There has been tremendous growth in the field of paediatric transcatheter interventions in the recent years. Reference Kenny and Hijazi1 Various tools are now available for use in different types of congenital cardiac defects. Konar-multifunctional occluder (Lifetech, Shenzhen, China) is a relatively new device which has been primarily marketed for use in ventricular septal defects. Reference Schubert, Kelm, Koneti and Berger2 It is a nitinol alloy occluder available in a wide variety of sizes with 1-2 mm increments. A hybrid design produced by amalgamation of single and double disc duct occluder devices with screws on either side ensures that the occluder can be implanted in both antegrade and retrograde fashion. Even though the larger sized variants have interwoven polytetrafluoroethylene fabric, the occluders maintain a relatively small profile and can be delivered by guiding catheters or 4–7 Fr sheaths.
The nomenclature Konar-“multifunctional” occluder suggests that it is useful in a variety of cardiac lesions along with ventricular septal defects. Over the years, devices like duct occluders and Amplatzer duct occluder II (Abbott, Plymouth, MN), etc., have been used in positions for which they were not primarily designed. Reference Vijayalakshmi, Narasimhan, Singh and Manjunath3,Reference El-Sisi, Sobhy and Jaccoub4 A truly multifunctional occluder is one which can be used in a wide array of cardiac lesions with adequate safety, efficacy and technical feasibility. Different physical attributes of the Konar-multifunctional occluder make it an interesting choice for a variety of cardiac lesions. In this study, we share our initial experience of usage of these occluders in lesions other than ventricular septal defects.
Materials and methods
This study is a retrospective review from two centres. Eight patients, where Konar-multifunctional occluders were used in locations other than ventricular septal defects, in between March 2019 and September 2019, were included in the study. Pre-procedure, all patients underwent detailed clinical evaluation followed by transthoracic echocardiography, 12-lead electrocardiogram, and Chest X-ray at the outpatient department. All patients were either symptomatic or had a haemodynamically significant lesion on echocardiography (chamber dilatation). The decision to use Konar-multifuctional occluder for a particular pathology over other available choices was guided by clinical judgement. The patients were analysed based on their demographic data, type and size of lesion, size of occluder used, procedural approach, as well as complications if any. The continuous variables (age, weight) were expressed in terms of median with range. The device sizing was based on standard established guidelines. For patent ductus arteriosus and aortopulmonary window, the appropriate occluder size was 1.5–2 mm more than the defect. For coronary fistula, the appropriate occluder was thought to be atleast one and half times the narrowest diameter.
All patients were followed up post procedure at regular intervals with transthoracic echocardiograms and 12-lead electrocardiograms for a minimum period of 6 months.
Results
Our cohort consisted of five female and three male children. The age ranged from 8 months to 7 years with a median age of 3.2 years. The median weight was 9 kg (range – 4.8– to 18 kg). All procedures were performed under local anaesthesia and intravenous sedation. Intubation was not needed in any of the cases. Anticoagulation during the procedure was maintained with intravenous heparin at 100 units/kg. Device deployment was done from the antegrade approach in majority (seven patients, 87.5%). Of them, femoral vein was used in all patients except one where right internal jugular venous approach was needed in view of interruption of inferior caval vein. Retrograde femoral arterial approach was used in one patient (12.5%) (Table 1). Out of total eight patients, six patients had patent ductus arteriosus, one patient had aorto-pulmonary window, and one patient had coronary arterio-venous malformation. Successful deployment was seen in all patients. Procedural success was not achieved in one patient, and deployed device was replaced by a different type of occluder. The fluoroscopy time ranged from 2.3 minutes to 23.8 minutes with a median fluoroscopy time of 5.2 minutes. The most commonly used occluder was 6 × 4 mm (three patients – 37.5%). There were no instances of device displacement or embolisation. No major rhythm-related issues occurred in any patient during or after the procedure.
Table 1. Demographic and procedural details

IVC, inferior caval vein; LDO, Lifetech duct occlude; LPA, left pulmonary artery; MFO, multifunctional occlude; PAH, pulmonary arterial hypertension; PDA, patent ductus arteriosus; PM-VSD, peri-membranous ventricular septal defect; RCA, right coronary artery; RV, right ventricle
Three of our patients had relatively small ductus arteriosus (Table 1 – patient 1, patient 2, patient 6). The ductal morphology in these patients resembled Krichenko types D and E with an elongated course ending with a constriction at the pulmonary end with or without narrowing at the aortic end. All of them were successfully occluded with 6 × 4 mm Konar-MF occluder. 4 Fr TorqVue sheath (Abbott Structural Heart, MN, USA) was used to deploy the devices from femoral venous route in two patients. The third child was 7 years old and weighed 18 kg, and a retrograde femoral arterial deployment was done using 5 Fr guide catheter (Fig 1a–c).

Figure 1. Lateral view aortogram showing elongated narrow ductus with small opening at pulmonary end (a); Retrograde deployment of Konar-MF occluder and subsequent pulmonary injection showing unobstructed flow to left pulmonary artery [white arrow] (b); Aortogram post-release depicting final position of device with no residual (c)
Other three children who had undergone ductal closure had unique issues. One of them had interruption of inferior caval vein and an associated moderate sized peri-membranous ventricular septal defect (5.2 mm). The ventricular septal defect was closed first using a 10 × 8 mm duct occluder (Lifetech, Shenzhen, China). The duct was conical (Fig 2a) and measured 3.8 mm and ideal for a duct occluder device (8 × 6 mm). We felt that tracking a 6 Fr sheath, from neck through the heart, across the duct in a small child (7.8 kg), with an existing device in situ, might be difficult. Retrograde approach was also not desirable considering the weight of the child. Hence, a 5 Fr right coronary guide was used instead to cross the ductus from the neck and successfully deploy a 8 × 6 mm Konar-MF occluder (Fig 2b). Another patient with a 3.5 mm conical ductus was initially planned for a duct occluder. In this child, negotiation of the 6 Fr delivery sheath across the internal iliac vein was extremely difficult. Angiogram done locally showed significant bilateral stenosis of internal iliac vessels at the joining site with inferior caval vein. A 5 Fr right coronary guide, however, could easily be passed across the narrowed vein and subsequently the defect was closed using an 8 × 6 mm Konar-MF occluder. The remaining child had a large almost tubular ductus (5.3 mm) with severe pulmonary arterial hypertension. There was ample space in the left pulmonary artery and hence a double disc device was preferred. A 10 × 8 mm Konar-MF occluder was used in this patient. After release of device there was minimal colour doppler flow turbulence in left pulmonary artery but without any significant gradient (<2 m/s).

Figure 2. Conical (Type A) patent ductus arteriosus seen on lateral view angiogram (a) ; Antegrade deployment of Konar-MF occluder from right internal jugular venous route with ventricular septal defect device in-situ [white arrow] (b)
The next patient in our series had a 4 mm intermediate-type aorto-pulmonary window. The defect was well away from coronary ostia and was suitable for percutaneous closure. After initial angiograms, it was decided to close the defect with an 8 × 6 mm Konar-MF occluder (Fig 3a). The device could be successfully deployed from the antegrade femoral venous route. Post-deployment angiogram showed good approximation of the occluder to the margins of the defect. But significant intra-device residual flow was present probably due to lack of fabric in the device (Fig 3b). Considering the high flow nature of the defect, it was decided to retrieve the device. It was subsequently closed using a 10 × 8 mm duct occluder (Lifetech, Shenzhen, China).
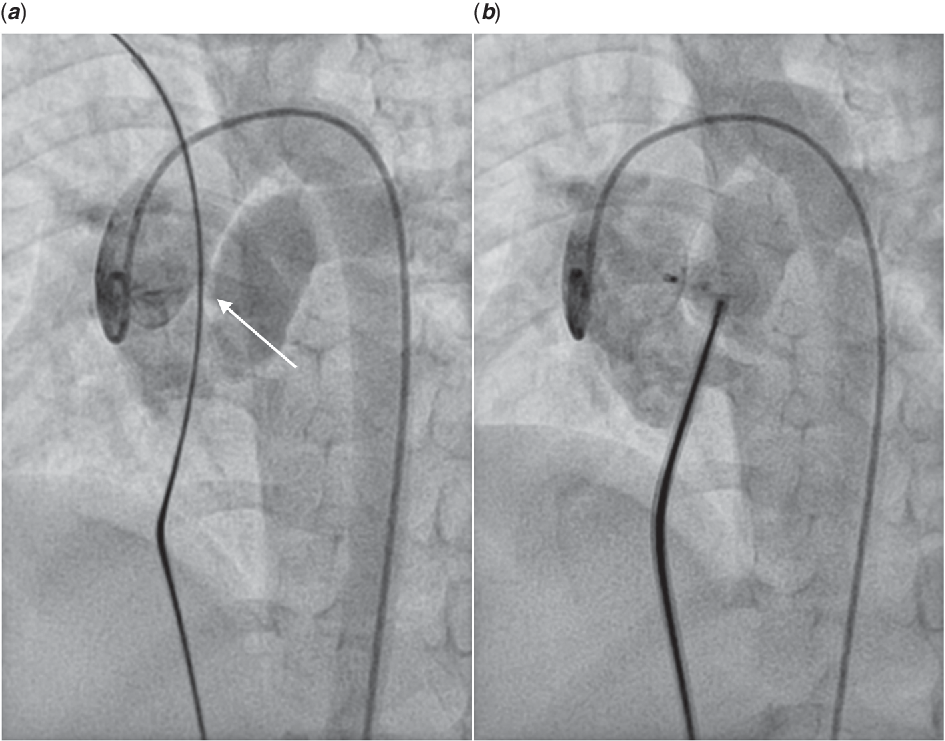
Figure 3. AP window closure using Konar-MF device: aortic root injection showed moderate sized intermediate type of AP window (white arrow) (a); Post device deployment aortic root injection showed stable device position with significant intra-device shunt (b)
The last child in our cohort had a haemodynamically significant coronary arterio-venous fistula. Suitability for percutaneous closure was assessed with transthoracic echocardiography and CT scan. The fistulous tract originated from left main coronary artery and drained into right ventricular apex with no major coronary branches arising out of it. The narrowest segment measured around 6.5 mm. Intra-procedure angiograms revealed similar findings (Fig 4a). A standard arterio-venous loop was created and a 6 Fr sheath was advanced from the femoral venous side to deploy a 12 × 10 mm Konar-MF occluder. Angiogram post device release showed complete abolition of the shunt (Fig 4b). The child was kept on anti-coagulant for 6 months and anti-platelet (Aspirin) for an indefinite period.

Figure 4. Coronary arterio-venous fistula closure using Konar-MF device: aortic root angiogram showed large coronary fistula from left main coronary artery to RV apex with no major side branches (a); Post-procedure angiogram showed complete occlusion of the fistulous tract by the device and unobstructed flow to the branches of left main coronary artery (b)
None of the patients had any major complications, prolonged stay, or any vascular injuries. All patients completed minimum 6-month follow-up and were doing well without any residual flows.
Discussion
Increasingly, more and more structural congenital cardiac defects are being tackled by transcatheter measures. Reference Kenny5 Konar-MF multifunctional occluder is a recent addition to a host of occluders already in vogue, for use in such conditions. It is a novel, hybrid bi-lobed occluder available in multiple size options and is primarily designed for closure of ventricular septal defects. It is soft, low to medium profile, malleable device with less clamping force, thus potentially reduces the risks of complete heart block. Initial reports from few studies with early and midterm follow-up show promising results in ventricular septal defects. Reference Tanidir, Baspinar and Saygi6,Reference Haddad, Daou and Saliba7 In this study, however, we have retrospectively looked at our non-ventricular septal defect usage of the multifunctional occluder and its potential scope in such circumstances.
Percutaneous closure has become the standard of care for majority of patients with patent ductus arteriosus over the last few decades. Reference Azhar, Abd El-Azim and Habib8 Duct occluder devices have stood the test of time as being safe and effective for majority of the ducts. Reference Parra-Bravo, Cruz-Ramírez and Toxqui-Terán9 Other devices like Duct Occluder II (Abbott, Plymouth, MN), Vascular Plug II (Abbott, Plymouth, MN), or embolisation coils have also been used sparingly in specific ductal morphologies. Reference Van Loozen, Sandoval and Delaney10 We have used Konar-MF occluders in six patients for specific indications. Use was guided by the ductal morphology in four of our patients. Some of them had elongated ductus with constriction at the pulmonary end or at aortic end (Krichenko types D and E). Reference Krichenko, Benson and Burrows11 In this type of ductal morphology, often it is extremely difficult to maneuver the delivery sheath across the ductus from the venous side and forcibly doing so may lead to catastrophic consequences. Hence, low-profile double disc devices like Duct Occluder II (Abbott, Plymouth, MN) have been sometimes used to close such ductus. Embolisation coils have also been commonly used in many such ducts but is much more technically demanding. Reference El-Said, Bratincsak and Foerster12 We found the Konar-MF device to be safe and effective and could be easily delivered through guide catheters or small sheaths from either arterial or venous side thus having similar advantages like the duct occluder II device. Double disc devices like Konar-MF are also potentially useful in short tubular ductus particularly those with severe pulmonary arterial hypertension. Reference Baruteau, Lambert and Riou13 This was seen in one of our patients where we effectively used a 10 × 8 mm occluder with satisfactory results.
We used the Konar-MF occluder in two patients with regular ductal morphology (conical; Krichenko type A). Both patients had antegrade approach-related issues. In the child with interrupted inferior caval vein, the use of Konar-MF occluder obviated the need for a larger sheath from neck in the presence of a ventricular septal defect device (Fig 2b). The procedure was safer and less cumbersome. The same can be said about the child with internal iliac vein stenosis where a smaller guide catheter could be passed easily to successfully close the ductus using a Konar-MF device. Inspite of our successful endeavors, we would not like to recommend the routine use of Konar-MF occluder for ductus closure. The risk of left pulmonary occlusion is a real concern for any double-disc device Reference Liddy, Oslizlok and Walsh14 and should always be kept in mind while deploying such a device, even when done as a bailout option.
Coronary arterio-venous malformation is a highly anatomically variable lesion and has a certain degree of unpredictability. Reference Jama, Barsoum and Bjarnason15 The multifunctional nature of the Konar-MF occluder was evident in our patient with coronary arterio-venous malformation. Even a large device (12 × 10 mm) could be very easily tracked through a 6 Fr sheath. After deployment the device conformed to the shape of the defect by its radial force losing its self-centering property. We found a similar report by Egorova et al. Reference Egorova, Ewert and Hadamitzky16 where Konar-MF occluder was successfully used to close a left circumflex to coronary sinus fistula. The effective use in such scenarios coupled with dual screw advantage makes the Konar-MF occluder potentially a very useful device for closure of various other vascular communications including high flow arterial collaterals as well as paravalvular leaks. In any such attempt, the size could be a limiting factor considering largest available occluder is of 14 × 12 mm variant. The other issue that we faced was in our child with aorto-pulmonary window where due to the residual intra-device flow, the 8 × 6 mm occluder had to be replaced inspite of proper sizing and successful deployment. Perhaps the presence of a fabric would have been useful in an occluder of such size, particularly in high flow settings. We believe a 10 × 8 mm Konar-MF which has fabric would have achieved instant occlusion. The decision of opting for duct occluder was an on table decision of the operator. Alternatively, we could have waited on a table for a few minutes as immediate intra-device flow is known to reduce with time as seen with closure of other high flow defects like ductus arteriosus.
In our experience, some of biggest advantages with a Konar-MF occluder lay in the technical feasibility and ease of use. Sizes upto 8 × 6 mm have a very low profile and could be delivered using slender delivery cables (0.025 in. nitinol or stainless steel delivery cables). We did a bench testing with all the available occluder sizes and found that the actual sheath and catheter compatibility were even lower than the company recommendations. We are publishing our findings for future references (Table 2). Considering that the occluder is primarily designed for ventricular septal defects, our observations showcase the versatile nature of its use.
Table 2. Bench testing results of Konar-MF occluders for sheath and guide catheter compatibility
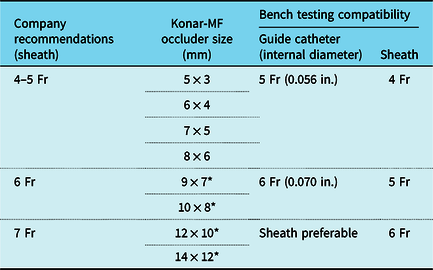
* Occluders have woven PTFE mesh.
Conclusion
To summarise, the Konar-MF occluder has quite a few interesting attributes which makes it a very exciting addition in the paediatric catheterisation laboratory. The asymmetric design with screw on either side provides both antegrade as well as retrograde delivery options in many complicated scenarios. The low-profile occluder also offers potential advantages in small children. With proper knowledge of anatomy and when used with proper judgement, in various situations, the occluder lives up to its “multifunctional” nomenclature. Production of larger sized variants and inclusion of fabric in some of the smaller sized occluders may further widen the scope of this device.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical Standards
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later ammendments.









