair, Castellanos-Gonzalez and White2015). NTZ is only partially absorbed from the gastrointestinal tract (Broekhuysen et al., Reference Broekhuysen, Stockis, Lins, De Graeve and Rossignol2000) and therefore has not an ideal biodisposition profile for treating a disseminated cryptosporidiosis. Efforts have, therefore, been made to go to better solubility of NTZ in trying to keep the active moiety of the drug. A new amino ester derivative, aminoxanide, has been developed which is, like NTZ, a pro-drug that delivers TIZ and which is water-soluble enough to be given by injection. Toxicity studies have been completed in dogs and rats with no adverse effects identified (Stachulski et al., Reference Stachulski, Swift, Cooper, Reynolds, Norton, Slonecker and Rossignol2017). Our study describes the activity of this l-tert-leucyl thiazolide against C. parvum infection. As a secondary objective, this study aimed to determine whether the systemic administration of TIZ, in the form of aminoxanide, could improve its efficacy compared to its oral administration, in the form of NTZ, and contribute to our understanding of the mechanism of action of thiazolides against Cryptosporidium infection. In order to meet these aims, we evaluated the efficacy of aminoxanide against C. parvum in vitro development in HCT-8 cells and then we compared its efficacy to NTZ in an immunosuppressed Mongolian gerbil (Meriones unguiculatus) model of C. parvum infection. The data presented here show that aminoxanide is not only effective in vitro against C. parvum but also in vivo with a comparable efficacy between a dose of 100 mg kg−1 day−1 for 5 days of aminoxanide administered parenterally and a dose of 400 mg kg−1 day−1 of NTZ given orally.
Materials and methods
Test agents
NTZ, TIZ and aminoxanide (l-tert-leucyl thiazolide) (Fig. 1) were supplied by Romark Laboratories L.C., Tampa, FL. For in vitro experiments, the test compounds were solubilized in dimethylsulphoxide (DMSO) at a maximum concentration of 5 g L−1 and stored at −20 °C until use. Final concentrations tested in cultures were 0.1, 1, 5 and 10 mg L−1 corresponding to a maximum DMSO concentration of 0.2% (v/v). Since NTZ spontaneously hydrolyses to TIZ in aqueous media alone and because aminoxanide is a prodrug of TIZ, for in vitro experiments, we chose to compare in vitro efficacy of aminoxanide with that of TIZ excluding that of NTZ since its in vitro efficacy has already been published (Gargala et al., Reference Gargala, Delaunay, Li, Brasseur, Favennec and Ballet2000).
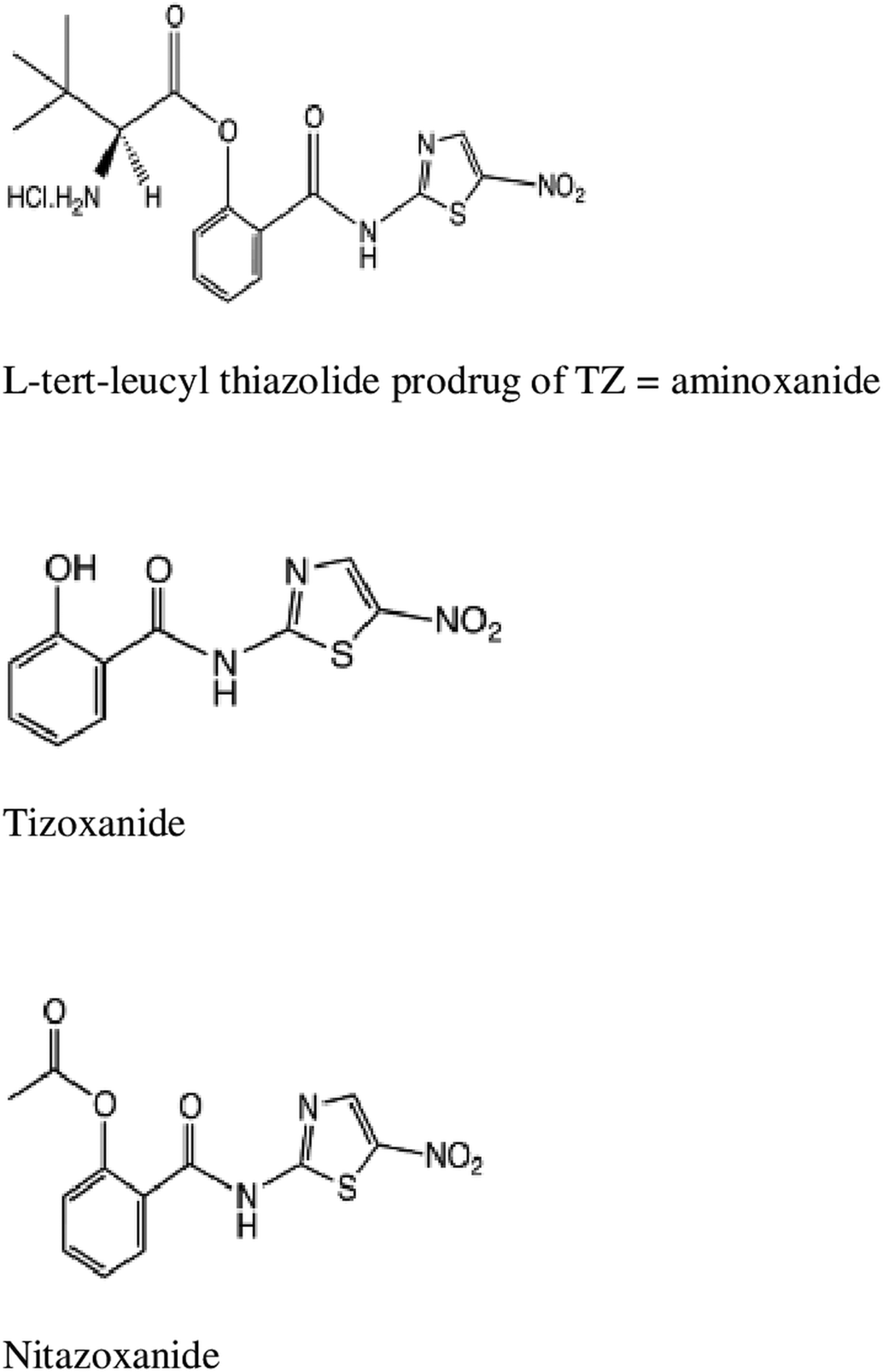
Fig. 1. Chemical structures of aminoxanide, TIZ and NTZ.
Origin of parasites
Cryptosporidium parvum oocysts of the Nouzilly strain were purified from feces obtained from calves experimentally infected with an isolate maintained by Laboratoire de Pathologie Aviaire, Institut National de Recherche Agronomique, Nouzilly, France. Feces were stored in a 2.5% K2Cr2O7 solution for less than 3 months and oocysts were isolated using diethyl ether and discontinuous sucrose density gradient (densities: 1.045 and 1.090) and were bleached before use (either before HCT-8 cell culture infection or before gerbil gavage).
In vitro infection of HCT-8 cells by C. parvum and assessment of agents' activities
The in vitro system has been previously used to screen compounds for their activity against C. parvum (Gargala et al., Reference Gargala, Baishanbo, Favennec, François, Ballet and Rossignol2005). HCT-8 cells (ATCC CRL 244, ATCC, MD, USA) were cultured to >80% confluence in 16-well tissue culture chambers (Labteck Chamber Slides, Nunc) in RPMI-1640 medium supplemented with final concentrations of 5% FCS, 1% non-essential amino acids, 100 000 units mL−1 of penicillin G and 100 mg L−1 of streptomycin. Cryptosporidium parvum oocysts were permitted to excystate in taurocholic acid (1.5%, vol/vol) for 90 min at 37 °C. Sporozoite suspensions were obtained after sieving through a 5 μm nucleopore filter and 2.5 to 5 × 105 freshly excysted sporozoites were added to each HCT-8 monolayer-containing well. Two hours later, culture supernatants containing unpenetrated sporozoites were removed and replaced by agent-containing or agent-free medium. The time-point for measurement of agent-induced growth inhibition was 48 h after adding parasite. Monolayers were fixed with methanol and C. parvum development was assessed by indirect immunofluorescence using a polyclonal hyperimmune rat serum. The inhibitory activity of the tested compounds was calculated as:

The 50% inhibitory concentration (IC50) was defined as the concentration of agent which resulted in a mean 50% inhibition of C. parvum development and values for aminoxanide and TIZ were determined using non-linear regression analysis (GraphPad Prism software, version 7.01). A 90% inhibitory activity (IC90) was similarly given. The mean IC50 and IC90 per compound are expressed as average values (mean and s.d.) from three independent experiments with 4 to 6 wells/concentration per experiment.
Host cell toxicity assay
Agent-induced effects on the viability of confluent HCT-8 cells were monitored by light microscopic inspection and a tetrazolium assay as previously described (Gargala et al., Reference Gargala, Le Goff, Ballet, Favennec, Stachulski and Rossignol2009). Briefly, HCT-8 cells were grown to >95% confluence in flat-bottomed 96-well microtitre plates thus limiting cell cycle progression, a prerequisite for thiazolide-induced cell death in rapidly dividing cells (Müller et al., Reference Müller, Sidler, Nachbur, Wastling, Brunner and Hemphill2008). Compound concentrations used for anticryptosporidial testing were added, and assay plates were incubated at 37°C under 5% CO2 for 48 h. To determine the 50% cytotoxic concentration for TIZ and aminoxanide (CC50 = drug concentration that reduced the cell viability by 50% compared to the cell-only control), the effect of agents on HCT-8 cell viability was determined using the CellTiter 96 AQueous non-radioactive cell proliferation assay kit (Promega, Madison, WI) according to the manufacturer's instructions. Each dilution was examined in triplicate. A selectivity index (SI) was calculated as the ratio of the C. parvum IC50 to the host cell CC50.
Animals
Six-week-old male and female Mongolian gerbils (M. unguiculatus) weighing 30–40 g were purchased from Janvier Labs (Le Genest St., Isle, France). Animals were group housed upon receipt in plastic cages protected by another top wrapped with sterile paper in order to comply with level II contamination requirements. Animals were handled according to the technical and ethical regulations of the French Ministry of Agriculture. All animals had access to water ad libitum. Animals were acclimated for 5–8 days prior to onset of immunosuppression.
Immunosuppression of Mongolian gerbils
Gerbils were immunosuppressed by a low-protein diet (white bread ad libitum) and intraperitoneal injection of dexamethasone (Qualimed, Puteaux, France) at a 25 mg kg−1 dose every 2 days for nine consecutive days before infection. This immunosuppression regimen was applied until the end of the experiment.
Route of administration of treatment to gerbils
NTZ given orally was suspended in DMSO. Aminoxanide was suspended in 0.9% NaCl for oral gavage or for parenteral (intramuscular or intraperitoneal) injection. The dose of 400 mg kg−1 of NTZ was selected based on results obtained in previous C. parvum infection efficacy studies in rat or gerbil models of C. parvum infection (Li et al., Reference Li, Brasseur, Agnamey, Leméteil, Favennec, Ballet and Rossignol2003; Gargala et al., Reference Gargala, François, Favennec and Rossignol2013). The dose of 50 mg kg−1 diluted in a volume of 0.05 mL of 0.9% NaCl for each twice-daily injection was chosen according to the maximum injectable volume tolerable by the quadriceps of the gerbil.
Study design
Two sets of experiments were performed in which inoculated oocyst number, onset and duration of treatment were similar. On day 0, each immunosuppressed gerbil received 2 × 105 C. parvum oocysts by oral gavage and allowed to develop infection for 24 h before treatment with the test drugs. On day 1 p.i., the animals were divided into treatment groups. The first experiment was conducted to test efficacy of aminoxanide only, to explore feasibility of its parenteral administration and to compare efficacy of parenteral with that of oral administration. One group of four infected gerbils was treated with a 200 mg kg−1 oral dose of aminoxanide, one group of four infected gerbils treated with a 20 mg kg−1 dose of intraperitoneal aminoxanide and one infected positive control group received orally administered DMSO. The second set of experiments was designed to confirm efficacy of parenteral aminoxanide and to compare it to that of NTZ. A first group of five gerbils was treated with a 400 mg kg−1 dose of NTZ given orally, a second group of six gerbils received a 100 mg kg−1 dose of aminoxanide given by intramuscular injection, a third group of six gerbils received a 100 mg kg−1 dose of intraperitoneal injected aminoxanide, a fourth group (vehicle group) of six infected gerbils received oral DMSO and was used as positive control and a fifth group was not-infected and not-treated, receiving only oral DMSO. Sixteen hours after they received the last dose of treatment, gerbils were anaesthetized by intraperitoneal injection of xylazine (Rompun®) and ketamine before bleeding via cardiac puncture. Gerbils were euthanized for quantitation of oocyst excretion in the caecal and colonic contents and for evaluation of mucosal C. parvum development and injury by histology examination.
Evaluation of agent efficacy on C. parvum by quantitation of oocyst excretion
To assess C. parvum infection in individual gerbils, feces were daily collected and oocysts were detected microscopically using the Heine staining method on a fecal smear. Briefly, observation of oocysts was made by staining fecal smears with Ziehl fuchsin and observation at 400× magnification under a phase-contrast microscope according to Heine (Reference Heine1982). The intensity of excretion was evaluated semi-quantitatively according to the average number of oocysts in 30 randomly selected fields. As in this model of C. parvum infection, gerbils do not exhibit diarrhoea, fecal excretion of oocysts may not reflect the actual intestinal C. parvum development. Thus, to finally assess the effectiveness of the test agents, the entire caecal and colonic contents were collected from each euthanized gerbil on 6th day p.i. Individual intestinal contents were homogenized vigorously for 60 s in 1.5 mL of deionized water, were suspended in 10% (w/v) formalin solution and homogenized. The suspension was vortexed for 30 s and allowed to settle for an additional 30 s. Ten percent of the suspension was diluted in phosphate-buffered saline (PBS) and centrifuged at 1800 g for 30 min. After washing, pellet was re-suspended in PBS and half of the oocyst containing pellet was incubated with an oocyst-specific monoclonal antibody conjugated with FITC (Crypt-a-Glo®, Waterborne Inc., New Orleans, LA) in the dark at 37°C for 30 min. A cytospin was prepared for each specimen, slides were mounted in buffered Mowiol (Calbiochem, La Jolla, CA), and oocysts were counted by epifluorescence microscopy at 400× magnification. All samples were analysed on the same day they were processed for immunofluorescent staining. Number of counted oocysts was expressed by 1 mL of colonic content to make comparison between gerbils in treated groups with the infected untreated control group and to calculate percent reduction of oocyst excretion.
Evaluation of histological lesions by microscopic examinations
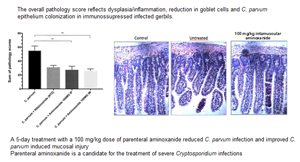
Gerbils were necropsied on 6th day p.i. and distal segments of ileum and jejunum were removed and fixed in 10% formalin, embedded in wax, processed, sectioned and stained with H&E or Giemsa. Sections were examined and scored by two independent observers blinded to the individuals in any given group. Villus heights (VH), crypt depths (CD) and all scores for each individual gerbil were acquired in jejunum and ileum sections on 20-well-oriented villus-crypt units (VCU) and were measured by light microscopy (100×) using the image analysis software Histolab 5.12.0 (Microvision, Evry, France). For each group, the VH/CD ratio was calculated as the mean VH divided by the mean CD. For scoring microscopic lesions, previously published scores (Lee et al., Reference Lee, Harwood, Girouard, Meyers, Campbell, Beamer and Tzipori2017) were applied for C. parvum colonization [percentage of villous enterocytes with at least one parasitic form on its apical surface was calculated from 20 microscopic fields (1250×) with at least 100 enterocytes observed on each field], villus changes (by measuring the VH/VCU ratio), cellular inflammation (by counting the number of infiltrated lymphocytes) and epithelial changes (percentage of goblet cells in the villus epithelium) for all samples. The scores were as follows: for C. parvum colonization, no parasite forms detected = 0; 1–40% epithelial surface infected = 1; 41–60% surface infected = 2; 61–80% surface infected = 3; 81–100% surface infected = 4; 81–100% surface infected = 5. For villus changes, ratio ⩾2.75 = 0; 2.50 ⩽ ratio < 2.75 = 1; 2.25 ⩽ ratio < 2.50 = 2; 2.00 ⩽ ratio < 2.25 = 3; 1.75 ⩽ ratio < 2.00 = 4; 1.50 ⩽ ratio < 1.75 = 5; for inflammation, 0–10 lymphocytes = 0; 11–20 = 5; 21–30 = 10; 31–40 = 15; 41–50 = 20; >50 = 25, for epithelial changes : 0–20% reduction in goblet cells = 0; 21–40% = 5; 41–60% = 10; 61–80% = 15; 81–100% = 20. The overall pathology score is an aggregate reflecting dysplasia/inflammation, reduction in goblet cells and C. parvum enterocyte colonization, for the two intestinal sites (jejunum and ileum) of each gerbil.
Statistical analysis
Considering the numbers of experimental data, the statistical comparison of the differences among experimental groups were performed using non-parametric Wilcoxon rank sums or Mann–Whitney tests (oocyst shedding, colonization and lesion scores). A P value of ⩽0.05 was considered significant. The statistical comparison of the differences among experimental groups was conducted using the statistical tools in GraphPad Prism 7.01 software (GraphPad Software, San Diego, CA, USA).
Results
Cell cytotoxicity of test agents
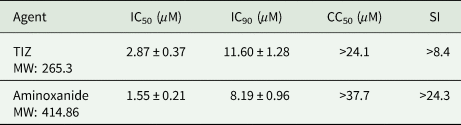
From preliminary studies, we verified that HCT-8 cell cytotoxicity was minimal after a 48 h contact with test agents. Cell viability was 85 and 86% at 1 mg L−1, 78 and 80% at 5 mg L−1, for TIZ and aminoxanide, respectively. At a 10 mg L−1 concentration, cell viability was 58 and 66%, giving a CC50 > 37.7 μ m and >24.1 μ m for TIZ and aminoxanide, respectively (Table 1).
Table 1. In vitro inhibitory concentrations (IC50) and IC90 of TIZ and aminoxanide against Cryptosporidium parvum development in HCT-8 cell cultures
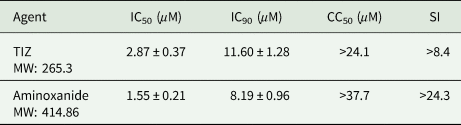
Agents were tested at concentrations ranging from 0.1 to 10 mg L−1. Inhibition was determined in a cell-based assay using confluent HCT-8 monolayers inoculated with C. parvum sporozoites. The mean IC50 and IC90 per agent are expressed as average values (mean ± s.d.) from pooled data of triplicate independent experiments. For each experiment, six culture wells were done for each drug concentration. The SI is defined as the ratio of the 50% cytotoxic concentration (CC50) to the 50% anticryptosporidial concentration (IC50). CC50 values are evaluated from three separate experiments (4 to 6 wells/concentration per experiment).
In vitro C. parvum growth inhibition by aminoxanide and TIZ
Table 1 summarizes results of in vitro experiments designed to evaluate efficacy of aminoxanide and TIZ on C. parvum development in HCT-8 cells and shows fifty percent (IC50) and ninety percent (IC90) inhibitory concentrations. Both agents were strong inhibitors of C. parvum in vitro development (maximum inhibition >99%) with an IC90 of 3.6 and 3.4 mg L−1 (11.60 and 8.19 μ m) for TIZ and aminoxanide, respectively. Not surprisingly knowing that aminoxanide is a prodrug of TIZ, aminoxanide had the same in vitro efficacy against C. parvum development as that of TIZ (P > 0.5).
In vivo efficacy of NTZ and aminoxanide against C. parvum infection in immunosuppressed Mongolian gerbils (M. unguiculatus)
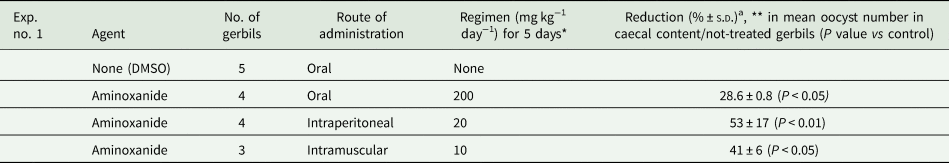
In a set of preliminary in vivo experiments whose results are detailed in Table 2, at a daily oral dose of 200 mg kg−1 for 5 days, oocyst excretion was reduced by 28.6% (±0.8) in treated gerbils compared with the untreated infected control group (P < 0.05). It is noteworthy that intraperitoneal aminoxanide at a 10-fold lower dose (20 mg kg−1) and intramuscular aminoxanide at a 20-fold lower dose (10 mg kg−1) produced a statistically significant reduction of oocyst excretion of 53% (±17) and 41% (±6), respectively, vs control infected gerbils (P < 0.01). In a second set of experiments whose results are detailed in Table 3, a daily 100 mg kg−1 i.p. or i.m. dose of aminoxanide reduced oocyst shedding by 72.1% (±13.4) and 72.5% (±2.0) (P > 0.05), respectively, compared to oocyst excretion in infected untreated control gerbils (P = 0.01, P = 0.014, respectively). NTZ exhibited a 75.5% (±13.9) mean reduction of oocyst excretion in treated gerbils vs control ones (P = 0.05) (Table 3). Treatment with i.m. or i.p. aminoxanide resulted in an oocyst shedding reduction comparable to that obtained with NTZ (P = 0.01). It is noteworthy that no animal was totally cured.
Table 2. First set of experiments: inhibitory effect of aminoxanide on C. parvum oocyst excretion in immunosuppressed infected Mongolian gerbils

a [[(Mean oocyst count in untreated animals) − (mean oocyst count in treated animals)]/(mean oocyst count in untreated animals)] × 100.
No statistically significant difference was found between oral and i.p. aminoxanide.
* Starting 24 h after infection with 2 × 105 oocysts.
** Evaluated 16 h after cessation of treatment.
Table 3. Second set of experiments: efficacy of NTZ and parenteral (i.m. or i.p.) aminoxanide on C. parvum oocyst excretion in immunosuppressed Mongolian gerbils

a [[(Mean oocyst count in untreated animals) − (mean oocyst count in treated animals)]/(mean oocyst count in untreated animals)] × 100.
* Starting 24 h after infection with 2 × 105 oocysts.
No statistical difference was found between treated experimental groups.
Gerbils were euthanized 6 days p.i. Microscopic examination of the small intestine sections including jejunum and ileum of the infected control group revealed severe alterations of the structure of the intestinal mucosa in the form of active ileitis in comparison with that of the non-infected control group. Representative photomicrographs of H&E-stained sections of the ileum from an infected and a non-infected gerbil are shown for comparison (Fig. 2A and B). Sections revealed that goblet cells (containing clear apical vacuoles) were decreased in all of the infected and not-treated gerbils, accompanied by a significant reduction in the height of the villi, infiltration of lamina propria with inflammatory cells, mainly lymphocytes while these changes did not occur in the control uninfected gerbils. Many small spherical protozoal particles (2–5 μm) were seen on the apical surface of epithelial cells from the ileal crypt epithelium of the infected gerbils. Ileum lesions from a gerbil infected with C. parvum and then treated with i.p. or i.m. aminoxanide are shown in Fig. 2D and E, respectively. A decrease in C. parvum colonization of enterocytes was attributable to NTZ or parenteral aminoxanide but certain parasitic forms are still present at the top of the villi (Figs 2B, 2C, 2D, 2E and 3A with P = 0.016 and P = 0.008 for i.p. and i.m. aminoxanide vs not-treated group, respectively), also consistent with oocyst counting in caecal and colonic contents. In the aminoxanide-treated groups (i.m. or i.p.) compared to the infected and untreated gerbils, the jejunum and ileum sections showed a drastic improvement in the C. parvum induced histological alterations consisting of a villous restoration and a less infiltration of the lamina propria by inflammatory cells (P = 0.008 for both groups of aminoxanide-treated gerbils) and goblet cell number restoration (P = 0.016 and P = 0.008 for i.p. and i.m. aminoxanide vs not-treated group, respectively) (Fig. 3B and C). The overall pathology score, an aggregate reflecting epithelial colonization (Fig. 3A), dysplasia/inflammation (Fig. 3B) and goblet cell restoration (Fig. 3C), was significantly reduced by aminoxanide treatment as compared to gerbils challenged with C. parvum alone (P < 0.01 for both i.m. and i.p. aminoxanide, Fig. 3D).

Fig. 2. Histology of the ileum of gerbils at 6 days p.i. Pieces from each ileum were fixed in 10% formalin and embedded in paraffin, and 5-μm sections were H&E stained. Representative aspects of ileal mucosa showing consistent features observed in each group: infected control gerbils (line A), infected NTZ-treated gerbils (line B), infected intraperitoneal aminoxanide-treated gerbils (line C), infected intramuscular aminoxanide-treated gerbils (line D), uninfected control gerbils (line E). Original magnification, 100× (left column); original magnification, 250× (central column); original magnification (right column). Marked villus atrophy, crypt hyperplasia and inflammatory infiltrates are seen in Cryptosporidium parvum-infected gerbils. A 5-day treatment with a daily 100 mg kg−1 dose of parenteral aminoxanide reduced C. parvum infection and improved C. parvum-induced mucosal injury. Black arrows point to typical C. parvum mucosal forms.
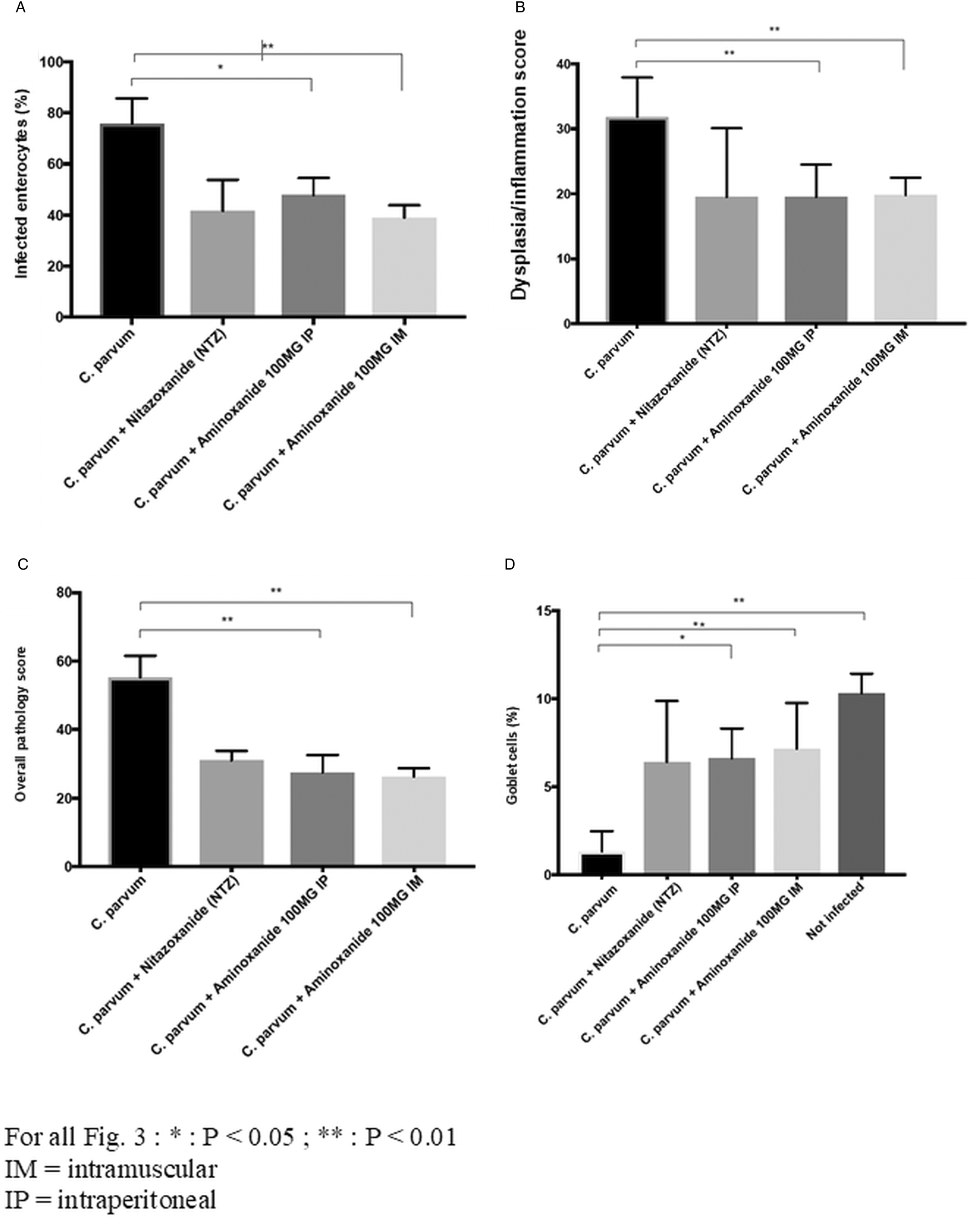
Fig. 3. Aminoxanide reduces severity of cryptosporidiosis. Aminoxanide or NTZ was administered to gerbils 1 day after C. parvum oocyst challenge (with 2 × 105 oocysts) and continued through 5 days post-challenge. At autopsy, 6 days a.i. (16 h after stopping treatment) a histological study was performed on the distal segments of the ileum and jejunum and scored. These histograms depict the scores obtained. Colonization and lesion scores were analysed using the Mann–Whitney test. *Cp for C. parvum infected. (A) C. parvum enterocyte colonization (**P < 0.01 for Cp*-not-treated vs Cp-intramuscular aminoxanide, *P < 0.05 for Cp-not-treated vs Cp-intraperitoneal aminoxanide). (B) Dysplasia/inflammation score (**P < 0.01 for Cp-not-treated vs Cp-intramuscular aminoxanide, **P < 0.01 for Cp-not-treated vs Cp-intraperitoneal aminoxanide). (C) Overall pathology score (**P < 0.01 for Cp-not-treated vs Cp-intramuscular aminoxanide, **P < 0.01 for Cp-not-treated vs Cp-intraperitoneal aminoxanide). (D) Reduction in goblet cells (**P < 0.01 for Cp-not-treated vs not-infected, *P < 0.05 for Cp-intraperitoneal aminoxanide vs not-infected, **P < 0.01 for Cp-intramuscular aminoxanide vs not-infected).
Discussion
Aminoxanide was able to significantly and dose-dependently inhibit in vitro complete development of the apicomplexan parasite C. parvum with a good toxicity profile since the IC50 for C. parvum was at least 20-fold lower than CC50 for HCT-8 cells. This absence of in vitro toxicity in the HCT-8 cell line is consistent with previous study with TIZ tested in MDCK cells (Tilmanis et al., Reference Tilmanis, van Baalen, Oh, Rossignol and Hurt2017) and its good safety profile in animal safety pharmacology and toxicology (Stachulski et al., Reference Stachulski, Swift, Cooper, Reynolds, Norton, Slonecker and Rossignol2017). Although in the first series of in vivo experiments, NTZ was not tested, previously published results using the same model interpret for less efficacy than NTZ when aminoxanide is administered orally at the same dose (i.e. 200 mg kg−1) (Gargala et al., Reference Gargala, François, Favennec and Rossignol2013). The results presented here revealed that parenteral aminoxanide at a 10-fold lower dose (i.e. 20 mg kg−1) was as effective as oral aminoxanide (at 200 mg kg−1) and this lack of efficacy after oral administration could be due to an unexpected lack of systemic absorption of the molecule from the digestive tract and a lack of biological action of the agent at the apical site of the enterocytes. Amino-acid based prodrug esters such as the antiviral agent valacyclovir have improved the oral bioavailability of acyclovir and greatly improved its aqueous solubility. Valacyclovir enters cells via the h-PEPT 1 transporter (Guo et al., Reference Guo, Hu, Balimane, Leibach and Sinko1999) and aminoxanide similarly, derivative of l-tert-leucine, which has been shown to improve intestinal absorption of TIZ is likely to enter enterocytic cells via an amino-acid transporter. As it has been shown previously in a rodent model that cryptosporidiosis was responsible for a major malabsorption of amino acids, including leucine (Barbot et al., Reference Barbot, Windsor, Rome, Tricottet, Reynes, Topouchian, Huneau, Gobert, Tome and Kapel2003; Topouchian et al., Reference Topouchian, Huneau, Barbot, Rome, Gobert, Tomé and Kapel2003), this amino-acid malabsorption is likely to be responsible for the lack of efficacy of oral aminoxanide in our model and this warrants other experiments targeting the pharmacology of aminoxanide in animal models of cryptosporidiosis. To confirm the hypothesis that control of C. parvum infection by aminoxanide would be related to systemic availability of this agent we therefore administered a 5-day treatment with an i.m. or i.p. dose of 100 mg kg−1 aminoxanide and oocyst shedding of treated gerbils was significantly reduced. Moreover, parenteral aminoxanide effectively reduced severe changes of the villous structure and intestinal mucosal inflammation induced by C. parvum and was at least as effective as NTZ given orally, although oocyst excretion was not completely inhibited. Cryptosporidium parvum resides under the apical membrane of epithelial cell in a parasitophorous vacuole and the route of drug uptake by this intracellular but extracytoplasmic protozoa is largely unknown (Griffiths et al., Reference Griffiths, Balakrishnan, Widmer and Tzipori1998; Tzipori and Griffiths, Reference Tzipori and Griffiths1998). It remains unclear whether the optimal anticryptosporidial agent should be absorbed systemically and/or retained in the gastrointestinal tract and this issue of great interest has been developed in recent papers (Castellanos-Gonzalez et al., Reference Castellanos-Gonzalez, White, Ojo, Vidadala, Zhang, Reid, Fox, Keyloun, Rivas, Irani, Dann, Fan, Dustin and Van Voorhis2013; Gorla et al., Reference Gorla, McNair, Yang, Gao, Hu, Jala, Haribabu, Striepen, Cuny, Mead and Hedstrom2014; Huston et al., Reference Huston, Spangenberg, Burrows, Willis, Wells and Van Voorhis2015; Arnold et al., Reference Arnold, Choi, Hulverson, Schaefer, Vinayak, Vidadala, McCloskey, Whitman, Huang, Barrett, Ojo, Fan, Maly, Riggs, Striepen and Van Voorhis2017; Jumani et al., Reference Jumani, Bessoff, Love, Miller, Stebbins, Teixeira, Campbell, Meyers, Zambriski, Nunez, Woods, McNamara and Huston2018). In our gerbil model, the efficacy of parenteral aminoxanide is at least equal to treatment with a 4-fold higher oral dose of NTZ. We could thus extrapolate that the anticryptosporidial activity of an orally administered dose of NTZ, rapidly deacetylated into TIZ which is also the active metabolite of aminoxanide, is only related to the systemic availability of TIZ. TIZ is extensively metabolized in the liver as its O-arylglucuronide (TIZ-Glu) which is excreted in both urine and bile (Rossignol and Stachulski, Reference Rossignol and Stachulski1999). NTZ has been reported to display antimicrobial activity similar to TIZ, while TIZ-Glu is largely inactive against a number of pathogens (Adagu et al., Reference Adagu, Nolder, Warhurst and Rossignol2002) and has been reported to retain only some moderate activity against extra-cellular forms of C. parvum (Rossignol and Stachulski, Reference Rossignol and Stachulski1999; Gargala et al., Reference Gargala, Delaunay, Li, Brasseur, Favennec and Ballet2000). We have been able to detect TIZ and TIZ-Glu in whole blood of gerbils 16 h after the tenth consecutive dose of parenteral aminoxanide indicating that glucuronidation does occur in gerbils (data not shown), although no conclusions can be drawn from our results on the pharmacokinetics of aminoxanide, it is very unlikely that biliary excretion of TIZ-Glu can act effectively in the intestinal lumen on C. parvum infection. Amixicile, another water soluble nitro-thiazolide derivative and nearly 100% absorbed appeared to concentrate in sites of local inflammation, reverse weight loss and facilitate Cryptosporidium clearance in a malnourished mouse model despite a lack of direct anticryptosporidial activity (Bartelt et al., Reference Bartelt, Bolick, Kolling, Stebbins, Huston, Guerrant and Hoffman2018). These data highlight the potential role for both direct and indirect therapies including thiazolides against cryptosporidiosis. However, nitro-thiazolides are strong inhibitors of pyruvate-ferredoxin oxidoreductase and can disrupt normal intestinal microflora if their biodisposition is primarily seen in the gastrointestinal tract, as documented by cases of enterocolitis in horses caused by NTZ (McClure and Palma, Reference McClure and Palma1999). As clearly shown with amixicile, by not accumulating in the colon, systemic bioavailable thiazolides that moreover spares resident flora would have potential application in the treatment of Cryptosporidium infection.
NTZ has been shown to have a broad-spectrum activity against anaerobic bacteria, parasites (protozoan and helminths) and viruses and, although the specific mode of action could differ depending on the organism targeted, this activity could be attributed to the fact that it targets host cell mechanisms rather than pathogens (Hemphill et al., Reference Hemphill, Mueller and Esposito2006; Rossignol, Reference Rossignol2014). Previous reports have clearly indicated that NTZ and other thiazolides do not exert an immediate toxic efficacy on apicomplexan parasites including C. parvum and the closely related Sarcocystis neurona and Neospora caninum (Esposito et al., Reference Esposito, Stettler, Moores, Pidathala, Müller, Stachulski, Berry, Rossignol and Hemphill2005; Gargala et al., Reference Gargala, Le Goff, Ballet, Favennec, Stachulski and Rossignol2009; Jumani et al., Reference Jumani, Bessoff, Love, Miller, Stebbins, Teixeira, Campbell, Meyers, Zambriski, Nunez, Woods, McNamara and Huston2018; Funkhouser-Jones et al., Reference Funkhouser-Jones, Ravindran and Sibley2020). As in the absence of a competent immune system, the effectiveness of an anticryptosporidial agent could only depend on its cidal activity, the static activity of NTZ could partly explain why its effectiveness depends so much on the immune status of infected humans (Jumani et al., Reference Jumani, Bessoff, Love, Miller, Stebbins, Teixeira, Campbell, Meyers, Zambriski, Nunez, Woods, McNamara and Huston2018). Studies have shown that the antiviral effects of NTZ and thiazolides result from an immunomodulatory activity involving the interferon (IFN) type I signal transduction pathways (Elazar et al., Reference Elazar, Liu, McKenna, Liu, Gehrig, Puglisi, Rossignol and Glenn2009; Trabattoni et al., Reference Trabattoni, Gnudi, Ibba, Saulle, Agostini, Masetti, Biasin, Rossignol and Clerici2016) and since studies have suggested that autocrine activation by type I IFN may help protect the epithelium early during cryptosporidial infection (Barakat et al., Reference Barakat, McDonald, Foster, Tovey and Korbel2009), we could assume that thiazolides can act on Cryptosporidium infection by similar indirect mechanisms and moreover without the risk of Cryptosporidium developing resistance.
Although an oral form of the ideal drug to treat intestinal cryptosporidiosis is desirable, Cryptosporidium can extend to mucosal epithelial surfaces of other tracts and/or organs (Clifford et al., Reference Clifford, Crook, Conlon, Fraise, Day and Peto1990; Kutukculer et al., Reference Kutukculer, Moratto, Aydinok, Lougaris, Aksoylar, Plebani, Genel and Notarangelo2003; Sponseller et al., Reference Sponseller, Griffiths and Tzipori2014) which can be involved in approximately 20% of immunocompromised patients (Hunter and Nichols, Reference Hunter and Nichols2002; Chen et al., Reference Chen, Keithly, Paya and LaRusso2002; Checkley et al., Reference Checkley, White, Jaganath, Arrowood, Chalmers, Chen, Fayer, Griffiths, Guerrant, Hedstrom, Huston, Kotloff, Kang, Mead, Miller, Petri, Priest, Roos, Striepen, Thompson, Ward, Van Voorhis, Xiao, Zhu and Houpt2015). Ideally, in patients with disseminated cryptosporidiosis or with severe watery diarrhoea who purge at very high rates, an injectable therapy acting at all sites of infection is desirable. In our study, gerbils received a daily dose of 100 mg kg−1 of aminoxanide and, using a dose area-based dose translation formula (Reagan-Shaw et al., Reference Reagan-Shaw, Nihal and Ahmad2007), this dose could be extrapolated to a feasible dose of 660 mg per day for a human of 60 kg. Moreover, a time release i.m. strategy might be beneficial for some patient populations. To our knowledge, aminoxanide and MMV665917, a new drug based on piperazine (Jumani et al., Reference Jumani, Bessoff, Love, Miller, Stebbins, Teixeira, Campbell, Meyers, Zambriski, Nunez, Woods, McNamara and Huston2018), are the only two agents that have been shown to inhibit C. parvum infection when parenteral administered in a rodent infection model.
In conclusion, additional studies with several doses of aminoxanide and several time points are needed but our preliminary study highlights this second-generation thiazolide as a valuable injectable form of TIZ, providing a clue to develop a parenteral treatment of cryptosporidiosis.
Acknowledgements
We are most grateful to our colleague and friend Dr Laetitia Le Goff, Univ. of Rouen, France, for her technical assistance with the animal experiments. Dr Le Goff passed suddenly in 2018 and will be sadly missed by all the laboratory team of Rouen.
Financial support
We are grateful to Romark Laboratories, LC for funding this study at the University of Rouen.
Conflict of interest
E. Diawara and G. Gargala have no commercial affiliation with Romark Laboratories, LC. J.F. Rossignol owns equity interests in Romark Laboratories, LC, the pharmaceutical company that owns the patent for nitazoxanide.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.