Introduction
Genetic control programmes like the sterile insect technique (SIT) that use irradiation to induce sterility often experience a reduced competitiveness of released insects (Dyck et al., Reference Dyck, Hendrichs and Robinson2005; Parker & Mehta, Reference Parker and Mehta2007). This reduction in competitiveness results from the somatic damage induced during the irradiation process (LaChance, Reference LaChance, Wright and Pal1967; Proverbs, Reference Proverbs1969), in addition to the costs of mass-rearing (Cayol, Reference Cayol, Aluja and Norrbom2000; Calkins & Parker, Reference Calkins, Parker, Dyck, Hendrichs and Robinson2005; Rull et al., Reference Rull, Brunel and Mendez2005), and other factors associated with transportation, packaging and release. SIT programmes can adopt different strategies to deal with a reduction in competitiveness. The loss of competitiveness is accepted and the reduction compensated for by increasing the ratio of irradiated males over wild ones for releases (Dyck et al., Reference Dyck, Hendrichs and Robinson2005). Alternatively, it is attempted to produce more competitive insects by lowering the irradiation dose, or irradiating the most competitive, normally fully mature, developmental stage for which somatic damage is decreased (Parker & Mehta, Reference Parker and Mehta2007).
For mosquitoes, the mating competitiveness of males irradiated as pupae was observed to be lower compared to males irradiated in the adult stage (Curtis, Reference Curtis1976; Andreasen & Curtis, Reference Andreasen and Curtis2005), and previous results from our laboratory confirmed these findings (Helinski & Knols, Reference Helinski and Knols2008). Anopheles arabiensis Patton males irradiated as pupae had a lower competitiveness compared to un-irradiated males, and this was more pronounced for a fully-sterilizing dose of 120 Gy (Helinski & Knols, Reference Helinski and Knols2008). In contrast, adult irradiation with a partially- (70 Gy) or fully-sterilizing dose resulted in equal competitiveness of irradiated males compared to un-irradiated males when tested at a 1:1:1 ratio in large cages (Helinski & Knols, Reference Helinski and Knols2008). An increase in the ratio of irradiated males can compensate for the loss in competitiveness, and this was demonstrated in An. pharoensis Theobald (Tantawy et al., Reference Tantawy, Abdel-Malek and Wakid1967) and An. quadrimaculatus Say (Davis et al., Reference Davis, Gahan, Weidhaas and Smith1959) after pupal stage irradiation. In the current study, the competitiveness of males irradiated as pupae was determined when the number of irradiated males was increased threefold (i.e. 3:1:1) compared to the un-irradiated males. It was hypothesized that at this higher ratio the proportion of females mated to irradiated males would increase compared to the results observed at the 1:1:1 ratio.
Despite the fact that adult irradiation results in more competitive males, the irradiation and transportation of pupae is highly preferred because of their robust nature and ease of handling. In previous studies, pupae have been routinely irradiated approximately 24 h after pupation (Davis et al., Reference Davis, Gahan, Weidhaas and Smith1959; Tantawy et al., Reference Tantawy, Abdel-Malek and Wakid1967; Abdel-Malek et al., Reference Abdel-Malek, Tantawy and Wakid1967, Reference Abdel-Malek, Wakid, Tantawy, El-Gazzar, Nehme and Hassan1975; Curtis, Reference Curtis1976; Helinski et al., Reference Helinski, Parker and Knols2006; Helinski & Knols, Reference Helinski and Knols2008). In a study where males were irradiated as slightly older pupae (i.e. 24–32 h), it was observed that their competitiveness remained below the competitiveness observed in males irradiated as young adults (<24 h) (Andreasen & Curtis, Reference Andreasen and Curtis2005). In the current study, we irradiated even older pupae by slowing down their development by cooling, such that a fully developed adult was present inside the pupal skin for irradiation. It was hypothesized that the irradiation of these older pupae would result in more competitive males compared to the irradiation of younger, not fully developed, pupae. In addition, cooling under hypoxia of insects prior to the irradiation process is routinely used in Tephritid fruit fly SIT programmes (i.e. 12–20°C) to slow down the metabolic rate and potentially reduce somatic damage (FAO/IAEA/USDA, 2003).
Material and methods
Mosquitoes
The mosquito strain used was the Dongola strain of Anopheles arabiensis. For a detailed description of rearing procedures, see Helinski et al. (Reference Helinski, Parker and Knols2006). Until experiments took place, insects were maintained in the insectary at 27±0.5°C and RH 82±2% in normal rearing cages (30×30×30 cm). Competition experiments were performed in large pyramid-shaped cages and measured 1.8(l)×1.2(w)×1.0(h) m. (MoTec Pop-Up, Brettschneider, Heimstetten, Germany); for a detailed description, see Helinski & Knols (Reference Helinski and Knols2008). Large cage experiments took place in the room used for larval rearing with slightly different conditions (24±0.5°C and RH 50±3%). Previous work had shown that survival and mating behaviour under these conditions were similar to what can be observed in the insectary (Helinski et al., Reference Helinski, Hood and Knols2008). The light regime in both rooms was L10:D12 plus a one hour computer-simulated dusk and dawn period. All adults were continuously supplied with 6% sucrose solution [w/v].
Irradiation
Insects were exposed to gamma rays generated by a cobalt-60 source with a dose rate of ca. 12 Gy min−1. Males were irradiated in the pupal stage, and irradiation procedures and handling methods were similar to those described by Helinski et al. (Reference Helinski, Parker and Knols2006) and Helinski & Knols (Reference Helinski and Knols2008). Irradiation doses were 70 Gy for a partially-sterilizing dose and 120 Gy for a fully-sterilizing dose. Dosimetry was used for each irradiation to verify the doses received by the batch (Helinski et al., Reference Helinski, Parker and Knols2006).
Collection of experimental material
Pupae were collected between 9 am and 3 pm and were not sexed prior to irradiation. Young pupae were irradiated aged 20–26 h after pupation as routinely performed (Helinski et al., Reference Helinski, Parker and Knols2006; Helinski & Knols, Reference Helinski and Knols2008); old pupae were irradiated aged 42–48 h. Old pupae were obtained by the artificial prolonging (i.e. normally pupae emerge approximately 30–36 h after pupation) of the pupal phase by cooling. On the morning after collection, the pupae (i.e. aged 18–24 h) were placed at 9 am in an incubator, and were cooled for 24 h until irradiation the next day at 9 am. The light regime in the incubator was synchronized to light conditions in the insectary and was L12:D12. Temperature in the incubator was 15.7±0.5°C during darkness and 18.7±0.5°C during the light phase as a result of the heat produced by the tube lights. After irradiation, pupae emerged in a normal rearing cage in the insectary. Young pupae emerged in the evening following irradiation and sexes were separated the next day; old pupae emerged within six hours after irradiation and sexes were separated the same day.
The effect of pupal cooling on adult emergence and longevity was tested for the following treatments: (i) control (i.e. no cooling), (ii) cooling (i.e. no irradiation), (iii) cooling and irradiation with 70 Gy or (iv) cooling and irradiation with 120 Gy. For each treatment, 100 pupae were used and treatments were replicated thrice; all pupae were derived from the same batch. Adult emergence was scored, and longevity of the emerged adults was determined routinely (i.e. at 24–48 h intervals) in normal rearing cages by the removal of dead insects until the majority of adults had died. In a separate experiment, a number of old pupae were fixed in 96% ethanol and dissected to determine pupal development.
Competition experiments
Irradiated males were introduced in the large cage together with un-irradiated males and females at the ratio of 3:1:1 and 75–100 insect were used for the lower ratio (i.e. 1). Mating took place for two nights, and mortality of males and females was scored after the experiment. The un-irradiated males in all experiments were derived from the same batch of pupae as the irradiated males. For the experiments with old pupae, the un-irradiated males used in the competition experiments were not cooled but left to emerge as normal. Females used as mates were of similar age as the males and separated to sex <18 h after emergence to ensure virginity. First, the competitiveness of males irradiated as young pupae was tested; subsequently, experiments with males irradiated as old pupae were performed. For both pupal age treatments, irradiation doses (i.e. 70 or 120 Gy) were tested pair-wise; and, for each dose, four replicates with different batches of males were carried out. Because only one large cage was available, doses could not be tested simultaneously, and experiments had to be performed in succession. The males introduced first were aged three days, while males introduced second were aged five days with the exception of replicate one for young pupae where all males were two days older. The order of experiments (i.e. dose tested first) was alternated between replicates.
Blood feeding and egg laying
Females were blood fed on membranes filled with heparinized human or defibrinated cow blood. If feeding was low, the unfed females were blood fed on the forearm of a human volunteer. Females were fed prior to introduction in the cage or afterwards, depending on time of introduction; females introduced with the first batch of males were fed afterwards, females introduced with the second batch before. Three to four days after blood feeding, fed females were individually placed in tubes (2.5×9 cm) for egg laying. For every egg batch, the hatch rate was determined after substantial time was given for hatching (i.e. 5–7 days).
To determine if an egg batch from the competition experiments was fathered by an irradiated or an un-irradiated male, the level of inherent (control) sterility in the colony, and the sterility induced by irradiated males in the absence of competition had to be determined. Males were mated against un-irradiated virgin females, and hatch rates of individually laid egg batches were determined (i.e. referred to as control data). Data for un-irradiated males, and males irradiated as young pupae, had already been collected in a previous study (Helinski & Knols, Reference Helinski and Knols2008); and data for males irradiated as old pupae were collected in the present study.
Statistics
Prior to analyses, data were checked for normality. Hatch data were analyzed according to procedures described previously (Helinski & Knols, Reference Helinski and Knols2008). Briefly, logistic regression analyses were used to classify the hatch rates as either deriving from irradiated or un-irradiated males (i.e. using the control data); this distribution was analysed using a replicated G-test for goodness of fit. If there was no heterogeneity between replicates of a treatment, data were pooled. General linear models (GLMs) were used to compare the proportion of un-irradiated batches between treatments, and means were separated using Tukey's honestly significantly different (HSD) and individual t-tests. To facilitate comparison with similar studies in the literature, the competitiveness index C (C=(# irradiated batches/# un-irradiated batches)×(N/S), where S is the number of sterile males and N is the number of un-irradiated males) was determined (Fried, Reference Fried1971; Hooper & Katiyar, Reference Hooper and Katiyar1971). Cumulative mortality data from the competition experiments were arcsine square root transformed and analysed using GLMs. Mortality data from the cooling experiment were analysed using Kaplan-Meier survival analysis with log-rank tests. All two-sided tests were performed using the SPSS software version 14 (SPSS Inc., Chicago, USA).
Results
Mean values throughout the text are reported±SEM. Dosimetry confirmed that all doses delivered were within a 5% error range.
Cooling
After placing pupae for 24 h at the lower temperature, upon dissection a fully developed adult was present inside the pupal skin; in contrast to the young pupae aged 20–26 h (fig. 1). After irradiation of the old pupae, the large majority of adults emerged within the first six hours. The cooling of pupae for 24 h had no impact on pupal emergence compared to a control without cooling, nor was there an effect on emergence of the combined treatment of cooling and irradiation with either dose (F(3, 8)=0.35, P>0.05). On average, emergence was 96±1%. The analysis of longevity was complicated by a rather large variation within treatments, and for all treatments replicates could not be grouped for statistical analysis. However, in table 1 it can be seen that cooling of pupae appeared to have no effect on mean male longevity compared to uncooled males. Survival times of males cooled as pupae and irradiated with 70 Gy were similar compared to uncooled males with the exception of replicate one, where survival was low. For 120 Gy, a trend towards a reduced survival was observed (table 1).

Fig. 1. Images of a young (i.e. 20–26 h) and old (i.e. 42–48 h) pupa used in the irradiation experiments. Location of the eye and wing are indicated. In the old pupa, development was near to completion and a fully developed adult (with scaled wings) was present inside the pupal skin; in the young pupa, development was still in progress.
Table 1. Effect of different pupal treatments (i.e. control (no cooling) and cooling for 24 h with or without irradiation with 70 or 120 Gy) on mean (±SEM) survival times of An. arabiensis males. Survival times were estimated from Kaplan-Meier survival analysis. For each replicate, approximately 100 insects were used and on average 54±2% were males. Replicates were not statistically similar (log-rank tests, data not shown) and data could not be pooled.

Sterility
The competitiveness of males irradiated as pupae at 20–26 h with 70 Gy varied considerably between replicates and data could not be pooled (table 2). Individual G-test results showed that only in the second replicate significantly fewer females were inseminated by irradiated males compared to un-irradiated males; in the other replicates, no significant differences were observed (table 2). For the 120 Gy experiments, replicates were statistically similar and data were pooled (table 2). Significantly fewer females were inseminated by irradiated males than un-irradiated males (table 2), and on average 81±5% of all egg batches were fathered by un-irradiated males (fig. 2).

Fig. 2. Proportion of egg batches (mean±SEM) fathered by un-irradiated males for young (i.e. 20–26 h, open bars) and old (i.e. 42–48 h, grey bars) pupae irradiation. Bars with the same letter are not statistically different from each other, P<0.05 (Tukey HSD).
Table 2. The number of egg batches fathered by un-irradiated or irradiated males in the competition experiments. Males were irradiated with 70 or 120 Gy as pupae either aged 20–26 h or 42–48 h. Irradiated males competed with un-irradiated males for females on a 3:1:1 ratio. Individual and pooled G-tests results are given, as well as the competitiveness (C) value (mean±SEM) based on the four replicates for each treatment when data could be pooled. The mean proportion of egg batches with intermediate hatch rate (i.e. between 20–60%) is presented.
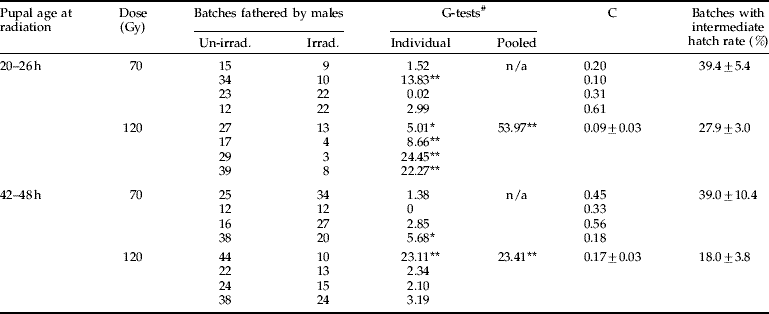
# Asterisks indicate significant differences between control and irradiated batches; *, P<0.05; **, P<0.01; n/a, not applicable (i.e. replicates not statistically similar).
The control data of older pupae (i.e. 42–48 h) gathered to construct regression models resulted in some interesting findings. Females mated to males irradiated as old pupae with 120 Gy produced a small number (i.e. 4%) of egg batches with a much higher hatch rate (i.e. 22–65%) than could be expected based on the data obtained from young pupae (Helinski & Knols, Reference Helinski and Knols2008). These data were removed from the logistic regression analysis to normalize the data (i.e. to prevent the threshold from becoming too liberal). For 70 Gy, the control data were similar compared to the data obtained from young pupae (Helinski & Knols, Reference Helinski and Knols2008).
The distribution of egg batches fathered by irradiated and un-irradiated males for the males irradiated as older pupae are shown in table 2. For 70 Gy, again replicates were not statistically similar and data could not be pooled. Individual results showed that males irradiated with 70 Gy fathered a similar number of egg batches compared to un-irradiated males, with the exception of the last replicate where un-irradiated males accounted for the majority of matings (table 2). For 120 Gy, the individual G-test results showed no significant differences in the number of females inseminated by irradiated or un-irradiated males in all replicates but the first, in which irradiated males inseminated significantly fewer females than un-irradiated males (table 2). When data were pooled, irradiated males performed overall worse than un-irradiated males (table 2), and 67±5% of all egg batches were fathered by un-irradiated males (fig. 2).
When comparing the performance of males that had been irradiated as young or old pupae, it appeared that in the latter treatment irradiated males performed slightly better (fig. 2, table 2). However, no statistically significant differences could be observed in the proportion of females inseminated by un-irradiated males for either dose when all data were combined (fig. 2). The only significant difference was observed between old pupae irradiated with 70 Gy and young pupae irradiated with 120 Gy (F(3,12)=4.59, P<0.05; fig. 2).
The proportion of egg batches with an intermediate hatch rate, defined as being between 20–60% (see Helinski & Knols, Reference Helinski and Knols2008) is shown in table 2. The proportion of egg batches with intermediate hatch rate was higher for competition experiments with 70 Gy compared to 120 Gy (table 2), but data were just not statistically different from each other (F(3,12)=3.58, P=0.051).
Mortality & egg laying
Cumulative mortality of males and females after two nights of competition was similar for all experiments (males: (F(3,12)=1.42, P>0.05); females: (F(3,12)=0.48, P>0.05)) and was 7±1% and 4±1% for males and females, respectively. The proportion of females laying eggs in individual tubes was similar for all experiments (F(3,12)=0.63, P>0.05); and, on average, 55±4% of the females laid eggs.
Discussion
At a three to one ratio, males irradiated as pupae aged 20–26 h with the partially-sterilizing dose of 70 Gy fathered an equal number of egg batches compared to un-irradiated males for the majority of replicates. The proportion of females mated by un-irradiated males was on average 57±9%. At the fully-sterilizing dose of 120 Gy, the un-irradiated males accounted for 81±5% of matings. Previous competition experiments on a 1:1 ratio in the large cage observed 73±2% and 84±3% of all matings to be fathered by un-irradiated males when competing with males irradiated in the pupal stage at 70 and 120 Gy, respectively (Helinski & Knols, Reference Helinski and Knols2008). Thus, it appeared that a threefold increase of males irradiated as pupae with 70 Gy resulted in an increase in the number of egg batches fathered by irradiated males. For 120 Gy, this increase was not observed and higher ratios are probably needed to increase the number of females mated by irradiated males (Davis et al., Reference Davis, Gahan, Weidhaas and Smith1959, Tantawy et al., Reference Tantawy, Abdel-Malek and Wakid1967). However, some caution in interpreting results from the different ratio experiments is needed, as conditions in the two ratio experiments, notably density and mating period, were higher and longer, respectively, in the 1:1 ratio study (Helinski & Knols, Reference Helinski and Knols2008). Nevertheless, the conditions presented in this study, with a mating period of two nights only, could be regarded as more representative of a natural situation, where released Anopheles males are likely to participate in few swarming events only.
The cooling of pupae for 24 h successfully delayed adult emergence, and a fully developed adult was present for irradiation. Cooling, either alone or in combination with radiation had no impact on adult emergence. Even though the analysis of longevity was complicated by large variation within replicates, mean survival data indicated that cooling had no effect on male longevity. Cooling in combination with irradiation resulted in a reduced longevity for 120 Gy, while for 70 Gy this was only observed for one of the replicates. In previous work, survival of males was similar to the control for 70 Gy. For 120 Gy, results varied; and a similar or reduced longevity compared to un-irradiated males was observed (Helinski, Reference Helinski2008).
Males irradiated as old pupae appeared slightly better competitors compared to males irradiated as young pupae based on the individual G-test results (table 2). However, no significant differences could be observed in the number of females inseminated between the two treatments for both doses when data was combined. In addition, males irradiated as older pupae appeared to have a lower mating competitiveness compared to males irradiated in the adult stage where an equal competitiveness to un-irradiated males was observed (Andreasen & Curtis, Reference Andreasen and Curtis2005; Helinski & Knols, Reference Helinski and Knols2008). Thus, the hypothesis that the irradiation of old pupae, in which a fully developed adult was present, would result in a similar level of male mating competitiveness compared to adult stage irradiation did not hold to the extent expected. However, the observation that cooling can be used to delay pupal emergence in An. arabiensis without any impact on adult emergence or longevity is useful for operational SIT programmes where pupae need to be transported (e.g. when the field site is not in the vicinity of the rearing facility) or stacked for further handling (Mutika et al., Reference Mutika, Opiyo and Robinson2001).
The data showed a greater variability in mating competitiveness for the 70 Gy irradiation treatments compared to 120 Gy for both young and old pupae irradiation experiments. It is assumed that the lack of variability for 120 Gy is caused by the fact that at this high dose all males will receive a considerable amount of somatic damage and, thus, little variation in mating competitiveness is seen. For the lower dose of 70 Gy, we hypothesize that not all males are damaged equally, which could have resulted in the greater variability observed for these experiments.
Surprisingly, a few egg batches with much higher hatch rate than expected were observed for females mated to males irradiated as old pupae with 120 Gy. For 70 Gy, this phenomenon was observed previously (Helinski & Knols, Reference Helinski and Knols2008); and again, in the present study, few egg batches with much higher than average hatch rate were observed. The biological relevance of these data is not clear but could indicate some radio-resistance in specific pupae at the time of radiation. This would be interesting to study in future experiments, but is complicated by the fact that single-pair matings in An. arabiensis are difficult to perform (M. Benedict, unpublished data).
The proportion of egg batches with intermediate hatch rates, a possible indicator for multiple mating events as previously discussed (Helinski & Knols, Reference Helinski and Knols2008), appeared higher for the competition experiments with 70 Gy compared to 120 Gy and in line with previous results observed at the 1:1 ratio experiments (Helinski & Knols, Reference Helinski and Knols2008). Multiple mating was shown to occur in small cage competition experiments in An. arabiensis using stable isotopes (Helinski & Knols, Reference Helinski and Knols2008), and a similar approach can be undertaken to determine the degree of multiple mating in the large cages.
It is concluded that the reduction in competitiveness of males irradiated as pupae can be compensated for by a threefold increase of irradiated insects at the partially-sterilizing dose of 70 Gy but not at the fully-sterilizing dose of 120 Gy. Irradiation of older pupae resulted in a marginal, but non-significant, improvement in competitiveness compared to the irradiation of young pupae for each dose. The beneficial effects of the irradiation of old pupae are small and probably do not outweigh the additional handling costs; however, cooling can be used as a tool in operational SIT campaigns to facilitate pupal handling.
Acknowledgements
The authors wish to thank S. Soliban for technical assistance, M. Dicke and two anonymous referees for constructive comments on the manuscript and the International Atomic Energy Agency for providing funds. BGJK is supported by a VIDI grant from the Netherlands Organization for Scientific Research (#864.03.004).






