Published online by Cambridge University Press: 03 February 2005
Sequences of the first internal transcribed spacer (ITS-1) and the D1-D3 domains of the large subunit (LSU) of the ribosomal DNA (rDNA) were determined for multiple specimens of 4 operational taxonomic units (OTUs) of the monogenean, Pseudorhabdosynochus lantauensis. OTUs were defined based on their collecting localities, host and/or morphological characteristics. All P. lantauensis specimens of one group (OTUs 1 and 3) differed in their sequences of the ITS-1 and partial LSU rDNA when compared with specimens of a second group (OTUs 2 and 4) by 12% and 2%, respectively. Results of the phylogenetic analyses of the LSU rDNA sequence data showed total (100%) bootstrap support for the separation of P. lantauensis into 2 distinct clades. At least 11 of the 18 nucleotide differences in the LSU sequence between the two P. lantauensis clades were derived (i.e. autapomorphic) characters when the morphologically distinct species, P. epinepheli and P. coioidesis, were used as outgroups. Furthermore, there were several autapomorphic character states for each P. lantauensis clade. This provides sufficient evidence to reject the null hypothesis that P. lantauensis represents a single species. Morphological and morphometric differences between these two clades provided additional strong support for the separation of P. lantauensis into two species. These two parasite species were found to co-exist on one of the two species of serranid fish (i.e. Epinephelus coioides) examined in the South China Sea (Guangdong Province, China).
Species of Pseudorhabdosynochus (Diplectanidae) occur on the gills of a variety of serranid fish, including those of Epinephelus (see Bu et al. 1999; Santos, Buchmann & Gibson, 2000). These fish are an important human food source and are cultured intensively in floating net cages in several countries, including China. However, infection by large numbers of Pseudorhabdosynochus in captive fish populations produces significant pathological effects and substantial economic losses (e.g., Leong, 1997; Vidal-Martínez & Mendoza-Franco, 1998). Given that serranid fish may be parasitized by several species of diplectanid (e.g., P. lantauensis, P. coioidesis and Diplectanum grouperi in E. coioides and E. aerolatus; see Bu et al. 1999), it is important that these parasites be identified in order to study their transmission patterns, epidemiology, and for the implementation of strategies to control the diseases they cause.
Pseudorhabdosynochus is characterized by the presence of a sclerotized, compartmental, bulb-shaped male copulatory organ (MCO). Identification of species within this genus is based on the size and shape of the MCO, vagina, squamodisc, ventral and dorsal bars, ventral and dorsal hamuli and the marginal hooklets, and on the number of rows of elements in the squamodisc (Beverley-Burton & Suriano, 1981; Kritsky & Beverley-Burton, 1986; Bu et al. 1999; Santos et al. 2000). However, there are sometimes difficulties in distinguishing among species with confidence because of the considerable within-species variation for some morphological characters (Santos et al. 2000). For example, the number of rows of elements in the squamodisc varies from 14–16 in P. amplidiscatus and P. capurroi, 14–17 in P. epinepheli and 15–16 in P. sulamericanus (see Santos et al. 2000). There are also reports of differences in the morphological measurements and/or descriptions of some species collected from different localities or hosts. For instance, Bu et al. (1999) noted differences in the shape of the dorsal bar and vagina of P. lantauensis from E. coioides (Malaysia and Indonesia) and E. aerolatus (Hong Kong) when compared to the original descriptions of Beverley-Burton & Suriano (1981) for P. lantauensis from E. brunneus and E. fario (Hong Kong). These differences were considered by Bu et al. (1999) to reflect geographical variation within the species or a consequence of comparing fixed with fresh specimens. Another possible explanation for these morphological differences is that P. lantauensis may represent two or more cryptic (i.e. genetically distinct but morphological similar) species.
DNA sequencing provides a valuable approach for the genetic characterization of parasite species (e.g. McManus & Bowles, 1996; Littlewood, Rohde & Clough, 1998) and for the detection of cryptic species (e.g. Andrews et al. 1998; Desdevises et al. 2000). Recent studies have demonstrated that sequences of the first internal transcribed spacer (ITS-1) and the partial large subunit (LSU) of the ribosomal DNA (rDNA) provide reliable genetic markers for the identification of monogenean species and for examining their phylogenetic relationships (e.g., Cunningham, 1997; Mollaret et al. 1997; Desdevises et al. 2000; Mollaret, Lim & Justine, 2000; Bentz et al. 2001; Chisholm et al. 2001; Whittington et al. 2004). In the present study, we compared the sequences of the ITS-1 and partial LSU rDNA, and the morphology of P. lantauensis from E. coioides and E. brunneus collected near Dayawan and Huidong (Guangdong Province, China) to test the null hypothesis that P. lantauensis from different hosts and sampling localities represents a single species.
Monogeneans were removed from the gills of 10 freshly killed fish. Some parasites were fixed in Bleasure's glue (Acacia gum 17·25%, glycerin 13·79%, chloral hydrate 34·48%, distilled water 34·48%) and their sclerotized parts examined using a dissecting microscope. All specimens (including those subsequently used for the DNA analyses) were identified morphologically to species level based on the existing keys and species descriptions (see Beverley-Burton & Suriano, 1981; Bu et al. 1999; Zhang, Yang & Liu, 2001). Measurements (in μm) of 22 specimens of P. lantauensis collected from 5 individuals of E. coioides and 5 individuals of E. brunneus from two locations in Guangdong, China (Table 1) were carried out as described previously (Bu et al. 1999). For the DNA analyses, 16 specimens (Psl 1-16) of P. lantauensis were divided into 4 operational taxonomic units (OTUs) based on host species, collecting localities (Dayawan or Huidong) and the size and shape of the vagina (e.g. presence/absence of a chelate diverticulum on the vagina) (Table 1). Specimens designated as OTU 1 and OTU 2 were collected from the same host individual (E. coioides) and locality (Dayawan), but differed in their morphology.

Genomic DNA was isolated from 24 individual specimens of Pseudorhabdosynochus (i.e. 4 P. coioidesis, 4 P. epinepheli, and 4 of each P. lantauensis OTU; Table 1). Each specimen was placed in 20 μl of lysis solution (proteinase K 20 μg/ml, Tween-20 0·45%, NP-40 0·45%, EDTA 1 mM, Tris-HCl 10 mM), and incubated at 65 °C for 1 h, followed by incubation at 95 °C for 15 min to inactivate the proteinase. This lysate (6 μl) was used as template in PCR reactions to amplify two DNA regions, the ITS-1 and the D1-D3 domains of the LSU rDNA. The ITS-1 was amplified using primers IT1 (forward; 5′-GTCGTAACAAGGTTTCCGTAGG-3′) and IT2 (reverse; 5′-GCTGCACTCTTCATCGACGCRCG-3′) (Ding et al. 2003), whereas the D1-D3 domains of the LSU rDNA were amplified using primers C1 (forward; 5′-ACCCGCTGAATTTAAGCAT-3′) and D2 (reverse; 5′-TGGTCCGTGTTTCAAGAC-3′) (Li, Liao & Yang, 2000). PCR reactions (50 μl) were performed in 1·5 mM MgCl2; PCR buffer (100 mM Tris-HCl, 500 mM KCl, 0·8% NP-40, pH 8·8) (TakaRa™); 200 μM of each dNTP; 0·8 μM of each PCR primer set and 2·5 units of Ex Taq polymerase (TakaRa™) in a thermocycler (MJ Research) using the following conditions: an initial denaturation at 94 °C for 5 min, followed by 30 cycles of 94 °C for 1 min (denaturation); 56 °C for 1 min (annealing) and 72 °C for 1 min (extension), followed by a final extension at 72 °C for 5 min. Control samples with host (fish) DNA or without genomic DNA (no-DNA controls) were included in each PCR run, but in each case, no amplicons were detected. Aliquots (5 μl) of amplicons were detected in 1% agarose gels, stained with ethidium bromide, and photographed using trans-illumination. The remaining 45 μl of each amplicon was purified over a spin column (Ultra-Pure™ PCR purification Kit, SBS) and subjected to automated DNA sequencing (ABI 373 DNA Sequencer, Shanghai United Gene Inc.) using the same primers (individually) as used for PCR.
Sequences were aligned using the computer program ClustalX (Thompson et al. 1997). Pairwise comparisons were made of the sequence differences (D) among P. lantauensis OTUs and between species using the formula D=1-(M/L), where M is the number of alignment positions at which the two taxa have a base in common, and L is the total number of alignment positions over which the two taxa are compared. Phenograms were constructed using the Unweighted Pair Group Method using Arithmetic averages (UPGMA; Sneath & Sokal, 1973). For the LSU rDNA sequence data, phylogenetic trees were constructed using a maximum parsimony (MP) analysis of the program PAUP* v.4b10 (Swofford, 1999). P. epinepheli (Pse 1-4) and P. coioidesis (Psc 1-4) were used as outgroups for these analyses. Characters were weighted equally and treated as unordered. Alignment gaps were treated as a fifth character state. A heuristic search with TBR-branch swapping was used to infer the shortest trees. A bootstrap analysis (using 1000 replicates) was conducted using heuristic searches and TBR branch-swapping with the MulTrees option, in order to determine the relative support for clades of the consensus tree.
Sequences of the partial LSU and complete ITS-1 rDNA were obtained for 4 specimens representing each of the 4 OTUs of P. lantauensis and 4 specimens of P. epinepheli and P. coioidesis. Sequences have been deposited in the EMBL, GenBank™ and DDBJ databases (Accession numbers AY553622-AY553624 and AY596179-AY596183). The lengths of the ITS-1 and the D1-D3 domains of the LSU for all parasite specimens ranged 467–534 bp and 884–896 bp, respectively. The ITS-1 and partial LSU sequences were aligned over a consensus length of 566 bp and 897 bp, respectively (sequence alignments available from corresponding author upon request). No sequence variation was detected in the ITS-1 and LSU rDNA among individuals of a P. lantauensis OTU, nor among individuals of P. epinepheli or P. coioidesis. The 4 P. lantauensis OTU1 specimens had identical ITS-1 and LSU sequences as the 4 P. lantauensis OTU3 specimens. Similarly, specimens of P. lantauensis OTU2 had identical ITS-1 and LSU sequences when compared to specimens of P. lantauensis OTU4. However, sequence differences were detected between these two P. lantauensis groups (i.e. OTUs 1 and 3 vs. OTUs 2 and 4). There were 62 (12%) nucleotide differences in ITS-1 sequence between the two groups, representing 46 substitutional changes (25 transitions, 21 transversions) and 16 indels (i.e. insertion-deletions). This difference was less than that (25%) detected between specimens of the 2 morphologically distinct species P. epinepheli and P. coioidesis. The phenogram depicting the genetic differences in ITS-1 sequence among specimens of Pseudorhabdosynochus revealed that P. lantauensis OTUs 1 and 3 were genetically more similar to P. lantauensis OTUs 2 and 4 than to either P. epinepheli or P. coioidesis (Fig. 1A). The topology of the phenogram derived from the LSU rDNA data topology (Fig. 1B) was similar to that for the ITS-1. There were, however, relatively fewer nucleotide differences in LSU sequence (2%) between P. lantauensis OTUs 1 and 3 and OTUs 2 and 4. These differences in LSU sequence corresponded to 17 substitutional changes (11 transitions, 6 transversions) and 1 indel.

Fig. 1. Phenograms depicting the average percentage differences in sequences for the (A) ITS-1 and (B) LSU rDNA (D1-D3 domains) among Pseudorhabdosynochus lantauensis OTUs, P. epinepheli and P. coioidesis.
A maximum parsimony analysis of the LSU sequence data yielded a single most parsimonious tree (Fig. 2) with a length of 123, a consistency index (excluding uninformative characters) of 0·87 and a retention index of 0·96. There was total statistical support (bootstrap values 100%) for the separation of P. lantauensis OTUs 1 and 3 and P. lantauensis OTUs 2 and 4 into 2 distinct clades. When P. epinepheli was used as the outgroup in the cladistic analysis, 17 of the 18 nucleotide differences between the two P. lantauensis clades were considered as derived (i.e. autapomorphic) characters (Fig. 2A), 11 for OTUs 1 and 3 (positions 193, 410, 494, 545, 546, 727, 804, 820, 872 and 829) and 6 for OTUs 2 and 4 (positions 619, 669, 772, 777, 808 and 837) (Fig. 2A). When P. coioidesis was used as the outgroup, 16 of the 18 nucleotide differences were considered as derived characters (Fig. 2B), 8 for OTUs 1 and 3 (positions 410, 494, 544, 669, 772, 804, 820 and 872) and 8 for OTUs 2 and 4 (positions 193, 546, 619, 727, 777, 808, 829 and 837) (Fig. 2B).

Fig. 2. Strict consensus trees, constructed using maximum parsimony and depicting the relationships of Pseudorhabdosynochus lantauensis OTUs 1–4, and the outgroups P. epinepheli (A) and P. coioidesis (B) based on sequence for the D1-D3 domains of the LSU rDNA. The derived character states (numbers correspond to sequence alignment positions) separating the two groups of P. lantauensis are shown on each branch. Numerals above the branches represent bootstrap values (%).
Table 2 shows the morphological measurements obtained for 22 specimens of P. lantauensis. Data for specimens representing OTUs 1 and 3 were combined, as were those for OTUs 2 and 4, based on the results of the molecular analyses and because there was no differences in measurements within each of these two groups. However, there were differences between the two groups (i.e. OTUs 1 and 3 vs. OTUs 2 and 4) in the distal length of the MCO and in the length of the vagina (Table 2). These structures were shorter in P. lantauensis OTUs 2 and 4. Also, P. lantauensis OTUs 2 and 4 had a larger body size (mean length×width: 611×184 μm vs. 533×164 μm), a greater ratio of the proximal to distal length in the MCO (1·9[ratio ]1–3·7[ratio ]1 vs. 0·8[ratio ]1–1·4[ratio ]1), a longer ventral bar and a larger squamodisc (mean length×width: 44×74 μm vs. 37×48 μm) than did P. lantauensis OTUs 1 and 3 (Table 2). Furthermore, the two P. lantauensis groups could be readily distinguished based on the shapes of the vagina (e.g. presence/absence of a chelate diverticulum on the vagina) and the dorsal bar (Fig. 3). All specimens of OTUs 1 and 3 had an overlapping dorsal bar and a longer, tubular vagina, whereas all specimens of OTUs 2 and 4 had a shorter, broader and more globular vagina (Fig. 3).
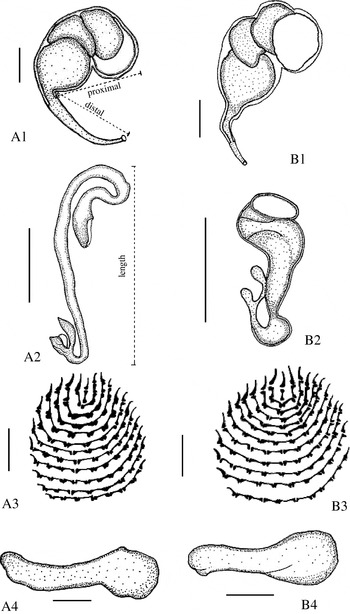
Fig. 3. Key morphological features of the two clades (A, OTUs 1 and 3; B, OTUs 2 and 4) of Pseudorhabdosynochus lantauensis. A1 and B1, male copulatory organs; A2 and B2, vagina; A3 and B3, squamodisc; A4 and B4, dorsal bar. Scale bars: 20 μm.
Table 2. Comparative measurements (μm) of Pseudorhabdosynochus lantauensis specimens used in present study (L, length; W, width.)

Sixteen specimens of P. lantauensis were divided into 4 OTUs based on host species, collecting localities (Dayawan or Huidong) and the shape and size of the vagina. OTUs 1 and 3 had 46 (12%) fixed differences in ITS-1 sequence when compared to OTUs 2 and 4. Similarly, there was 2% fixed sequence difference between two groups of P. lantauensis (i.e. OTUs 1 and 3 vs. 2 and 4). Although the magnitudes of difference in ITS-1 and LSU sequence among the two groups of P. lantauensis OTUs was considerably lower than that between the two morphologically distinct species, P. epinepheli or P. coioidesis (25% and 9%, respectively), they were within the range of sequence differences detected among closely related species of monogeneans (e.g. Cable et al. 1999; Bentz et al. 2001; Cunningham et al. 2001; Matejusová et al. 2001; Huyse & Volckaert, 2002; Whittington et al. 2004) and other platyhelminthes (e.g. Jousson, Bartoli & Pawlowski, 2000). However, it has been argued (e.g. Nadler et al. 2000) that the phylogenetic species concept should be used for species delineation rather than a comparative (i.e. yardstick) approach based on the magnitude of sequence differences.
Cladistic analyses were conducted on the D1-D3 domains of the LSU rDNA sequence data using P. epinepheli or P. coioidesis as outgroup to define autapomorphic (i.e. derived) character states within different lineages of P. lantauensis. The LSU was chosen for these analyses rather than the ITS-1 because of the greater confidence in comparing homologous characters (i.e. based on a more reliable sequence alignment). The results of the cladistic analyses showed that there was total statistical support (bootstrap values of 100%) for the separation of P. lantauensis into 2 distinct clades (i.e. OTUs 1 and 3 vs. OTUs 2 and 4). Furthermore, 17 of the 18 fixed nucleotide differences between these two groups were autapomorphic characters (11 for OTUs 1 and 3 and 6 for OTUs 2 and 4) when P. epinepheli was used as the group. There were 16 autapomorphic characters (8 for each P. lantauensis clade) when P. coioidesis was defined as the outgroup. The presence of autapomorphic character states for each of the two P. lantauensis clades provides strong evidence to reject the null hypothesis that P. lantauensis represents a single species.
Further evidence that P. lantauensis represents more than one species is provided by morphological and morphometric data. The two species could be readily distinguished from each other based on the difference in shape and size of terminal genitalia. P. lantauensis OTUs 1 and 3 possessed a differently shaped dorsal bar and a longer, tubular vagina, whereas P. lantauensis OTUs 2 and 4 had a shorter, broader and more globular vagina. The ratio of the proximal to distal length of the MCO was always higher in P. lantauensis OTUs 2 and 4 (1·9[ratio ]1–3·7[ratio ]1) than in P. lantauensis OTUs 1 and 3 (0·8[ratio ]1–1·4[ratio ]1), and the squamodisc of the former was wider than that of the latter. Previous studies have demonstrated that the characteristics of the MCO and vagina are of significance in delineating species within Pseudorhabdosynochus (see Beverley-Burton & Suriano, 1981; Kritsky & Beverley-Burton, 1986; Santos et al. 2000). Although the morphology of the haptor (including the hamuli, bar and hooklet) has also been considered important for distinguishing species of monogeneans (Yamaguti, 1963), there was overlap in the ranges of the length measurements for the haptor between P. lantauensis OTUs 1 and 3 and 2 and 4. This demonstrates that these structures are not useful diagnostic characters for this species complex. However, the shape of dorsal bar is one important morphological character for the delineation of the two P. lantauensis species (Bu et al. 1999).
The morphological features of P. lantauensis OTUs 1 and 3 were the same as those in the description of P. lantauensis from E. brunneus and E. farrio from Hong Kong (see Beverley-Burton & Suriano, 1981), whereas P. lantauensis OTUs 2 and 4 corresponds to the morphological description of this species from E. coioides collected in waters of Malaysia and Indonesia (see Bu et al. 1999). The morphometric measurements of P. lantauensis OTUs 2 and 4 are also in agreement with those of Bu et al. (1999). In contrast, specimens of P. lantauensis OTUs 1 and 3 were significantly larger than those recorded by Beverley-Burton & Suriano (1981). This may reflect methodological differences (i.e. measurements on fixed vs. fresh material) or the possibility of the presence of a third species within the P. lantauensis complex. Further study is therefore needed to elucidate the number of species within the P. lantauensis complex. This can be achieved by comparing, in a cladistic manner, the LSU and ITS-1 rDNA sequences for a large number of P. lantauensis from all geographical localities and species of hosts.
In conclusion, the combined molecular and morphological data obtained during the present study provide strong evidence to support the hypothesis that P. lantauensis from serranid fish collected in the South China Sea within Guangdong Province (China) represents at least 2 species. Both species were collected in sympatry from fish near Huidong. P. lantauensis OTUs 2 and 4 was only found in E. coioides (about 2000 individuals in 4 hosts), while P. lantauensis OTUs 1 and 3 was only detected in E. brunneus (about 1000 individuals in 5 hosts). In contrast, both parasite species (i.e. 42 and 27 individuals of P. lantauensis OTUs 1 and 2, respectively) were found in one E. coioides collected near Dayawan (i.e., located 60 km from Huidong). Detection of the presence of at least 2 co-existing species within what is currently considered as P. lantauensis has major implications for studying the transmission patterns and ecology of this socio-economically important group of parasites.
Project support was provided by grants from the National Natural Science Foundation of China (Grant No. 30170124) to A.X.L., and from the China National Funds for Distinguished Young Scientists (No. 30225033) to X.Q.Z. The authors are grateful to Professor Zhao-Rong Lun for advice and suggestions, and to Professor Jian-Ying Zhang for providing some references.

Table 1. The number, host species and collection localities in Guangdong Province (China) for Pseudorhabdosynochus specimens used in the molecular and morphological analyses
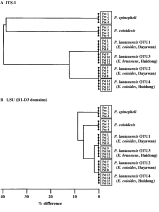
Fig. 1. Phenograms depicting the average percentage differences in sequences for the (A) ITS-1 and (B) LSU rDNA (D1-D3 domains) among Pseudorhabdosynochus lantauensis OTUs, P. epinepheli and P. coioidesis.

Fig. 2. Strict consensus trees, constructed using maximum parsimony and depicting the relationships of Pseudorhabdosynochus lantauensis OTUs 1–4, and the outgroups P. epinepheli (A) and P. coioidesis (B) based on sequence for the D1-D3 domains of the LSU rDNA. The derived character states (numbers correspond to sequence alignment positions) separating the two groups of P. lantauensis are shown on each branch. Numerals above the branches represent bootstrap values (%).

Fig. 3. Key morphological features of the two clades (A, OTUs 1 and 3; B, OTUs 2 and 4) of Pseudorhabdosynochus lantauensis. A1 and B1, male copulatory organs; A2 and B2, vagina; A3 and B3, squamodisc; A4 and B4, dorsal bar. Scale bars: 20 μm.

Table 2. Comparative measurements (μm) of Pseudorhabdosynochus lantauensis specimens used in present study