Introduction
Italian ryegrass [Lolium perenne L. spp. multiflorum (Lam.) Husnot] is a winter annual or biennial (DiTomaso and Healy, Reference DiTomaso and Healy2007), and it is commonly cultivated as pasture forage, turf, and cover crop in many parts of the world. L. multiflorum is also a weedy species of worldwide occurrence, infesting roadsides, agronomic crops, orchards, and vineyards and competing efficiently for environmental resources (Hashem et al. Reference Hashem, Radosevich and Dick2000).
In the United States, California leads the nation in the production of almonds [Prunus dulcis (Mill.) D. A. Webb var. dulcis], clingstone peaches [Prunus persica (L.) Batsch.], dried plums (Prunus domestica L.), and walnuts (Juglans regia L.), among other nuts and fruits (USDA 2017). In the Central Valley of California, fruit and nut growers routinely mow between tree rows to reduce weed competition, but rely heavily on herbicides for weed control within the tree line. A typical herbicide program in most of these crops consists of a PRE/POST tank mix applied in late fall before rainfall of one or a mixture of the herbicides listed in Table 1, followed by a burndown treatment in early spring (March to April) and a second burndown treatment (often glyphosate or paraquat) in summer or fall after harvesting operations are completed. Glyphosate is the most widely used herbicide in California (California Department of Food and Agriculture 2015), due to its efficacy, broad-spectrum weed control, and safety. However, populations of L. multiflorum exhibiting resistance to glyphosate have been reported in almond orchards and vineyards (Jasieniuk et al. Reference Jasieniuk, Ahmad, Sherwood, Firestone, Perez-Jones, Lanini, Mallory-Smith and Stednick2008). Since the first report of glyphosate-resistant L. multiflorum in California, several other cases of herbicide resistance in this species have been identified (Heap Reference Heap2018).
Table 1 List of herbicides (including trade names and manufacturers’ details) used in greenhouse and field experiments.

The overreliance on herbicides has selected for herbicide-resistant L. multiflorum populations around the world (Preston et al. Reference Preston, Wakelin, Dolman, Bostamam and Boutsalis2009). This widespread resistance may be associated with the obligate-outcrossing, self-incompatible breeding system of L. multiflorum. This breeding strategy facilitates the dispersal of herbicide-resistance genes within and among populations (Loureiro et al. Reference Loureiro, Escorial and Chueca2016), resulting in the selection of multiple herbicide–resistant biotypes in response to continued selection pressure (Mahmood et al. Reference Mahmood, Mathiassen, Kristensen and Kudsk2016).
Herbicide-resistant weeds increase weed control costs (Mueller et al. Reference Mueller, Mitchell, Young and Culpepper2005). Weed interference in orchard crops may result in reduced performance and mortality of young trees (Belding et al. Reference Belding, Majek, Lokaj, Hammerstedt and Ayeni2004), as well as reduced fruit size, total yield, and fruit number in older trees (MacRae et al. Reference MacRae, Mitchem, Monks, Parker and Galloway2007). Even if the direct effects of weed competition can be overcome with additional water or fertilizer inputs, efficiency of many field operations is reduced. Adequate herbicide-resistance management programs are necessary for growers to maintain economic sustainability even after evolution of herbicide-resistant weeds in their fields. Using multiple herbicides with different sites of action within a cropping season delays the evolution of resistance and mitigates the direct and indirect impacts of herbicide-resistant weeds (Diggle et al. Reference Diggle, Neve and Smith2003). Therefore, diversified management practices are crucial for both proactive and reactive weed management.
Recently, poor control of L. multiflorum with paraquat was observed in a prune orchard in Hamilton City, CA. Due to the presence of glyphosate-resistant L. multiflorum in the orchard, paraquat had been applied several times each year for at least 8 yr as the main weed management tool, primarily to reduce interference with irrigation practices. The objectives of this research were to characterize the response of a multiple herbicide–resistant population of L. multiflorum to POST herbicides, study the mechanisms of resistance involved in this field-selected population of L. multiflorum, and evaluate chemical control options with PRE herbicides currently registered in orchard cropping systems in California.
Material and Methods
Greenhouse Studies
Seeds of suspected multiply resistant L. multiflorum seeds (a population hereinafter referred to as “PRHC”) were collected in April 2015 from plants that survived a burndown treatment with paraquat in a 28-ha prune orchard (39.752°N, 122.016°W) near Hamilton City, CA. The field collection was made by harvesting mature seeds from individual plants on grids of approximately 30 by 30 m (near every fifth tree in every fifth row) from across the orchard and bulking all samples. A previously characterized glyphosate-susceptible biotype of L. multiflorum (S) (Jasieniuk et al. Reference Jasieniuk, Ahmad, Sherwood, Firestone, Perez-Jones, Lanini, Mallory-Smith and Stednick2008) was used for comparison purposes. Preliminary herbicide screening indicated that the S biotype was susceptible to clethodim, fluazifop-P-butyl, glufosinate, glyphosate, paraquat, pyroxsulam, rimsulfuron, and sethoxydim (unpublished data). Studies in this research focused on herbicides commonly used by perennial crop growers in California (Table 1).
PRHC and S seeds were germinated in petri dishes by alternating 5 C in darkness (14 h) with 25 C in light (10 h) until desired germination was achieved. Seedlings were then transplanted to 5 by 5 by 10 cm pots filled with commercial potting media, with each pot receiving 1 plant to minimize intraspecific competition and optimize spray coverage. When plants reached the BBCH-23 stage (3 visible tillers) (Hess et al. Reference Hess, Barralis, Bleiholder, Buhr, Eggers, Hack and Stauss1997), dose–response experiments were carried out with POST herbicides registered in orchard systems (Table 2). Each herbicide experiment included seven doses ranging from 0.125X to 8X the recommended use rate. A nontreated control treatment was included for comparison in each herbicide experiment. Treatments were applied with a spray cabinet equipped with an even flat-fan spray nozzle (8002E, Teejet®, Spraying Systems, Wheaton, IL), calibrated to deliver 187 L ha−1. After treatment, plants were maintained in a greenhouse under natural light, supplemented with artificial light, with a total daylight period of 11 h. Pots were arranged in a completely randomized design with four replications. Visual assessments were performed at 7, 14, 21, and 28 d after treatment (DAT) using a scale that ranged from 0 to 100, where 0 represented absence of injury and 100 represented complete plant death. Following the final visual evaluation, aboveground biomass of each plant was collected and dried, and the dry weight was recorded. Several individual plants from the field population of PRHC were grown to maturity, and the seeds produced were used to repeat the dose–response experiments. Data were pooled between experiments in agreement with a Levene’s ANOVA test for homoscedasticity of variance. Data were fit to log-logistic models, and the Akaike information criterion was used for comparison among several commonly used preselected models. The outliers were identified by comparing the semi-studentized residuals with a cutoff value based on a t-distribution with α=0.05. Normality of residues and homoscedasticity of variance were assessed at a 5% level of significance. In cases in which the assumptions underlying nonlinear regression were not met (i.e., correct mean function, variance homogeneity, normally distributed errors, mutually independent errors), the data were transformed with an optimal lambda obtained from a log-likelihood function (Box and Cox Reference Box and Cox1964; Kniss and Streibig Reference Kniss and Streibig2015). Finally, data were fit to a three- or four-parameter log-logistic model (in agreement with the Akaike information criterion results), and the herbicide rate that reduced plant biomass by 50% (GR50) was compared within each dose–response experiment and between PRHC and S using Student’s t-test. The resistance index (RI) is reported as a means of comparison between the biotypes (the PRHC GR50 was compared with the S GR50). Statistical analysis was performed using the R software (R Core Team 2017).
Table 2 Herbicide rates used in greenhouse POST dose–response experiments with multiple herbicide–resistant and susceptible L. multiflorum.

a Ammonium sulfate at 1% was added to all treatments. Nonionic surfactant at 0.25% was added to clethodim, fluazifop-P-butyl, glyphosate, paraquat, pyroxsulam, and rimsulfuron. Crop oil concentrate was added at 1% to sethoxydim.
Field Studies
An experiment was conducted twice to evaluate PRE herbicides commonly used by fruit and nut growers (Table 3) in the prune orchard near Hamilton City, CA, where failures to control PRHC with paraquat were observed. Experiments were initiated in November and December of 2015, spaced 3 wk apart in two different blocks in the same orchard with similar soil characteristics (Wyo silt loam with pH 5.9, 1.8% organic matter, 33% sand, 46% silt, and 21% clay). Approximately 3 wk and 1 wk before the start of each experiment, all plots were treated with a tank mix of glyphosate+glufosinate (1,260+1,160 g ha−1), to ensure complete absence of weeds in the plots and, consequently, improve PRE herbicide application uniformity. A CO2-pressurized backpack sprayer calibrated to deliver 187 L ha−1 through three flat-fan extended-range 8002 nozzles (TeeJet®, Spraying Systems, Wheaton, IL) was used to apply the treatments. The experimental design was a randomized complete block design with four replications and 17 treatments, including a nontreated control. Visual injury assessments were performed at 30, 60, 90, 120, and 150 DAT using a scale that ranged from 0 to 100, where 0 represented absence of injury and 100 represented complete plant death. Aboveground biomass was collected at 150 DAT from 1-m−2 quadrats randomly placed in each plot, then dried, and the weight was recorded. The original data did not meet the assumptions of ANOVA (i.e., normality of residues and homoscedasticity of variances); therefore, visual injury assessment and biomass data were subjected to Box-Cox transformations (Box and Cox Reference Box and Cox1964) based on a log-likelihood function built from the original data sets. Upon data transformation, the assumptions of ANOVA were met, and a multiple comparison test was performed with a Tukey’s post hoc test, with the overall experiment-wise type I error rate corrected for 17 treatments using the Bonferroni correction and α=0.05.
Table 3 Visual injury and biomass of multiple herbicide–resistant L. multiflorum after PRE herbicide treatments in a prune orchard in Hamilton City, CA, during 2015 to 2016.
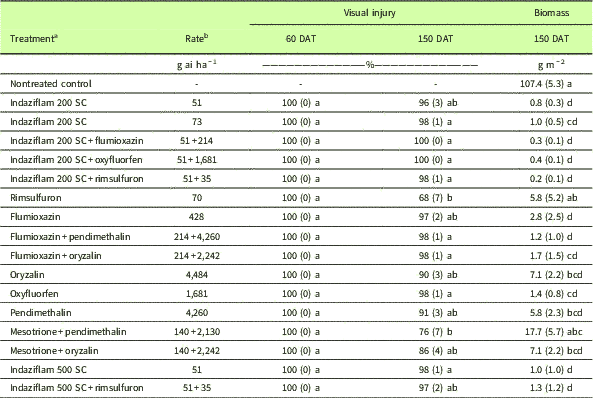
a Plots were treated with a tank mix of glufosinate+glyphosate (1,680+1,260 g ha−1) before treatment. Glufosinate at 1,680 g ha−1, glyphosate at 1,260 g ha−1, ammonium sulfate at 1%, and nonionic surfactant at 0.25% were added to all treatments.
b Means (±SE) followed by same letter within a column are not statistically different.
Laboratory Studies
To determine whether polymorphism at position 106 of the EPSPS was involved in the resistance to glyphosate in PRHC, RNA was extracted (RNeasy Plant Mini Kit, Qiagen, Germantown, MD) from young leaves of untreated PRHC and S individuals from the field population; this was followed by cDNA synthesis (QuantiTect Reverse Transcription Kit, Qiagen).
Forward (AWF, 5′-AACAGTGAGGAYGTYCACTACATGCT-3′) and reverse (AWR, 5′-CGAACAGGTGGGCAMTCAGTGCCAAG-3′) degenerate primers were used (Adu-Yeboah et al. Reference Adu-Yeboah, Malone, Gill and Preston2014) to amplify a 310-bp fragment of the EPSPS gene containing the encoded position 106, known to cause resistance to the herbicide glyphosate (Powles and Yu Reference Powles and Yu2010). A polymerase chain reaction (PCR) composed of 1X PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTP mix, 0.2 µM AWF, 0.2 µM AWR, 50 ng of cDNA, and 2 U Taq DNA polymerase was performed with cDNA from four PRHC and S individuals. PCR cycles were as follows: an initial denaturation step at 94 C for 2 min, followed by 35 cycles of 94 C for 30 s, 60 C for 30 s, and 72 C for 1 min, with a final extension step at 72 C for 5 min.
Upon reaction completion, 5 µl of individual PCR products were run in a 2% agarose gel, and the remaining products were sequenced with BigDye® Terminator v. 3.1 (Life Technologies, Burlington, Canada) and ABI Prism® 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Results and Discussion
Greenhouse results indicated that PRHC had a high RI when treated with clethodim (RI=10), fluazifop-P-butyl (RI=12), glyphosate (RI=10), paraquat (RI=19), and pyroxsulam (RI=20), and a moderate RI for rimsulfuron (RI=2) and sethoxydim (RI=3) (Table 4). However, statistically different GR50 values between PRHC and S were only observed for clethodim, glyphosate, and paraquat. Conversely, both biotypes responded similarly to glufosinate (RI=1). Because dose–response error estimates for fluazifop-P-butyl were large, the RI was not significantly different between PRHC and S, even though field rates of this herbicide controlled S, whereas high rates (up to 8X) did not control PRHC (unpublished data). Similar observations were made for pyroxsulam, for which PRHC exhibited lower biomass reductions compared with S at field rates of this herbicide (unpublished data). Although the statistical criteria adopted in this research did not indicate differences between PRHC and S when treated with fluazifop-P-butyl or pyroxsulam, it should be noted that field rates of these herbicides did not control PRHC, whereas S was controlled, and these responses were inheritable and reproducible across experiments. Because L. multiflorum is an outcrossing, self-incompatible weed species and PRHC is a field population, individuals within the population could have different mechanisms of resistance to fluazifop-P-butyl and pyroxsulam, increasing variability in the response to these herbicides. Although PRHC was less susceptible to pyroxsulam compared with S in the greenhouse study, this herbicide is not registered in prunes and was not tested in the field. Herbicides in the same chemical family of pyroxsulam (e.g., penoxsulam, flazasulfuron), however, are widely used in several other fruit and nut crops, and might have previously exerted selection pressure on L. multiflorum in this region.
Table 4 Dose–response experiments with a multiple herbicide–resistant (PRHC) and a susceptible (S) population of L. multiflorum.

a Ammonium sulfate at 1% was added to all treatments. Nonionic surfactant at 0.25% was added to clethodim, fluazifop-P-butyl, glyphosate, paraquat, pyroxsulam, and rimsulfuron. Crop oil concentrate was added at 1% to sethoxydim.
b Herbicide rate to reduce plant biomass by 50% (GR50) compared with a nontreated control.
c RI, resistance index: ratio between GR50 of PRHC and S.
d Y=d/{1+exp[b(log x − log e)]} or Y=c+(d − c) exp[−exp[b(log x − e)]}, where b is the relative slope around e, c and d are the lower and upper limits, and e is the dose required to reduce dry weight by 50% (GR50).
EPSPS sequences from PRHC exhibited single-nucleotide polymorphisms at position 106. Surprisingly, all PRHC individuals sequenced presented heterozygous EPSPS, one allele exhibiting alanine at position 106 (Pro-106-Ala) and the other a serine (Pro-106-Ser), whereas S contained wild-type EPSPS alleles (proline at position 106) in both alleles. EPSPS duplication, which has been shown to be involved in glyphosate resistance in L. multiflorum populations from Arkansas (Salas et al. Reference Salas, Dayan, Pan, Watson, Dickson, Scott and Burgos2012), has not been assessed in this population. L. multiflorum is a diploid, obligate-outcrossing plant species (Loureiro et al. Reference Loureiro, Escorial and Chueca2016), and the pollen-mediated movement of resistance alleles from glyphosate-resistant plants evolved independently in the field might explain the presence of a heterozygous EPSPS gene for resistance to glyphosate in PRHC.
Resistance to glyphosate can involve restricted herbicide movement (i.e., absorption and translocation) (Brunharo et al. Reference Brunharo, Patterson, Carrijo, de Melo, Nicolai, Gaines, Nissen and Christoffoleti2016), enhanced herbicide metabolism (Carvalho et al. Reference Carvalho, Alves, González-Torralva, Cruz-Hipolito, Rojano-Delgado, De Prado, Gil-Humanes, Barro and Castro2012), mutations in the target enzyme (Karn and Jasieniuk Reference Karn and Jasieniuk2017), and increased expression and amplification of the EPSPS gene (Gaines et al. Reference Gaines, Zhang, Wang, Bukun, Chisholm, Shaner, Nissen, Patzoldt, Tranel, Culpepper, Grey, Webster, Vencill, Sammons, Jiang, Preston, Leach and Westra2010). The mechanisms associated with paraquat resistance in PRHC have been characterized and involve the vacuolar sequestration of paraquat away from the chloroplast (Brunharo and Hanson Reference Brunharo and Hanson2017). The mechanisms involved in the resistance to clethodim have not been investigated in PRHC; however, several target-site mutations in the ACC gene are known to cause resistance to field rates of clethodim (Délye et al. Reference Délye, Matéjicek and Michel2008; Powles and Yu Reference Powles and Yu2010). Non–target site mechanisms of resistance to acetyl-CoA carboxylase inhibitors (i.e., enhanced herbicide metabolism) have been identified in grass weed species (Hidayat and Preston Reference Hidayat and Preston1997; Wang et al. Reference Wang, Lin, Chiang and Wang2017) and might also be involved in the resistance to clethodim in PRHC.
Treatments with PRE herbicides provided high levels of weed control up to 150 DAT (Table 3). Tank mixes containing indaziflam and flumioxazin, as well as sole applications of indaziflam (51 and 73 g ai ha−1), flumioxazin (428 g ai ha−1), oryzalin (4,484 g ai ha−1), oxyfluorfen (1,681 g ai ha−1), and pendimethalin (4,260 g ai ha−1) provided >90% weed control. Conversely, rimsulfuron alone (70 g ai ha−1) and mesotrione+pendimethalin (140+2,130 g ai ha−1) provided visual injury lower than 80% to L. multiflorum. Rimsulfuron has a half-life as low as 5.6 d under field conditions (Schneiders et al. Reference Schneiders, Koeppe, Naidu, Horne, Brown and Mucha1993), and this short persistence may have contributed to the relatively poor control observed at 150 DAT. Rimsulfuron, however, may be an option when an herbicide with a short residual activity is practical, because it did control L. multiflorum at acceptable levels up to 60 DAT (Table 3). Mesotrione is an herbicide with primarily broadleaf activity (Mitchell et al. Reference Mitchell, Bartlett, Fraser, Hawkes, Holt, Townson and Wichert2001), and its association with low rates of oryzalin and pendimethalin only provided marginal weed control (<90%). Biomass data were comparable to visual control evaluations (Table 3).
In a seedling germination study conducted in Oregon (Ghersa et al. Reference Ghersa, Martínez-Ghersa, Brewer and Roush1994), L. multiflorum seeds germinated from August through April, and in some instances germination was observed even with limited incidence of sunlight (10% of full sunlight), a condition commonly found in orchard and vineyard systems. This nonuniform germination pattern of L. multiflorum poses challenges to growers who rely solely on POST herbicides for weed management, as it requires multiple applications to provide acceptable control. In this context, tank mixes of PRE herbicides with residual activity, like those studied in the present research, can be extremely important for managing multiple cohorts of weeds with nonuniform germination and emergence patterns.
Glyphosate-resistant L. multiflorum populations are widespread in California, and the overreliance on POST herbicides from a variety of chemical classes has selected for a multiple herbicide–resistant population. Greenhouse data indicate that glufosinate, rimsulfuron, and sethoxydim may be adopted as POST chemical alternatives to control PRHC, although resistance to these herbicides has been reported in L. multiflorum and other weed species in other parts of the world (Heap Reference Heap2018). Resistance to glyphosate in PRHC is due to double amino acid substitutions in the EPSPS, where one allele presented a proline-to-alanine substitution and the other a proline-to-serine substitution at position 106. Fruit and nut tree growers have a number of PRE herbicides registered in their crops that are effective options to control L. multiflorum, notably tank mixes that provide residual activity and high control levels, such as those containing indaziflam and flumioxazin.
Author ORCIDs
Caio A. C. G. Brunharo, http://orcid.org/0000-0001-9735-1648; Bradley D. Hanson, http://orcid.org/0000-0003-4462-5339.
Acknowledgments
This research was supported, in part, by funding from the Almond Board of California, the California Dried Plum Board, and the California Walnut Board. CACGB acknowledges the support of the National Council for the Improvement of Higher Education (CAPES) through a scholarship for his Ph.D studies and a Jastro Shields Scholarship from the Plant Science Department at University of California, Davis. The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.






