Published online by Cambridge University Press: 06 August 2004
The bacterial flora of the intestine plays an important role in the virulence caused by Entamoeba histolytica. Cysteine proteinase (CP), an amoebic virulence factor, plays a major role in host cell destruction. The mechanism of increased virulence following bacterial co-association is not understood. We studied CP of E. histolytica HM1:IMSS which was co-associated with Escherichia coli K12 strain pre-incubated with GalNAc or CP specific inhibitor E 64. Co-association of E. histolytica with bacteria enhanced CP activity 3·6-fold as assessed by azocasein assay and substrate gel electrophoresis showed bands at molecular weights of 28, 35 and 56 kDa. Northern and Western blot analysis showed increase in ehcp2 and ehcp5 gene expression. Trophozoites co-associated with E. coli showed greater cytotoxicity of BHK cells by a 51Cr release assay than trophozoites that had not been co-associated; this enhancement was abolished by E-64 treatment. The killing of BHK 21 targets by E. histolytica was characterized by DNA laddering which was not inhibited with E-64. GalNAc pre-incubation of trophozoites reduced cytotoxicity and DNA laddering, while E. coli co-associated E. histolytica showed smearing with faint laddering of BHK implicating both necrosis and apoptosis. Hence, bacterial co-association increases CP activity and CP gene expression and contributes to the necrosis of the target cell.
Entamoeba histolytica, an intestinal parasite, is responsible for amoebic dysentery and liver abscesses. It is a major cause of morbidity and mortality worldwide (WHO, 1997). Prolonged axenic cultivation decreases the virulence of E. histolytica (Mattern et al. 1982); this is restored by manipulations such as in vitro incubation of trophozoites with hamster serum, cholesterol, serial passage through hamster liver (Lushbaugh et al. 1978; Gupta et al. 1998; Das et al. 1976) as well as in vitro co-association with certain strains of E. coli (Sinha et al. 1997; Ghosh et al. 1998). The mechanism of increased virulence following bacterial co-association is not well understood.
Trophozoites of virulent amoebic strains cause lesions of the bowel by attaching to the epithelium through Gal/GalNAc inhibitable lectins (McCoy et al. 1994), degrading the extracellular matrix through the action of cysteine proteinases (Keene et al. 1990) and lysing epithelial cells via an amoebapore (Leippe, 1997). Cysteine proteinases (CP) of E. histolytica have been shown to be involved in the in vitro cytopathic effect of the parasite as well as degradation of extracellular matrix (Keene et al. 1990; Jacobs et al. 1998); the cytopathic activity depends upon high enzymatic activity of CP (Gadasi & Kobiler, 1983; Jacobs et al. 1998). CP has also been implicated in parasite virulence since blocking of CP activity by E-64 reduces the liver abscess formation in SCID mice (Stanley et al. 1995).
Of the several CP genes and proteins, EhCP5 and EhCP112, have been shown to localize on the surface of E. histolytica and hence may play a role in mediating virulence (Jacobs et al. 1998; Garcia-Rivera et al. 1999).
Approximately 90% of total CP activity present in a lysate of cultured E. histolytica trophozoites is present in the 3 cysteine proteinases EhCP1, EhCP2 and EhCP5 (Bruchhaus et al. 1996). How important their role is in the cytopathogenic activity of the parasite is not clear. Antisense inhibition of ehcp5 gene expression did not affect its cytopathic or haemolytic activity while inhibiting phagocytosis by the parasite. This CP-deficient parasite failed to induce liver lesion in a hamster model (Ankari et al. 1998, 1999). Inoculation of ehcp5 deficient E. histolytica in a human intestinal xenograft model in SCID mice induced significantly less gut inflammation than that caused by virulent amoeba (Zang et al. 2000).
In the host, the parasite mediates its virulence in the presence of the natural gut flora and bacterial associates support in vitro growth. The role of these bacteria in parasite virulence is intriguing. Bacterial co-association increases in vitro cytopathic activity (Sinha et al. 1997); however, the mechanism by which it does this is not clear. In this study we have investigated the effect of co-association of E. coli K 12 strain with E. histolytica on amoebic CP activity and gene expression and the role of co-association in target cell lysis.
Axenic Entamoeba histolytica trophozoite strain HM1:IMSS clone 6 was maintained in TYI-S-33 medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml) and 15% (v/v) adult bovine serum (Biological Industries, Haemek, Israel), as previously described (Diamond et al. 1978).
BHK-21 cell line (National Center for Cell Sciences, Pune, India) was cultured in RPMI-1640 (Gibco BRL, Rockville, USA) supplemented with 2 mML-glutamine, 25 mM HEPES buffer, 0·2% sodium bicarbonate, 50 μM 2-mercaptoethanol and 10% foetal calf serum (FCS) (Biological Industries, Haemek, Israel) with antibiotics (penicillin 100 U/ml and streptomycin 100 μg/ml). The cultures were maintained in 6-well tissue culture plates (Nunc, Rosklde, Denmark) at 37 °C in humidified 5% CO2 atmosphere.
Amoebic trophozoites were harvested by chilling the culture tube for 5 min on ice, followed by centrifugation at 280 g for 8 min at 4 °C and incubation with log-phase grown E. coli at a ratio of 1[ratio ]1000 (amoeba: bacteria) for 90 min at 37 °C. Then 200 μM E-64 [L-trans-epoxysuccinyl-leucyl-amido- (4-guanidino) butane], (Sigma, St Louis, USA) a specific and irreversible inhibitor of CP (Barrett et al. 1982) was added to the culture medium for 24 h to block the CP activity. The viability of trophozoites at the end of the incubation was 94% by the trypan blue (Sigma, St Louis, USA) exclusion method.
The BHK-21 monolayer was washed with RPMI-1640 without FCS. E. histolytica trophozoites were incubated with 1·0 mM GalNAc (Sigma, St Louis, USA) to block E. histolytica lectin or 200 μM of E-64 (Sigma, St Louis, USA) or co-associated with log-phase grown E. coli. The trophozoites were added to the BHK-21 monolayer (1[ratio ]10 E. histolytica[ratio ]BHK-21 ratio) and incubated at 37 °C in humidified 5% CO2 atmosphere for 60 min. Each well was washed with chilled PBS to dissociate the trophozoites from the BHK-21 monolayer.
The proteolytic activity of amoebic proteinase was assayed as described earlier (Scholze & Tannich, 1994; Gupta et al. 1998). CP acts on the chromogenic substrate azocasein (Sigma, St Louis, USA) to release low molecular weight soluble peptides into the supernatant fluid, which gives a colour reaction that is measured at 440 nm (A440). Briefly, 250 μl of 0·2% azocasein (w/v) in 100 mM phosphate buffer, pH 6·5, was incubated with 150 μl crude amoebic lysate at 37 °C for 30 min. The reaction was stopped by adding 10% trichloroacetic acid (TCA) for 15 min at room temperature. The mixture was centrifuged at 1400 g for 5 min at room temperature and 1·2 ml of supernatant was collected in a vial containing 1·4 ml of 1 M NaOH, mixed and the absorbance read at 440 nm.
One unit of enzyme activity was defined as the amount of enzyme required to cause a unit increase in the absorbance across a 1 cm path length at 440 nm. (Sarath et al. 1989).
The molecular weight pattern of CP was assessed by gelatin substrate gel electrophoresis as described earlier (Hellburg, Leippe & Bruchhaus, 2000). Ten μg protein of crude lysates (control and experimental) were electrophoresed at 50 V on 4 °C on a 10% polyacrylamide gel co-polymerized with 0·2% gelatin (Sigma, St Louis, USA). SDS was removed by 2 washings in 2·5% Triton X-100 for 30 min each at room temperature. The gel was incubated in developing buffer (20 mM DTT and 100 mM sodium acetate, pH 4·2 with 1% Triton X-100) at 37 °C with continuous shaking for 3 h and stained with Coomassie blue for 30 min. Zones of protease activity was visualized as clear bands against a blue background.
Northern blot of E. histolytica with and without E. coli co-association was probed with ehcp1, ehcp2 ehcp5 and 18S rRNA probes. RNA was isolated using TrizolLS (Gibco BRL, Rockville, USA) according to the manufacturer's instruction. A sample of 20 μg total RNA was separated on 1% agarose–formaldehyde gel and transferred onto nylon membrane. The membrane was hybridized with α 32P labelled ehcp1, ehcp2 and ehcp5 probe and 18S rRNA probe at 42 °C for 18 h and the blot washed with 0·2X SSC with 0·1% SDS at 37 °C. The blot was exposed to Kodak X-ray film at −70 °C for 1 week (Sambrook, Fritsch & Maniatis, 1989).
Crude lysates (20 μg protein) of trophozoites (control and experimental) were added to sample buffer (10% glycerol, 2% SDS, 50 mM Tris–HCl pH 6·8) with 10 mM β-mercaptoethanol and 50 μM E-64, 10 mMp-hydroxy methyl benzoate, 3 mM iodoacetamide, heated at 95 °C for 2 min and then chilled in ice. After electrophoresis, the gel was transferred at 0·65 mA/cm2 of gel at 4 °C onto nitrocellulose membrane and probed with polyclonal antibody specific for EhCP1, EhCP2 and EhCP5 (kindly gifted by Iris Brauchhaus, Germany). The blots were developed by the chemiluminescence system according to the manufacturer's instructions (Amersham Pharmacia Biotech Ltd, Uppsala, Sweden) (Sambrook et al. 1989).
Cell cytotoxicity was tested by 51Cr cytotoxicity assay. BHK 21 was seeded at 50×104 cells/well and allowed to grow overnight in 96-well flat-bottom culture plates (Nunc, Rosklde, Denmark) to reach confluence. The medium was removed and 50 μCi/ml 51Cr labelled sodium chromate (BARC, Trombay, India) was added to the culture medium and incubated at 37 °C in a humidified 5% CO2 atmosphere for 2 h. The plate was washed twice to remove unincorporated 51Cr. E. histolytica trophozoites with and without bacterial co-association or with GalNAc treatment or E-64 treatment were added to the BHK monolayer (1[ratio ]10 Eh[ratio ]BHK-21 ratio) and incubated at 37 °C in humidified 5% CO2 atmosphere for 60 min. Released 51Cr in the supernatant was counted in a Multigamma Counter (LKB Wallace, New Jersey, USA). All the experiments were done in triplicate and repeated 3 times. Percentage cell cytotoxicity was calculated as follows

BHK-21 cells were plated in a 6-well plate (Nunc, Rosklde, Denmark) at a cell density of 2×106 cells/well and allowed to grow to 60–70% confluence at 37 °C and 5% CO2. The monolayer was washed with RPMI without FCS. E. histolytica trophozoites with and without bacterial co-association or with GalNAc or E-64 treatment were added to the monolayer (1[ratio ]10 Eh[ratio ]BHK-21 ratio) and incubated for 1 h at 37 °C and 5% CO2. Adhered trophozoites were detached by chilling the slide at 4 °C and washing with chilled PBS. A positive control was generated by exposure of BHK 21 cells to UV (256 nm) light for 10 min followed by incubation at 37 °C and 5% CO2 for 16 h.
Small DNA fragments were isolated as described earlier (Tolskaya et al. 1995). Cells were detached by mild trypsinization and collected by centrifugation at 2800 g for 10 min. The cells were resuspended in buffer containing 20 mM EDTA, 10 mM Tris–HCl (pH 7·4) and 0·5% Triton X-100 and kept at 0 °C for 20 min. The cell lysate was centrifuged at 18000 g at 4 °C for 20 min to remove intact chromatin. SDS was added to the supernatant to give a final concentration of 1%, followed by phenol–chloroform deproteinization. Nucleic acids were precipitated with chilled absolute ethanol at −20 °C and the pellet dissolved in 10 μl of water. RNA was removed by treatment with 1 μl of RNase A (10 μg/ml) at 37 °C for 1 h. Glycerol was added to give a final concentration of 8% and samples were subjected to electrophoresis on 2% agarose gel.
BHK-21 cells were plated in 8-chamber slides (Nunc, Rosklde, Denmark) at a cell density of 5×104 cells/chamber and allowed to grow up to 60–70% confluence at 37 °C and 5% CO2. The monolayer was washed with RPMI without FCS. Amoeba trophozoites were added to the monolayer at a ratio of 1[ratio ]10 (trophozoites[ratio ]BHK-21) and incubated for 60 min at 37 °C and 5% CO2. Adhered trophozoites were removed by chilling the slide at 4 °C and washed with chilled PBS. A positive control was generated by exposure to UV (256 nm) light for 10 min followed by incubation at 37 °C and 5% CO2 for 16 h. Cells were fixed with chilled in 70% ethanol for 20 min and washed with PBS and stained with 50 μg/ml propidium iodide with 10 μg/ml RNase A for 30 min in the dark. The slide was mounted with 50% glycerol and examined under a fluorescent microscope.
Comparisons between groups were performed by Mann–Whitney sign test and P values <0·05 were considered as statistically significant.
The protease activity of E. histolytica was 16·8±7·4 U/mg of protein; this was almost completely abolished by E-64 (4·0±1·4 U/mg protein) (P<0·005). A 3·6-fold increase in protease activity (60·2±4·8 U/mg protein) was seen when trophozoites were co-associated for 90 min with E. coli K-12 strain as compared to E. histolytica alone (P<0·005); and this reduced 2·5-fold when E-64 pre-treated E. histolytica were co-associated with E. coli (6·7±1·2 U/mg protein) (P<0·005) (Fig. 1).

Fig. 1. Protease activity using the azocasein assay and percentage cytotoxicity measured by the 51chromium release assay of Entamoeba histolytica (Eh), E-64 and GalNAc treated (Eh+E 64; Eh+GalNAc), E. histolytica co-associated with Escherichia coli (Eh+Ec), and E-64 treated E. histolytica co-associated with E. coli ((Eh+E 64)+Ec). The bars represent the mean of 5 experiments, done in triplicate ±S.D.
Crude lysates of E. histolytica with and without co-association with bacteria were subjected to gelatin substrate gel electrophoresis. E. histolytica co-associated with E. coli showed more intense bands at 29, 35 and 56 kDa as compared to E. histolytica without bacterial co-association (Fig. 2). The most prominent band was in the region of 56 kDa, which represents neutral CP. No gelatin degradation was seen in E. coli alone and E-64 treated E. histolytica. The SDS–PAGE confirmed that protein loading in all the lanes was equal.

Fig. 2. SDS–PAGE (upper) and substrate gel (lower) of crude lysates of Entamoeba histolytica under different experimental conditions. Electrophoresis was performed on 10% SDS–PAGE co-polymerized with 0·1% gelatin. Lane 1: E. histolytica; Lane 2: E. histolytica pre-incubated with E-64 (200 μM); Lane 3: E-64 treated trophozoites co-associated with Escherichia coli; Lane 4: E. histolytica co-associated with E. coli for 90 min at 37 °C; Lane 5: E. coli and Lane M: molecular weight marker.
E. histolytica trophozoites co-associated with E. coli K12 strain showed a more intense band than E. histolytica alone, when hybridized with ehcp2 and ehcp5 probes; there was no difference in band intensity with ehcp1 probe. The 18S rRNA band intensity was equal in both (Fig. 3). Densitometry analysis showed that bacterial co-association increased ehcp2 gene expression by 2·4-fold over that of E. histolytica alone; there was also a 1·8-fold increase in ehcp5 gene expression but there was no change in ehcp1 gene expression.

Fig. 3. Northern blot of Entamoeba histolytica with (Lane 1) and without (Lane 2) Escherichia coli co-association probed with ehcp1, ehcp2, ehcp5 and 18S rRNA (A). Bar diagram shows ratio of band intensity of CP to 18S rRNA probe (B).
The translational modification in CP gene expression was assessed by Western blot, using antibodies specific to EhCP1, 2 and 5. Expression of E. histolytica EhCP2 and EhCP5 was increased by co-association with E. coli K12 while expression of EhCP1 showed no difference. Densitometry analysis showed that E. coli co-association caused a 2·0-fold increase in EhCP5 and a 2·6-fold increase in EhCP2 gene expression (Fig. 4).

Fig. 4. SDS–PAGE (upper) and Western blot (lower) of crude lysate of Entamoeba histolytica without (Lane 1) and with Escherichia coli co-association (Lane 2). (A) Lane M is molecular weight marker. Electrophoresis was performed on 12% SDS–PAGE and probed with EhCP1, EhCP2 and EhCP5 specific antibody. Bar diagram shows ratio of band intensity of Western blot of EhCP5 E. histolytica co-associated with E. coli to E. histolytica alone (B).
The cytotoxicity mediated by E. histolytica alone was 34·5±3·7% and when trophozoites were pre-incubated with E-64, it was 16·1±9·4% (P<0·0001) (Fig. 1). Trophozoites pre-treated with 1·0 M Gal/NAc showed reduced cytotoxicity as compared to untreated trophozoites (34·5±3·7% vs. 12·6±3·1%; P<0·0001) while co-association of E. histolytica with E. coli increased cytotoxicity to 57·2±9·6% (P<0·0001). The increase seen with E. coli co-association was reduced to 33·5±25·8% (P<0·005) when E. histolytica was pre-incubated with E-64 before co-association. This value was similar to that of untreated trophozoites. All the experiments were performed in triplicate and repeated 5 times.
BHK 21 monolayer co-cultured with E. histolytica trophozoites showed a DNA laddering pattern which was similar to that of positive control generated by UV. When trophozoites pre-treated with E-64 were added to BHK, the pattern of DNA fragmentation was similar to that when untreated E. histolytica were added. However, these cells showed reduced laddering when they were exposed to GalNAc treated trophozoites. When E. histolytica co-associated with E. coli K12 strain were co-cultured with BHK cells, smearing rather than a ladder pattern was observed (Fig. 5).

Fig. 5. DNA laddering pattern of BHK 21 cell lines run on a 2% agarose gel. DNA laddering pattern of BHK 21 cells co-cultured with Entamoeba histolytica (E his), E-64 treated E. histolytica (E his+E 64), GalNAc treated E. histolytica (E his+GalNAc) and Escherichia coli co-associated E. histolytica (E his+E coli). Positive control generated by UV treatment; negative control without any treatment.
The BHK-21 cell line was co-cultured with E. histolytica trophozoites and stained with propidium iodide for nuclear morphology. The oval nuclei of BHK 21 cells showed a uniform staining (Fig. 6A), while in the positive control the nuclei appeared fragmented (Fig. 6B). Following contact with E. histolytica trophozoites nuclei were rounded and fragmented, as was seen in the positive control (Fig. 6C) while the DNA fragmentation was not affected by incubation of trophozoites with E-64 (Fig. 6D). Nuclear fragmentation was not seen when trophozoites were pre-incubated with GalNAc (Fig. 6E). When the BHK 21 monolayer was incubated with E. coli co-associated trophozoites, the change in morphology was more prominent with only a few remaining normal cells. (Fig. 6F), and with the majority of cells detached from the surface of the slide.

Fig. 6. Propidium iodide staining of BHK-21 monolayer. (A) BHK-21 monolayer (negative control), (B) UV-treated BHK-21 cells (positive control), (C) BHK-21 cells co-cultured with Entamoeba histolytica trophozoites, (D) BHK-21 cells co-cultured with E. histolytica trophozoites pre-treated with E-64, (E) BHK-21 cells co-cultured with E. histolytica trophozoites pre-incubated with GalNAc, (F) BHK-21 cells co-cultured with E. histolytica trophozoites co-associated with Escherichia coli K12 strain. (Magnifications 40×.)
The bacterial flora of the intestine plays an important role in amoebic virulence. A number of studies have shown that association of axenically grown E. histolytica trophozoites with certain bacteria enhances its virulence (Anaya et al. 1997); however, the biological basis has not been well understood. We have demonstrated for the first time that short-term co-association of axenically grown trophozoites of E. histolytica HM1[ratio ]IMSS with E. coli K-12 strain results in increased CP activity, ehcp2 and ehcp5 gene expression and target cell cytotoxicity. This may be the mechanism for the increased virulence.
Co-association of E. histolytica with E. coli resulted in a 3·6-fold increase in E-64 inhibitable protease activity. The protease activity was of 29, 35 and 56 kDa molecular weights in substrate gels suggesting involvement of more than one CP. ACP3 (EhCP1) has been shown to have 2 distinct activities at 27–30 kDa (Luaces & Barrett, 1988), EhCP2 at 35 kDa, EhCP5 at 29 kDa (Hallberg et al. 2000) and neutral CP has been reported to have protease activity at 56 kDa (Keene et al. 1986). The increased CP activity following co-association therefore involved EhCP1, 2, 5 and neutral CP. We have previously shown that the activity at 56–66 kDa increases after passage of E. histolytica through hamster liver, which in turn was related to an increased virulence of the E. histolytica (Gupta et al. 1998).
The increased CP activity following co-association that we found was accompanied by increased expression of CP at mRNA and protein level. Northern blot analysis showed a 2·4-fold increase in ehcp2 gene expression and a 1·8-fold increase in ehcp5 gene expression following bacterial co-association; however, there was no difference in ehcp1 gene expression. This was supported by the observation of an approximately 2·6-fold increase in EhCP2 and a 2·0-fold increase in EhCP5 expression of the protein. There was no increase in expression of EhCP1. EhCP5 has been shown to be the only CP beside EhCP112 which is expressed on the parasite cell surface and is not found in the non-pathogenic strain E. dispar (Willhoeft et al. 1999). EhCP2 has been reported to be involved in destruction of CHO cell targets by amoeba (Hellberg et al. 2001). Although there was only a 1·8-fold ehcp5 and a 2·4-fold ehcp2 gene transcript increase, the 3·5-fold increase in CP activity can be attributed to the increased expression of both EhCP2 and 5 genes. This has also been supported by the substrate gel which has shown bands corresponding to other proteases, i.e. EhCP1, EhCP2, EhCP5 and neutral CP. Increased virulence following co-association of axenically grown E. histolytica trophozoites with certain bacteria has been shown previously (Sinha et al. 1997; Ghosh et al. 1998).
Measurement of E. histolytica-mediated cytotoxicity by a chromium release assay, showed an increase following co-association with E. coli which was inhibitable by pre-treatment of the parasite with E-64. This observation is similar to the earlier report of increased cytopathic effect on a BHK-21 cell line (Bracha & Mirelmand, 1984; Sinha et al. 1997). When trophozoites transfected with EhCP5 antisense were evaluated for cytotoxicity effect, their functional competence was comparable to that of control parasites although 90% of the CP activity was lost. The cytopathic effect was inhibitable by E-64 suggesting that it was mediated by non-EhCP5 proteases. These transfectants did not cause abscess formation in hamsters and failed to induce gut inflammation in the SCID mouse xenograft model (Ankari et al. 1998, 1999; Zang et al. 2000). Trophozoites with over-expressed ehcp2 had increased production of CP and increased cytopathic activity; but this increase did not increase the erythrophagocytosis or liver abscess formation (Hellburg et al. 2001). Thus, the available evidence indicates that cysteine proteinases are involved in causing lesions and that more than one CP is implicated. Our observations that co-association of E. histolytica and E. coli results in increased CP activity and target cell killing, both of which are E-64 inhibitable, clearly implicates the increased CP activity following bacterial co-association in the increased target cell cytotoxicity.
Killing of BHK 21 targets by E. histolytica was characterized by a DNA laddering pattern in BHK cells, which was not inhibited by E-64 treatment of E. histolytica trophozoites. However, cytotoxicity was E-64 inhibitable suggesting that cytotoxicity of target cells was not mediated by apoptosis. Both target cell cytotoxicity by E. histolytica trophozoites as well as DNA fragmentation and PI staining was inhibited by pre-treatment of trophozoites with Gal/GalNAc. Hence, lectin mediated cell-to-cell contact is a critical event in target cell killing. The role of amoebic lectin has been well investigated and blocking of these lectins with Gal/GalNAc has been previously shown to reduce both adherence and cytotoxicity (McCoy et al. 1994; Ravdin, 1986). Findings similar to ours have been reported by Huston et al. (2000), who showed that while Gal/GalNAc inhibited apoptosis of Jurkat cells, E-64 failed to do so.
Contact of E. histolytica co-associated with E. coli with BHK cells showed smearing rather than a ladder pattern in the DNA fragmentation assay. This may be attributed to the increased CP activity of trophozoites following bacterial co-association. It has been previously reported that target cell killing by contact was apoptotic while purified amoebapore or amoebic cell lysate causes necrotic death. (Berninghausen & Leippe, 1997).
In conclusion, we have shown that axenically grown trophozoites co-associated with bacteria result in increased CP activity of trophozoites. This is accompanied with enhanced ehcp5 and ehcp2 gene expression and cytotoxic capability. It may be suggested from these data that normal contact between trophozoites and host cells results in apoptotic death of the latter through lectin-Gal/GalNAc interactions. On the other hand, phagocytosis of the bacteria results in increased expression of CP and necrotic killing of the surrounding cells. The gut milieu is rich in bacterial growth and their role in amoebic disease has not been understood. How relevant our observations are to the in vivo situation can only be speculated upon and needs further studies in animal models for amoebiasis.
The authors would like to thank Professor E. Tannich, Bernard Nocht Institute for Tropical Medicine, Hamburg, Germany, for providing the ehcp1, 2 and 5 probe. This work was partially supported by a grant from the Department of Science and Technology, Government of India.
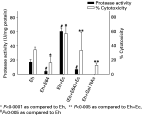
Fig. 1. Protease activity using the azocasein assay and percentage cytotoxicity measured by the 51chromium release assay of Entamoeba histolytica (Eh), E-64 and GalNAc treated (Eh+E 64; Eh+GalNAc), E. histolytica co-associated with Escherichia coli (Eh+Ec), and E-64 treated E. histolytica co-associated with E. coli ((Eh+E 64)+Ec). The bars represent the mean of 5 experiments, done in triplicate ±S.D.

Fig. 2. SDS–PAGE (upper) and substrate gel (lower) of crude lysates of Entamoeba histolytica under different experimental conditions. Electrophoresis was performed on 10% SDS–PAGE co-polymerized with 0·1% gelatin. Lane 1: E. histolytica; Lane 2: E. histolytica pre-incubated with E-64 (200 μM); Lane 3: E-64 treated trophozoites co-associated with Escherichia coli; Lane 4: E. histolytica co-associated with E. coli for 90 min at 37 °C; Lane 5: E. coli and Lane M: molecular weight marker.

Fig. 3. Northern blot of Entamoeba histolytica with (Lane 1) and without (Lane 2) Escherichia coli co-association probed with ehcp1, ehcp2, ehcp5 and 18S rRNA (A). Bar diagram shows ratio of band intensity of CP to 18S rRNA probe (B).

Fig. 4. SDS–PAGE (upper) and Western blot (lower) of crude lysate of Entamoeba histolytica without (Lane 1) and with Escherichia coli co-association (Lane 2). (A) Lane M is molecular weight marker. Electrophoresis was performed on 12% SDS–PAGE and probed with EhCP1, EhCP2 and EhCP5 specific antibody. Bar diagram shows ratio of band intensity of Western blot of EhCP5 E. histolytica co-associated with E. coli to E. histolytica alone (B).
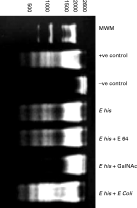
Fig. 5. DNA laddering pattern of BHK 21 cell lines run on a 2% agarose gel. DNA laddering pattern of BHK 21 cells co-cultured with Entamoeba histolytica (E his), E-64 treated E. histolytica (E his+E 64), GalNAc treated E. histolytica (E his+GalNAc) and Escherichia coli co-associated E. histolytica (E his+E coli). Positive control generated by UV treatment; negative control without any treatment.

Fig. 6. Propidium iodide staining of BHK-21 monolayer. (A) BHK-21 monolayer (negative control), (B) UV-treated BHK-21 cells (positive control), (C) BHK-21 cells co-cultured with Entamoeba histolytica trophozoites, (D) BHK-21 cells co-cultured with E. histolytica trophozoites pre-treated with E-64, (E) BHK-21 cells co-cultured with E. histolytica trophozoites pre-incubated with GalNAc, (F) BHK-21 cells co-cultured with E. histolytica trophozoites co-associated with Escherichia coli K12 strain. (Magnifications 40×.)