INTRODUCTION
Schizophrenia is a severe and heterogeneous mental illness affecting more than 21 million people worldwide (World Health Organization, 2004), listed into the top 10 medical disorders causing functional impairment (Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011). Behavioral, relational, and cognitive impairments precede the disease onset (Cannon et al., Reference Cannon, Caspi, Moffitt, Harrington, Taylor, Murray and Poulton2002). Thus, clarifying the role of premorbid variables with a direct impact on the individual’s functioning might constitute a key issue in order to better understand schizophrenia’s development, course, and the heterogeneity of the manifestations.
Premorbid adjustment (PA) is a multidimensional concept underlying a broad set of abilities, such as an individual’s academic, occupational, social, and intellectual functioning preceding disease onset (Cannon-Spoor, Potkin, & Wyatt, Reference Cannon-Spoor, Potkin and Wyatt1982). Indeed, poor PA during childhood and adolescence in patients with schizophrenia is well documented (Cannon et al., Reference Cannon, Caspi, Moffitt, Harrington, Taylor, Murray and Poulton2002; Reichenberg et al., Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray, Poulton and Moffitt2010) and related to earlier disease onset, worse prognosis (Rabinowitz, De Smedt, Harvey, & Davidson, Reference Rabinowitz, De Smedt, Harvey and Davidson2002; Addington & Addington, Reference Addington and Addington2005; Strauss et al., Reference Strauss, Allen, Miski, Buchanan, Kirkpatrick and Carpenter2012), more severe negative symptoms (Ayesa-Arriola et al., Reference Ayesa-Arriola, Rodriguez-Sanchez, Perez-Iglesias, Gonzalez-Blanch, Pardo-Garcia, Tabares-Seisdedos, Vázquez-Barquero and Crespo-Facorro2013; Galderisi et al., Reference Galderisi, Bucci, Mucci, Kirkpatrick, Pini, Rossi, Vita and Maj2013), and worse functional and treatment outcomes (Galderisi et al., Reference Galderisi, Bucci, Mucci, Kirkpatrick, Pini, Rossi, Vita and Maj2013; Buonocore, Bechi et al., Reference Buonocore, Bechi, Uberti, Spangaro, Cocchi, Guglielmino, Bianchi, Mastromatteo, Bosia and Cavallaro2018; Buonocore et al., Reference Buonocore, Bosinelli, Bechi, Spangaro, Piantanida, Cocchi, Bianchi, Guglielmino, Mastromatteo, Cavallaro and Bosia2018). More in detail, literature recognized two key domains: social PA (Social PA) and academic PA (Academic PA), both related to acute and persistent negative symptoms (Strauss et al., Reference Strauss, Allen, Miski, Buchanan, Kirkpatrick and Carpenter2012; Chung et al., Reference Chung, Tang, Hui, Wong, Chan, Lee and Chen2013) and to cognitive deficits (Rund et al., Reference Rund, Melle, Friis, Johannessen, Larsen, Midboe, Opjordsmoen, Simonsen, Vaglum and McGlashan2007; Barajas et al., Reference Barajas, Usall, Banos, Dolz, Villalta-Gil, Vilaplana, Autonell, Sánchez, Cervilla, Foix, Obiols, Haro and Ochoa2013), respectively.
Moreover, the study of PA may enhance the comprehension of the post onset manifestations of the disease, in line with the hypothesis that schizophrenia’s clinical heterogeneity begins early, even several years before disease onset (Chung et al., Reference Chung, Tang, Hui, Wong, Chan, Lee and Chen2013; Horton, Tarbox, Olino, & Haas, Reference Horton, Tarbox, Olino and Haas2015). As a matter of the fact, some studies reported that some patients would exhibit no functional impairment prior to schizophrenia onset, while others would show poor PA during childhood and adolescence (Cole, Apud, Weinberger, & Dickinson, Reference Cole, Apud, Weinberger and Dickinson2012; Horton et al., Reference Horton, Tarbox, Olino and Haas2015). Therefore, recent studies focused on PA trajectories, and they identified three groups of patients (Cole et al., Reference Cole, Apud, Weinberger and Dickinson2012; Chung et al., Reference Chung, Tang, Hui, Wong, Chan, Lee and Chen2013; Horton et al., Reference Horton, Tarbox, Olino and Haas2015): a stable-good group (i.e., patients with overall good PA), a stable-poor group (i.e., patients with widespread functional impairment since childhood), and a “deteriorating” group (i.e., patients with good PA during childhood, which progressively became poorer until schizophrenia onset). Such groups exhibited differences in cognition, symptomatology, and functioning after the onset of the illness. In particular, patients belonging to the stable-good group showed a mild overall impairment, that means less severe symptoms, higher educational attainment, better functional outcome, and a greater performance on core cognitive domains, such as processing speed, executive functions, verbal fluency, and logical memory tasks (Cole et al., Reference Cole, Apud, Weinberger and Dickinson2012; Chung et al., Reference Chung, Tang, Hui, Wong, Chan, Lee and Chen2013). By contrast, patients belonging to the “deteriorating” group were significantly younger at disease onset, with a poorer quality of life and more severe positive and negative symptoms with respect to the other groups (Addington & Addington, Reference Addington and Addington2005; Cole et al., Reference Cole, Apud, Weinberger and Dickinson2012). Lastly, patients belonging to the stable-poor group showed more negative symptoms, lower educational attainment, poorer functional outcome, and worse performance on cognitive tasks, such as processing speed and executive function measures (Dickinson, Ramsey, & Gold, Reference Dickinson, Ramsey and Gold2007; Mesholam-Gately, Giuliano, Goff, Faraone, & Seidman, Reference Mesholam-Gately, Giuliano, Goff, Faraone and Seidman2009; Cole et al., Reference Cole, Apud, Weinberger and Dickinson2012; Chung et al., Reference Chung, Tang, Hui, Wong, Chan, Lee and Chen2013).
In sum, these studies highlight that different cognitive and functional profiles could be found back in time in patients’ life.
Another aspect that could contribute to explain the heterogeneity of the manifestations after the onset of the illness is the presence of autistic traits (ATs). Noteworthy, recent research identified a group of patients exhibiting neurodevelopmental abnormalities that are typical of autism spectrum disorder (ASD) (i.e., difficulties in social interactions, communication, emotion processing, and motor abnormalities) (King & Lord, Reference King and Lord2011; Kastner et al., Reference Kastner, Begemann, Michel, Everts, Stepniak, Bach, Poustka, Becker, Banaschewski, Dose and Ehrenreich2015). It was also reported that childhood-onset schizophrenia is preceded by an ASD diagnosis in 30%–50% of the cases (Rapoport, Chavez, Greenstein, Addington, & Gogtay, Reference Rapoport, Chavez, Greenstein, Addington and Gogtay2009) and that some patients with schizophrenia with more pronounced negative symptoms show a noticeable autistic-like phenotype, characterized by difficulties in interpersonal relationships, receptive language, and adjustment, as well as a delayed motor development (Bastiaansen et al., Reference Bastiaansen, Meffert, Hein, Huizinga, Ketelaars, Pijnenborg, Bartels, Minderaa, Keysers and de Bildt2011). Moreover, available evidences propose the presence of overlapping pathogenic mechanisms, suggesting that ASD and schizophrenia may share similar biological alterations in pathways of brain development underlying the phenotypic spectrum of these disorders (Burbach & van der Zwaag, Reference Burbach and van der Zwaag2009; Kushima et al., Reference Kushima, Aleksic, Nakatochi, Shimamura, Okada, Uno, Morikawa, Ishizuka, Shiino, Kimura, Arioka, Yoshimi, Takasaki, Yu, Nakamura, Yamamoto, Iidaka, Iritani, Inada, Ogawa, Shishido, Torii, Kawano, Omura, Yoshikawa, Uchiyama, Yamamoto, Ikeda, Hashimoto, Yamamori, Yasuda, Someya, Watanabe, Egawa, Nunokawa, Itokawa, Arai, Miyashita, Kobori, Suzuki, Takahashi, Usami, Kodaira, Watanabe, Sasaki, Kuwabara, Tochigi, Nishimura, Yamasue, Eriguchi, Benner, Kojima, Yassin, Munesue, Yokoyama, Kimura, Funabiki, Kosaka, Ishitobi, Ohmori, Numata, Yoshikawa, Toyota, Yamakawa, Suzuki, Inoue, Nakaoka, Goto, Inagaki, Hashimoto, Kusumi, Son, Murai, Ikegame, Okada, Kasai, Kunimoto, Mori, Iwata and Ozaki2018). Given this scenario, it is possible to hypothesize that patients belonging to a stable-poor PA group, that is, patients exhibiting developmental impairments preceding schizophrenia onset, might present ATs.
Moreover, a core common cognitive feature of both schizophrenia and ASD concerns the impairment of theory of mind (ToM), although with some differences. ToM’s development starts in early childhood and continues through adolescence (Baron-Cohen, Reference Baron-Cohen1998). It has thus been suggested that children with autism might never completely acquire ToM abilities. By contrast, patients with schizophrenia might show intact mentalizing abilities until their first clinical manifestation of the disorder (Frith & Corcoran, Reference Frith and Corcoran1996). However, recent research highlighted that ToM impairment is widely heterogeneous in schizophrenia. In fact, although many patients present a poor performance on ToM tasks, a non-negligible group of patients reaches a within-normal range or close to normal performance on mental state attribution tasks (Brune & Schaub, Reference Brune and Schaub2012; Rocca et al., Reference Rocca, Galderisi, Rossi, Bertolino, Rucci, Gibertoni, Montemagni, Sigaudo, Mucci, Bucci, Acciavatti, Aguglia, Amore, Bellomo, De Ronchi, Dell’Osso, Di Fabio, Girardi, Goracci, Marchesi, Monteleone, Niolu, Pinna, Roncone, Sacchetti, Santonastaso, Zeppegno and Maj2016; Bechi et al., Reference Bechi, Bosia, Agostoni, Spangaro, Buonocore, Bianchi, Cocchi, Guglielmino, Mastromatteo and Cavallaro2018). Therefore, it could be hypothesized that also the heterogeneity of post onset ToM abilities starts early in the life, it could be linked to different PA trajectories and to the presence or absence of ATs in individuals with schizophrenia.
Given this scenario, the aim of the present study is three-fold. Firstly, it aims at identifying different premorbid trajectories in 97 patients with schizophrenia. Secondly, it aims at analyzing if different premorbid trajectories could correspond to different severities of psychopathology, as well as to different post onset cognitive and ToM abilities, also compared with healthy controls’ performance in the latter cited domains. Lastly, it aims at investigating the presence of ATs in the sample’s groups.
MATERIALS AND METHODS
Participants
Ninety-seven outpatients with schizophrenia were recruited in the psychiatric rehabilitation service of the IRCCS San Raffaele Scientific Institute. All patients met DSM-IV-TR criteria for schizophrenia, as determined by trained psychiatrists by using clinical interviews and were clinically and pharmacologically stabilized. In addition, data regarding 66 healthy controls were collected in a previous study (Anselmetti et al., Reference Anselmetti, Bechi, Bosia, Quarticelli, Ermoli, Smeraldi and Cavallaro2009). All controls underwent a screening visit and structured interviews by a trained psychiatrist in order to confirm the absence of any DSM-IV-TR disorder. Exclusion criteria for both patients and controls were co-morbid diagnosis on Axis I or II, substance dependence or abuse in the past year, major neurological illness, and perinatal trauma.
All subjects provided written informed consent to a protocol approved by the local Ethical Committee, which followed the principles of the Declaration of Helsinki.
Assessments
Patients underwent a broad battery aimed at assessing actual functioning in cognition, ToM, psychopathology, ATs, and retrospective premorbid functioning. Healthy controls were evaluated for cognition and ToM with the same tests. All tasks were administered by trained psychologists, except for the Positive and Negative Syndrome Scale (PANSS), which is used to derive PANSS Autism Severity Score (PAUSS), which was administered by trained psychiatrists. All data were collected at subjects’ enrollment.
Cognition was assessed with the Italian version of the Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al., Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour2004; Anselmetti et al., Reference Anselmetti, Poletti, Ermoli, Bechi, Cappa, Venneri, Smeraldi and Cavallaro2008). The battery consists of the following tests: list learning (verbal memory); digit sequencing (working memory); token motor task (psychomotor speed and coordination); symbol coding (processing speed); controlled oral words association test and category instances (verbal fluency); and Tower of London (executive functions). In light of this study’s purposes and of their primary role for ToM abilities, only processing speed and executive functioning tasks were administered (Bora, Yucel, & Pantelis, Reference Bora, Yucel and Pantelis2009; Piovan, Gava, & Campeol, Reference Piovan, Gava and Campeol2016). Raw scores were used for statistical analyses.
ToM abilities were assessed with the ToM Picture Sequencing Task (PST) (Brune, Reference Brune2003), which is composed of a sequencing task (i.e., a measure of non-verbal and affective ToM processes) and a questionnaire (i.e., a “cold ToM” measure). More in detail, the PST consists of six cartoon picture stories depicting (1) two scenarios where two characters cooperate, (2) two scenarios where one character deceives a second one, and (3) two scenarios where two characters cooperate in order to deceive a third one. In the sequencing task, four cards are presented face down in mixed order and the subject is asked to turn the cards over and to order them in a logical sequence of events. Two points are given when the first and the last cards are correctly ordered, and one point each is given when the two middle cards are correctly ordered. Furthermore, a 23-question ToM Questionnaire is administered to assess the subject’s ability to appreciate the mental states of each character involved in the cartoon stories. The questions refer to the mental states of the characters according to different levels of complexity and include first- to third-order false belief questions, questions concerning cheating detection, and two reality questions aimed at excluding major attentional problems. An answer is considered incorrect and scored 0 whether it includes errors about the story’s facts or inappropriate inferences on characters’ mental states, motivations, or beliefs. A previous study by Bechi et al. (Bechi et al., Reference Bechi, Riccaboni, Ali, Fresi, Buonocore, Bosia, Cocchi, Smeraldi and Cavallaro2012) confirmed the reliability and the good internal consistency (Cronbach’s α coefficient = .86) of the PST. In light of this study’s purposes, the variables of interest were the PST Total First-Order Beliefs Score, the PST Total Questionnaire Score, the PST Total Sequencing Score, and the PST Total Score. In particular, the PST Total First-Order Beliefs Score was included in order to clarify whether this basic mentalizing ability is impaired in patients with schizophrenia, similarly to patients with ASD.
PA was assessed with the Premorbid Adjustment Scale (PAS) (Cannon-Spoor et al., Reference Cannon-Spoor, Potkin and Wyatt1982), a retrospective interview focused on the individual’s social and academic achievements preceding illness onset. The PAS assesses PA during childhood (up to 11 years), early adolescence (12–15 years), late adolescence (16–18 years), and adulthood (19 years and above). Five domains are assessed (i.e., sociability and withdrawal, peer relationships, scholastic performance, adaptation to school, and social-sexual functioning) and rated from 0 (normal adjustment) to 6 (severe impairment). The adult PAS data were excluded from the current study because of uncertainties regarding its validity (van Mastrigt & Addington, Reference van Mastrigt and Addington2002; Horton et al., Reference Horton, Tarbox, Olino and Haas2015) and the risk to include measure after the disease onset. Thus, mean maladjustment ratings were calculated only for childhood, early adolescence, and late adolescence. Furthermore, as in prior studies (Allen, Frantom, Strauss, & van Kammen, Reference Allen, Frantom, Strauss and van Kammen2005; Bucci et al., Reference Bucci, Mucci, Piegari, Nobile, Pini, Rossi, Vita, Galderisi and Maj2016), separate scores were also calculated for Social and Academic PA at each developmental level, by averaging the sociability and withdrawal, peer relationships, and social-sexual functioning items (i.e., Social domain), scholastic performance and adaptation to school items (i.e., Academic domain).
Psychopathology was assessed with the PANSS (Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987), which evaluates the severity of positive, negative, and general psychopathology.
ATs and their severity were assessed with the PAUSS (Kastner et al., Reference Kastner, Begemann, Michel, Everts, Stepniak, Bach, Poustka, Becker, Banaschewski, Dose and Ehrenreich2015). Specific items of the PANSS are summed to create three sub-scales: (1) “Difficulties in Social Interaction” Score (items 1 ‘blunted affect’, 3 ‘poor rapport’, and 4 ‘social withdrawal’ from the Negative Scale); (2) “Difficulties in Communication” Score (items 5 ‘difficulties in abstract thinking’ and 6 ‘lack of spontaneity and flow of conversation’ from the Negative Scale); and (3) “Stereotypies/Narrowed Interests” Score (item 5 ‘mannerism’ and 15 ‘preoccupation’ from the General Scale, and item 7 ‘stereotyped thinking’ from the Negative Scale). Higher PAUSS scores represent a higher severity of the autistic-like phenotype.
Data Analysis
A two-step cluster analysis was conducted in order to identify groups of patients with different premorbid trajectories. PAS mean scores in childhood, early adolescence, and late adolescence were considered as clustering variables. Bayesian information criterion (BIC) values showed a first maximum of 169.52 for the two-cluster solution, a second maximum BIC of 172.76 for the three-cluster solution, and a third maximum BIC of 188.14 for the four-cluster solution. Given the very slight difference in BIC values, we opted for the three-cluster solution, according to studies where PA has been divided into three groups (Cole et al., Reference Cole, Apud, Weinberger and Dickinson2012; Chung et al., Reference Chung, Tang, Hui, Wong, Chan, Lee and Chen2013; Horton et al., Reference Horton, Tarbox, Olino and Haas2015).
Differences between groups in Social and Educational premorbid functioning were analyzed with repeated measures analyses of variance (ANOVAs) (3 × 3, p < .05, two-tailed), entering PAS Social and PAS Academic scores as dependent variables, time as fixed factor (with the three levels: childhood, early adolescence, and late adolescence) and PA groups (i.e., deteriorated, stable-poor, and stable-good) as independent variable. Fisher’s Least Significant Difference (Fisher LSD) test followed.
ANOVAs were performed on demographic, clinical, cognitive, and socio-cognitive, in order to evaluate the differences among patients’ groups and the control group, when applicable. Chi-squared test was applied on dichotomous variables. The significance of p value was corrected according to Bonferroni’s method and was set at .002.
Fisher LSD post hoc test was then launched, when applicable.
All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) software, version 22.
RESULTS
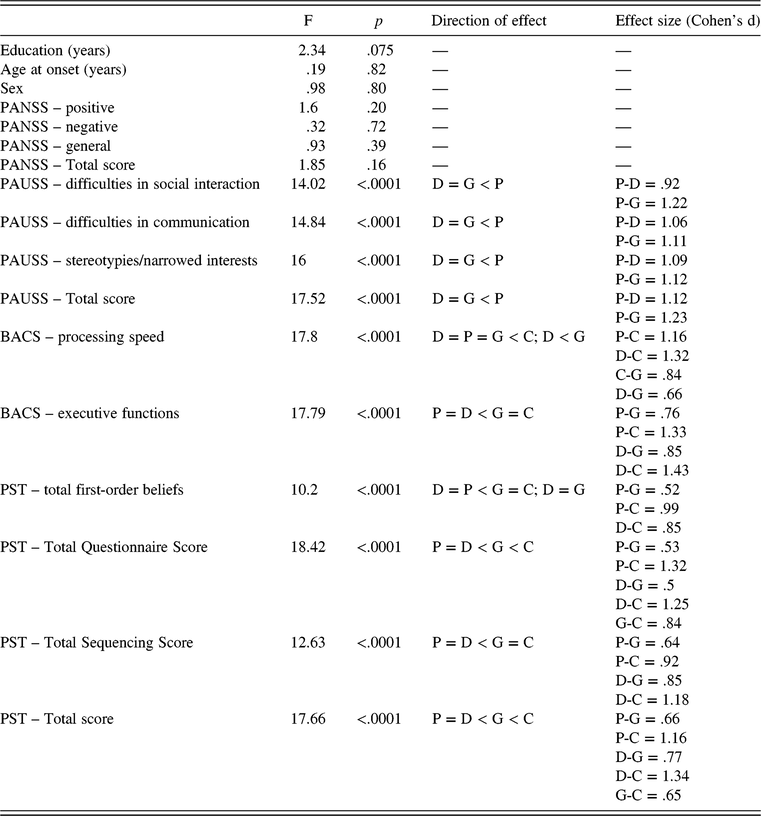
Table 1 shows demographic, clinical, premorbid, neurocognitive, and social cognitive data of patients, stratified by groups, and of healthy controls.
Table 1. Demographical, clinical, premorbid, neurocognitive, and social cognitive data

Data are given as mean (standard deviation).
PANSS = Positive and Negative Syndrome Scale; PAUSS = PANSS Autism Severity Score; BACS = Brief Assessment for Cognition in Schizophrenia; PST = Picture Sequencing Task; PAS = Premorbid Adjustment Scale.
The two-step cluster analysis on patients’ mean PA scores produced three groups with 36, 28, and 33 members each. The “silhouette measure of cohesion and separation” (i.e., a measure of clusters’ goodness of fit) stands at .5, suggesting a reasonable cluster structure. Group 1 (stable-poor group) included patients characterized by poor PA in childhood, which remained poor also across early and late adolescence. Group 2 (“deteriorating” group) included patients exhibiting a good PA during childhood, which got a further decline across both early and late adolescence. Group 3 (stable-good group) included patients with good PA during all developmental stages.
Global premorbid trajectories of the three groups are displayed in Figure 1.

Fig. 1. Groups’ trajectories during developmental stages. Trajectories show differences during childhood, adolescence, and late adolescence measured by PAS mean scores during these developmental stages.
Premorbid trajectories of PAS Academic and Social Scores also follow a pattern similar to mean PA, with stable-good, stable-poor, and “deteriorating” PA trajectories as depicted in Figures 2 and 3.

Fig. 2. Groups’ trajectories in Social PAS during developmental stages. Trajectories show differences during childhood, adolescence, and late adolescence measured by PAS Social scores during these developmental stages.

Fig. 3. Groups’ trajectories in Academic PAS during developmental stages. Trajectories show differences during childhood, adolescence, and late adolescence measured by PAS Academic scores during these developmental stages.
Repeated measures ANOVAs showed significant time (childhood, early adolescence, and late adolescence) × PA groups (i.e., deteriorated, stable-poor, and stable-good) interactions on PAS Social score (F[4,188] = 5.93, p = .0001) and PAS Academic score (F[4,188] = 7.11, p < .0001). Fisher LSD test showed that PAS Academic and Social Scores were significantly different between the stable-poor and the stable-good groups during all developmental stages. By contrast, as far as the “deteriorating” group is concerned, Fisher LSD test showed that PAS Social Scores were significantly different from the stable-poor and the stable-good groups during all developmental stages, except for childhood, where no significant differences between the “deteriorating” and the stable-good group emerged. Furthermore, the “deteriorating” group’s Academic Scores turned out to be significantly different from the stable-poor group during childhood and adolescence and from the stable-good group only during adolescence and late adolescence (see Table 2).
Table 2. Differences between stable-poor, stable-good, and “deteriorating” groups in Social and Academic premorbid functioning

P= stable-poor PA group; D= “deteriorating” PA group; G= stable-good PA group; PAS = Premorbid Adjustment Scale.
a p < .05.
Table 3 summarizes ANOVAs between patients’ groups and healthy controls. Significant differences, effect sizes, and directions of effects, evaluated with Fisher LSD when applicable, are reported. Main significant differences between groups were observed in PAUSS (Difficulties in Social Interaction, Difficulties in Communication, and Stereotypes/Narrowed Interests scores), BACS (Working Memory, Processing Speed, and Executive Functions scores), and PST (Total first-order beliefs, Total Questionnaire, Total Sequencing, and Total scores).
Table 3. Differences between stable-poor, stable-good, and “deteriorating” groups and healthy subjects

ANOVA = analysis of variance; P = stable-poor PA group; D = “deteriorating” PA group; G = stable-good PA group; C = controls; PANSS = Positive and Negative Syndrome Scale; PAUSS = PANSS Autism Severity Score; BACS = Brief Assessment for Cognition in Schizophrenia; PST = Picture Sequencing Task.
DISCUSSION
The present study aimed to analyze the influence of premorbid functioning and ATs on cognitive and social cognitive outcomes in a sample of patients with schizophrenia.
In line with previous studies (Cole et al., Reference Cole, Apud, Weinberger and Dickinson2012; Chung et al., Reference Chung, Tang, Hui, Wong, Chan, Lee and Chen2013; Horton et al., Reference Horton, Tarbox, Olino and Haas2015), we identified three groups of patients characterized by different PA trajectories, encompassing Social and Academic PA domains. We observed a stable-good PA group (i.e., patients with overall good PA), a stable-poor PA group (i.e., patients with widespread functional impairment since childhood), and a “deteriorating” PA group (i.e., patients with good PA during childhood, which progressively became poorer until schizophrenia onset).
More in detail, the stable-good group exhibited good Social and Academic PA, significantly different from that one characterizing the poor-stable group (during all developmental stages) or the “deteriorating” group (during adolescence and late adolescence).
As we hypothesized, those groups differed in actual social and cognitive functions, suggesting an influence of different premorbid functionings.
Concerning the stable-good PA group, our findings show that patients belonging to this group obtained better scores in actual cognitive functioning tasks, than the other patients groups. Indeed, their executive performance was similar to healthy controls, while processing speed performance was significantly worse than controls.
These results are in line with previous studies, suggesting that an impairment in speed-dependent functions would constitute a stable trait and an abnormal core cognitive process in schizophrenia even in high functioning patients (Leeson et al., Reference Leeson, Barnes, Harrison, Matheson, Harrison, Mutsatsa, Ron and Joyce2010; Bechi et al., Reference Bechi, Bosia, Agostoni, Spangaro, Buonocore, Bianchi, Cocchi, Guglielmino, Mastromatteo and Cavallaro2018).
As far as ToM is concerned, patients belonging to the stable-good group showed higher scores with respect to the other two groups on all PST Scores. When compared to healthy controls, patients in the stable-good group obtained lower scores in the PST Total Questionnaire (i.e., a measure of “cold” or cognitive ToM) and the PST Total Score, while no significant differences in the PST Total Sequencing Score (i.e., a measure of “hot” or affective ToM) emerged. These findings further stress that ToM impairment is multifactorial and derives from the interplay between multiple variables, which include, but are not limited to, cognitive factors such as preserved executive functions, which seem to crucially contribute to affective ToM processes (Piovan et al., Reference Piovan, Gava and Campeol2016; Bechi et al., Reference Bechi, Bosia, Agostoni, Spangaro, Buonocore, Bianchi, Cocchi, Guglielmino, Mastromatteo and Cavallaro2018). However, we suggest that the premorbid trajectory that characterizes the stable-good group might also help to explain the sparing of affective ToM processes. In fact, patients belonging to such group had the opportunity to acquire and exercise social skills (which are essential for ToM processes) during all their developmental stages. By contrast, cognitive ToM processes seem to be mainly influenced by disease onset.
As far as the “deteriorating” group is concerned, these patients present good Social and Academic PA during childhood that deteriorates from adolescence to late adolescence.
Our findings highlight that patients belonging to the “deteriorating” PA group after the onset of the illness do not statistically differ from those belonging to the stable-poor one, neither in ToM processes (affective, cognitive, and global), nor in processing speed and executive functions.
Therefore, despite they show a good Social and Academic PA during childhood, their global functioning declines during adolescence leading to a significant cognitive and social cognitive dysfunction after onset. Thus, these results suggest that adolescence period might be crucial in determining the subsequent illness outcomes.
The second main hypothesis of our study pertained the influence of ATs on cognitive and social cognitive functioning in schizophrenia. Our findings highlight that patients belonging to the stable-poor PA group show significantly higher PAUSS scores (a measure of ATs) compared to the other two groups of patients. Those patients are characterized by a compromised Social and Academic PA since childhood and show bad performance in cognitive and social cognitive tasks after schizophrenia onset.
Unexpectedly, the stable-poor PA group showed a widespread impairment of mentalizing abilities. Above all, this group presents lower first-order false beliefs abilities than other groups. This function requires the ability to recognize that different people may present different thoughts about the same situation, and many studies demonstrated that usually it is not impaired in schizophrenia, while it could be impaired in ASD (Baron-Cohen et al., Reference Baron-Cohen, Leslie and Frith1985). Furthermore, it is fundamental to remind that first-order false beliefs comprehension is completely acquired at the age of 4 in typically developing children (Baron-Cohen, Reference Baron-Cohen2001).
As a matter of fact, taking into account the limits of a retrospective study, the observation of premorbid functioning trajectories suggests that subjects included in the stable-poor PA group never had the opportunity from childhood to develop and acquire those abilities necessary for a gratifying and satisfying role functioning. Therefore, we can hypothesize that they developed insufficient and not adequate social and cognitive abilities from childhood, and these skills probably decline after the onset of the illness. This leads to the hypothesis that it could be traced a subset of patients with schizophrenia exhibiting behavioral traits typical of ASD since childhood. In other words, our findings could confirm the hypothesis of the existence of overlapping features between ASD and a group of patients with schizophrenia.
In line with our findings, a recent study by Barlati (Barlati, Deste, Gregorelli, & Vita, Reference Barlati, Deste, Gregorelli and Vita2018) reported that patients with ASD symptoms seem to present specific clinical (i.e., duration of illness, negative symptoms, and general psychopathology) and cognitive (i.e., working memory and processing speed) features.
Surprisingly, no differences emerged between groups concerning positive, negative, and general symptomatology as assessed with the PANSS. This negative result is in line with previous research (Cole et al., Reference Cole, Apud, Weinberger and Dickinson2012) that did not report any significant differences between groups in psychopathological variables. Another possible explanation for this lack of difference may be related to the characteristics of our sample, consisting of clinically stabilized subjects, with low PANSS total score.
In sum, these results further highlight the strong heterogeneity that characterizes schizophrenia pathology and underpins both premorbid and post-onset features, such as cognition and social-cognition. An innovative aspect concerns the finding that patients with a stable-poor premorbid trajectory also present autistic-like behaviors and a severe ToM impairment encompassing basic mentalizing processes, such as first-order beliefs, which are usually not impaired in schizophrenia.
Indeed, this study presents some limitations that must be taken into account: (i) the relatively small sample size might hamper the strength of our conclusions; (ii) cognitive assessment was limited to processing speed and executive functions; (iii) social cognitive assessment was limited to ToM abilities, excluding the other domains; (iv) the assessment of ATs through the PAUSS, which have been criticized since it assumes concordance between autism and negative symptoms; and (v) the cross-sectional and retrospective nature of functional assessment could have influenced our results. Future studies should take into account the role of environmental and traumatic factors, which might help to clarify further the issue of the “deteriorating” premorbid trajectory.
In conclusion, these data suggest the existence of a distinct group of patients with schizophrenia exhibiting autistic-like traits, characterized by a particular premorbid trajectory associated with specific cognitive and socio-cognitive profiles. In other words, such patients present intermediate features between the two diagnostic categories, that is, ASD and schizophrenia. To the best of our knowledge, to date very few studies focused on these patients (Barlati et al., Reference Barlati, Deste, Gregorelli and Vita2018) and this is the first study specifically aimed at analyzing their premorbid trajectory and their socio-cognitive abilities. The analysis of overlapping features between schizophrenia and ASD might have intriguing implications in both research settings and clinical practice. On the one hand, they may represent a possible underpinning endophenotype; on the other hand, the identification of such patients is of major relevance for rehabilitation approaches, in order to implement cutting-edge, person-centered interventions.
ACKNOWLEDGMENTS
The authors acknowledge Ms Eleonora Rossi for her support in proofreading and English revision. The Authors declare that there is no conflict of interest. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors have nothing to disclose.