Introduction
Benthic marine communities along the northern half of the western Antarctic Peninsula (WAP) possess rich and diverse assemblages of macroalgae and invertebrates (De Broyer et al. Reference De Broyer, Koubbi, Griffiths, Raymond, d'Udekem d'Acoz and van de Putte2014). Predominantly brown but also many red macroalgae dominate shallow benthic communities on hard substrates along the WAP, often covering over 80% of the seafloor with standing biomass levels similar to those in temperate kelp forests (Wiencke & Amsler Reference Wiencke, Amsler, Wiencke and Bischof2012, Wiencke et al. Reference Wiencke, Amsler, Clayton, De Broyer, Koubbi, Griffiths, Raymond, d'Udekem d'Acoz and Van de Putte2014). Several species of large, perennial brown algae are particularly abundant. These include Desmarestia menziesii J. Agardh and/or Desmarestia anceps Montagne that typically dominate biomass in shallower waters down to approximately 10–15 or 20 m, with Himantothallus grandifolius (A. Gepp & E.S. Gepp) Zinova dominating from 10–15 or 20 m down to 30 or 40 m, or sometimes even greater depths (Wiencke & Amsler Reference Wiencke, Amsler, Wiencke and Bischof2012, Wiencke et al. Reference Wiencke, Amsler, Clayton, De Broyer, Koubbi, Griffiths, Raymond, d'Udekem d'Acoz and Van de Putte2014). It is common to find one or two of these three species of brown algae covering nearly 100% of the benthos in some locations.
Mesograzers such as amphipods and small gastropods are very abundant in Antarctic communities in terms of both species richness and numbers of individuals (Amsler et al. Reference Amsler, McClintock and Baker2014). The current understanding of the shallow-water, macroalgal-dominated community along the WAP indicates that interactions between macroalgae, microalgae, and amphipod mesograzers are among the dominant factors shaping community structure, and the present researchers have described the macroalgal-amphipod relationship as a community-wide mutualism (Amsler et al. Reference Amsler, McClintock and Baker2014). Although the vast majority of the large Antarctic macroalgae are chemically defended from the amphipods (Amsler et al. Reference Amsler, McClintock, Baker and Amsler2008, Reference Amsler, McClintock and Baker2014, Aumack et al. Reference Aumack, Amsler, McClintock and Baker2010, Núñez-Pons et al. Reference Núñez-Pons, Rodríguez-Arias, Gómez-Garreta, Ribera-Siguán and Avila2012), the amphipods benefit from the association because the chemically defended macroalgae they live on provide associational defence from predatory fish (Zamzow et al. Reference Zamzow, Amsler, McClintock and Baker2010). In turn, the amphipods greatly benefit the large macroalgae by grazing down their associated epiphytic microalgae and filamentous macroalgae (Aumack et al. Reference Aumack, Amsler, McClintock and Baker2011a, Reference Aumack, Lowe, Amsler, Amsler, McClintock and Baker2017, Amsler et al. Reference Amsler, McClintock and Baker2012, Reference Amsler, McClintock and Baker2014). Although filamentous algae are common in high-energy areas of the upper intertidal zone where mesograzers have little access to them, they are very uncommon in the subtidal zone except growing endophytically within chemically defended macroalgae (Peters Reference Peters2003, Amsler et al. Reference Amsler, Amsler, McClintock and Baker2009). This is probably a consequence of the high densities of amphipods in the subtidal zone (Peters Reference Peters2003, Amsler et al. Reference Amsler, Amsler, McClintock and Baker2009, Reference Amsler, McClintock and Baker2012). In a mesocosm aquarium experiment using tanks with and without natural densities of amphipods, in tanks lacking amphipods filamentous endophytes were able to grow out which, along with heavy growths of diatoms, heavily fouled the surfaces of their hosts within seven weeks (Aumack et al. Reference Aumack, Amsler, McClintock and Baker2011a). Correspondingly, gut content analyses of macroalgal-associated subtidal amphipods found diatoms to be the most abundant food item in most amphipod species while filamentous algae constituted between 6% and 16% of the gut contents, even though they rarely grow to sizes visible to divers (Aumack et al. Reference Aumack, Lowe, Amsler, Amsler, McClintock and Baker2017). By dramatically reducing epiphytic microalgae and filamentous algae which otherwise would compete with the ecologically dominant macroalgae, the hugely abundant amphipods clearly play an important role in their communities.
There are also several reports of gastropod mesograzers being numerous in association with the larger WAP macroalgae (Richardson Reference Richardson1977, Picken Reference Picken1979, Reference Picken1980, Iken Reference Iken1999, Amsler et al. Reference Amsler, Huang, Engl, McClintock and Amsler2015). Amsler et al. (Reference Amsler, Huang, Engl, McClintock and Amsler2015) analysed the gastropod fauna associated with the same individual macroalgae from which Huang et al. (Reference Huang, Amsler, McClintock, Amsler and Baker2007) enumerated amphipods. Of the eight macroalgal species sampled, while common, gastropod densities per unit biomass were generally an order of magnitude lower than amphipod densities (Amsler et al. Reference Amsler, Huang, Engl, McClintock and Amsler2015). The large, sheet-like red alga Gigartina skottsbergii Setchell & NL Gardner supported relatively higher gastropod densities and relatively lower amphipod densities compared to the other algal species. This matches casual observations made by the present authors from nature for large bladed species, particularly H. grandifolius, which is the dominant deeper-water brown alga. This species forms blades that lie decumbent along the bottom, are commonly 5–10 m or longer and up to nearly a metre wide. These blades can cover close to 100% of the bottom. Because of the size of H. grandifolius, it was not practical for Huang et al. (Reference Huang, Amsler, McClintock, Amsler and Baker2007) to sample it for amphipod enumeration, but qualitative observations (authors' personal observations) indicate that these algae support noticeably higher densities of gastropods than amphipods. Moreover, these larger bladed species have much higher densities of the larger macroalgal-associated gastropods in the community, Margarella antarctica Lamy 1905 and Nacella concinna Strebel 1908, which are only rarely found on the more highly branched macroalgal species (Amsler et al. Reference Amsler, Huang, Engl, McClintock and Amsler2015, authors' personal observations).
The goal of the present study was to begin to address the question of whether or not gastropods play a role in WAP benthic communities analogous to that demonstrated for amphipods, particularly in terms of providing benefits to large-bladed algal species, such as the ecologically dominant H. grandifolius. Do gastropods at their natural densities on H. grandifolius reduce fouling from microscopic and filamentous algal biofoulers? If so, are there differences in the grazing effectiveness of different gastropod species? The study also begins to address the question of whether or not gastropods might gain some degree of refuge from predators on H. grandifolius, as amphipods and presumably gastropods do, on branched, chemically defended macroalgae.
Materials and methods
Outdoor mesocosm experiment
The experiment utilized ten white, slightly translucent plastic aquaria (mesocosms) covered on top by neutral density greenhouse cloth that reduced the surface solar irradiance by 75% to mimic a subtidal light field (Fig. S1). The mesocosm aquaria were 73.5 cm on each internal, bottom side with a water depth of 20 cm controlled by a standpipe. The mesocosms were situated on an outside deck at Palmer Station, Antarctica (64°46ʹ28.5ʺS, 64°03ʹ16.4ʺW) with full, natural sunlight, and plumbed with unfiltered, ambient flow-through seawater pumped directly from the ocean approximately 30 m away. Plankton mesh (63 µm, regularly cleaned) was placed over the water inlets and standpipe drains to prevent additional mesograzers coming in with the unfiltered water or the intended mesograzers escaping through the standpipes. This is exactly the same setup as used previously in an experiment with amphipod mesograzers by Aumack et al. (Reference Aumack, Amsler, McClintock and Baker2011a) except for lower water depths/standpipe heights and more transparent greenhouse cloth covers. The ten mesocosm aquaria were randomly assigned (drawn by lot) as either an experimental mesocosm with gastropods or a control mesocosm with no gastropod mesograzers. Each mesocosm had a length of plastic angle stock at the bottom to which a braided, nylon rope was attached. Three individual macroalgal pieces were attached to each rope as described below.
H. grandifolius individuals (n = 15) were collected on 17 March 2018 at approximately 12–18 m depth from the south-eastern portion of Bonaparte Point on Anvers Island, Antarctica (64°46ʹ45.2ʺS, 64°03ʹ17.3ʺW). Sections of each thallus, approximately 0.75 m in length, were removed from the lower portion of the blade but well above the meristem (leaving the alga still attached to the substrate with an intact meristem). The thallus sections were transferred to buckets of seawater and transported to the Palmer Station aquarium facility. Thallus sections were maintained in an indoor, flow-through filtered seawater tank with lighting until the experiment was initiated on 24 March.
From the centre of each of the 15 H. grandifolius sections, two roughly rectangular strips (92.3 ± 2.4 g; mean ± 1 s.e.) were cut using a razor blade. Each strip had an approximately 1 cm ×3 cm rectangular tab extending out from the centre of one short side. After removing excess water with a salad spinner, each piece was weighed (to the nearest one-hundredth of a gram) on a top-loading balance and then the tab on the thallus strip was fitted between braids of the nylon rope and further secured with a small plastic cable tie. The duplicate strips from each individual thallus were randomly assigned to one of 15 possible locations (three rope positions in each of five mesocosms for each treatment) in either an experimental or control mesocosm. The rope was anchored at either end to a length of plastic angle stock. The anchored ropes with thallus strips were submerged in a shallow aquarium until all three positions on the rope were full and then they were carefully transferred into the bottom of the appropriate mesocosm tank (Fig. S1).
Gastropods for the experiment were collected by divers brushing individuals off the thalli of H. grandifolius and into fine-mesh bags at multiple sites within 3.5 km of Palmer Station during the two-week period preceding the experiment. The numbers of gastropods used in the mesocosms were determined from collections of H. grandifolius made in 2017 at four sites in the same vicinity and at two different depths (9 m and 18 m). At each depth, divers collected five individual H. grandifolius, each of which were carefully enveloped in a fine-mesh bag (made from sheer curtain cloth) and transferred to Palmer Station in buckets of seawater. Thereafter, all gastropods were quantitatively removed from the thalli and preserved for later identification and enumeration. Total gastropod numbers per algal biomass from seven of the depth-site samples were very similar, and accordingly the data from each were combined to determine the species and numbers of gastropods to be used for the experiment. A total of 30 taxa were identified to species, in addition to a number of juveniles too small to identify and a few individuals that were unidentifiable.
The total surface area available to the gastropods in the experimental mesocosm aquaria, combining both the bottom and side walls of the tanks along with the H. grandifolius thalli, was calculated to be equivalent to approximately the surface area of 0.9 kg of H. grandifolius thallus (at 0.054 g thallus cm−2). The nine most numerically abundant gastropod species from the 2017 collections represented 91.2% of the total number of gastropods. The mean numbers of each of these species kg−1 of H. grandifolius thallus were added to each experimental tank to approximate the number of total gastropods on the calculated 0.9 kg thallus equivalent. Each mesocosm tank received 32 Skenella umbilicata Ponder 1983, 22 Eatoniella calignosa Smith 1875, 15 M. antarctica, 6 Laevilacunaria antarctica Martens 1885, 3 Eatoniella cana Ponder 1983, 2 Cyclostrema meridionale Melvill & Standen 1912, 1 Laevilitorina calignosa Gould 1850, 1 Eatoniella kerguelenensis regularis Smith 1915, and 1 N. concinna.
After three weeks, the ropes containing H. grandifolius in each mesocosm were transferred briefly and with great care to an indoor seawater table while each mesocosm tank was cleaned. Gastropods on the thalli were carefully removed. After the algae had been returned to the clean mesocosm, a fresh set of gastropods in the same numbers as added originally and collected as described above were added back to the mesocosm tanks.
The experiment ran for 52 days (takedown started on the evening of 14 May and concluded on the morning of 16 May). Each rope of H. grandifolius was carefully transferred to an indoor seawater table. Gastropods on the thallus strips were removed and the strip carefully transferred to a salad spinner for removal of excess water. The strips were weighed to the nearest one-hundredth of a gram on a top loading balance and then transferred back to the seawater table where the percentage cover of long, filament-like diatom chains on each side of each strip was estimated. Thereafter, three pairs of 15 mm diameter discs (sized to fit easily under a coverslip on a standard microscope slide) were gently cut from the longitudinal centre of each strip with a cork borer. The pairs of discs were removed from the approximate centres of the proximal, centre, and distal thirds of the strip (Fig. S2). The discs were transferred to microscope slides and placed under glass cover slips with one of each disc pair positioned such that what had been facing up or down in the mesocosm was facing up on the slide for imaging. Using a compound microscope at 100× magnification, the percentage cover of diatoms on the entire surface of the disc was estimated and other observations made, as described in the Results section. Percentage cover data from the three discs per side of each of the three thallus strips in each mesocosm were averaged and used as a single replicate for statistical analyses (i.e. each mesocosm was a single replicate for statistical analyses).
Incubator experiment
This experiment utilized a Percival LT36VL lighted incubator (Percival Scientific, Inc.) maintained at 1°C ± 0.5°C with a photoperiod of 14:10, L:D and irradiance of 40 μmol quanta m2 sec−1. Algae and gastropods were maintained in 350 ml Qorpak® jars fitted with Petri dish lids. Each jar initially contained 175 ml of Provasoli's enriched seawater media (PES; Provasoli Reference Provasoli, Watanabe and Hattori1968). The media was changed after 14 days, and after 28 days 50 ml of double-enriched PES was added. On a daily basis, each jar was agitated by hand and its position within the incubator rotated.
Individuals of H. grandifolius (n = 6) were collected on 16 March 2018 at approximately 12–18 m depth from the north-eastern corner of Litchfield Island, Antarctica (64°46ʹ06.7ʺS, 64°05ʹ01.9ʺW). Sections of each thallus approximately 0.75 m in length were removed from the lower portion of the blade but well above the meristem (leaving the alga still attached to the substrate with an intact meristem). The thallus sections were transferred to buckets of seawater and transported to the Palmer Station aquarium facility. Thallus sections were maintained in an indoor, flow-through filtered seawater tank with lighting until the experiment was initiated between 18 and 30 March.
From each thallus section, three pairs of 7.5 cm diameter discs (approximately 5 g wet weight; sized to fit into the Qorpak® jars) were cut from adjoining positions on the thallus. One disc in each pair was placed into a jar with gastropods from a single species and the other into a gastropod-free control jar. Three gastropod species (Fig. S3) were chosen for the experiment with the numbers of individuals per jar chosen so as to represent approximately the same cumulative gastropod mass. The resulting gastropod density was considerably higher than the natural densities utilized in the mesocosm experiment. Margarella antarctica (six individuals per jar) was chosen as the largest of the common gastropods observed on H. grandifolius in nature. Eatoniella calignosa (50 individuals per jar) was chosen as the most common species in the most common genus and E. kerguelenensis regularis (12 individuals per jar) as the species with the largest individuals in the most common genus.
The experiment ran for seven weeks. At that point, a pair of 15 mm diameter discs was gently cut from each 7.5 cm thallus disc and analysed microscopically as in the mesocosm experiment. Early in the experiment, all the gastropods in one of the M. antarctica jars died (for no discernible reason) so the sample size for that species was five rather than six paired jars.
Behavioural experiment
Twelve H. grandifolius individuals were collected, six each on 10 April and 6 May 2018 from the north-eastern corner of Litchfield Island. They were held in flowing seawater for four days when a pair of 10 cm diameter discs (sized for the experimental seawater pans) was cut from each individual. Thereafter, one disc from each pair was maintained in a fine mesh bag in flow-through seawater while the other disc was exhaustively extracted in three changes (24 h each) of 1:1 dichloromethane:methanol followed by three changes (24 h each) of 1:1 methanol:water. The discs were then rehydrated in seawater for three to six days prior to being used in the behavioural experiments.
For the behavioural experiments, the extracted and intact thallus discs weighed down with two small rocks were placed in separate pans of shallow seawater under even illumination in a constant temperature room (1°C). Small individual Odontaster validus Koehler 1906 sea-stars were placed at the centres of the discs and their behaviour monitored using a video camera mounted above the pans. One replicate experiment with discs from the same original individual H. grandifolius thallus was run each day with each started at approximately the same time of day to control for any potential diurnal activity patterns in the sea-stars. No sea-star was used more than once. Sea-star radii ranged from 9–22 mm (mean 13.8 mm) but the sea-stars compared in any given replicate only varied by more than 2 mm on one occasion (22 vs 16 mm). The recorded videos were used to determine which sea-star in a pair left the thallus disc first, and to determine the total length of time it took for each sea-star to do so.
Statistical analyses
Statistical analyses utilized SPSS v.25 (IBM Corporation) unless otherwise noted. All percentage data were arcsine-square root transformed prior to analysis. Mesocosm data for the percentage cover of filament-like diatoms and overall percentage cover of diatoms on the upper surfaces of the thalli had no variation in the control mesocosms (all 100% cover) and as such comparisons with thalli from mesocosms with gastropods were made with the nonparametric Mann-Whitney U test. Other mesocosm experiment data were checked for equality of variance with a Levine's Test, and then compared with t-tests. Incubator experiment data and residence time data in the behavioural experiment were compared using paired t-tests. Data for the behavioural experiment regarding which of the paired sea-stars left the disc first were compared using a chi-squared test from VassarStats (http://vassarstats.net/).
Results
Mass increase over time in the mesocosm experiment was significantly greater (t8 = 5.960, P < 0.0005) in the gastropod-free control mesocosms compared to those with gastropods present (Fig. 1). Long filament-like chains of pennate diatoms (Fig. S4) covered 100% of the top surfaces of all 15 thallus strips in the control mesocosms, but less than 10% of the top surfaces in mesocosms with gastropods (7.5% ± 2.93%, mean ± 1 s.e.; range 0–15% by mesocosm, 0–25% by individual strip; P = 0.008; Fig. 1). The diatom chains were observed on the bottoms of only two individual thallus strips, both in the same gastropod-free control mesocosm. In one case, the entire bottom had chains but they were not as long or as densely packed as observed on the tops of thallus strips. In the other, diatom chain coverage was only 15%.
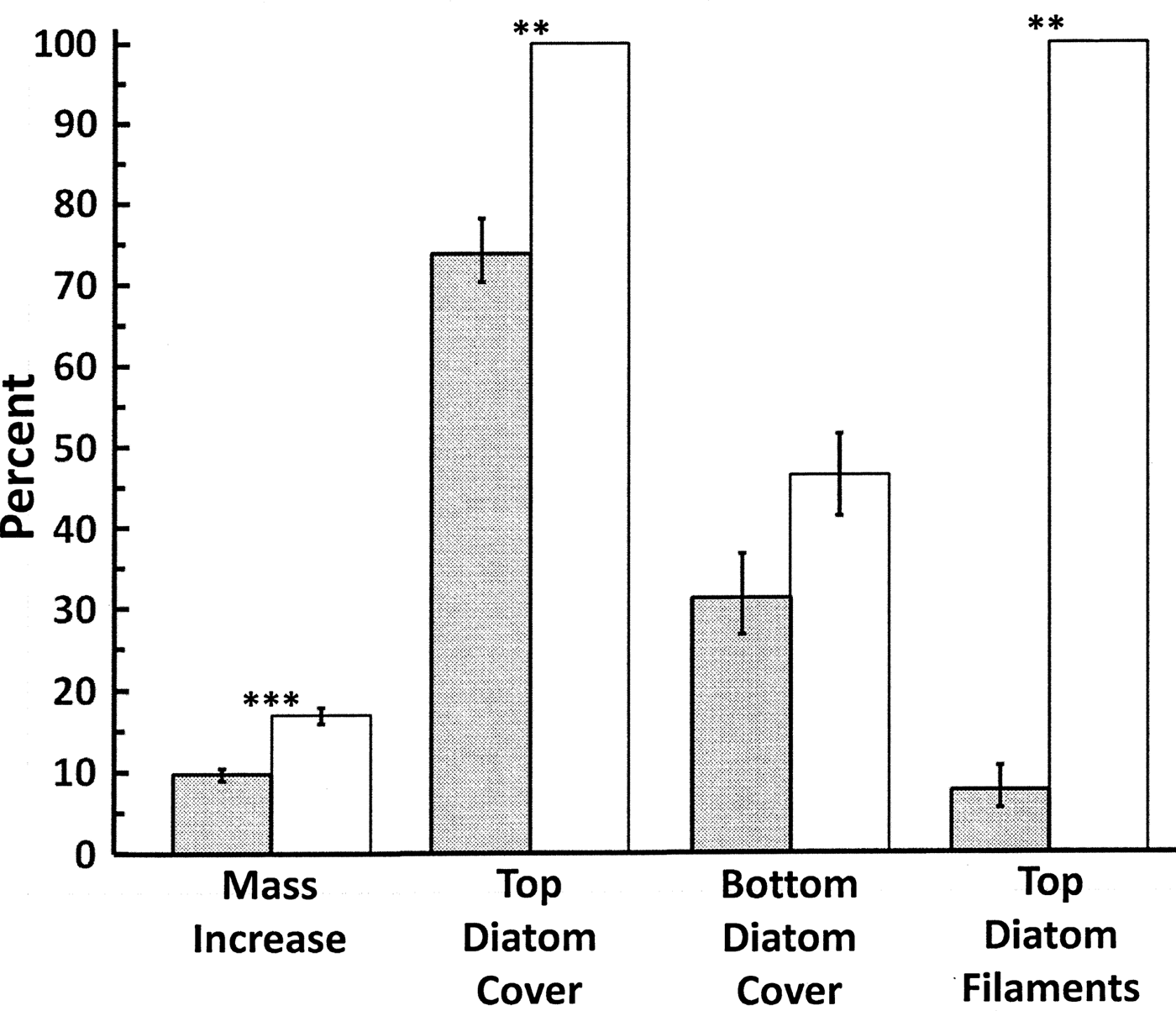
Fig. 1. Percentage increase in the mass of H. grandifolius thallus strips and associated diatoms in the mesocosm experiment along with percentage cover of single-celled diatoms on the top and bottom of the thallus strips and the percentage cover of filament-like diatoms on the tops of the thallus strips. Shaded bars indicate experimental mesocosms with gastropods, open bars indicate gastropod-free control mesocosms. Means ± 1 s.e. ** indicates P < 0.01. *** indicates P < 0.001.
In microscopic observations of thallus discs from the mesocosms, single-cell diatoms covered 100% of the top surfaces of controls compared to 73.8% ± 4.17% (mean ± s.e.) in experimental mesocosms with gastropods (Fig. 1, P = 0.008). While not quantifiable, the layers of diatoms in the controls consistently appeared to be much thicker than in the experimental thalli. Discs from the experimental thalli usually had some areas with 100% cover but others had lower or very low cover as would be consistent with recent grazing by small gastropods. Single-cell diatom coverage was lower and more variable on the bottom surfaces of the thallus discs and although there was a trend for higher coverage in the controls (Fig. 1), this did not reach statistical significance (t8 = 2.024, P = 0.078).
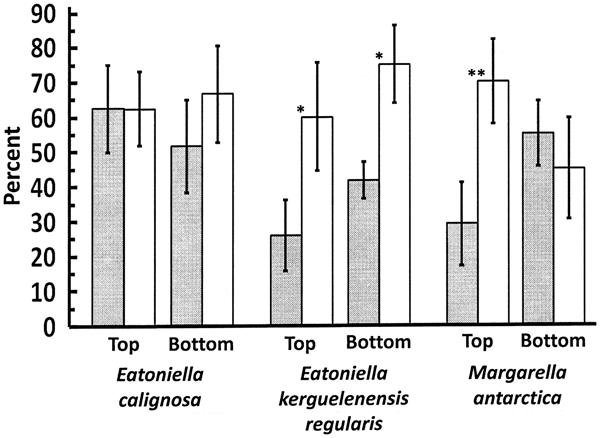
In microscopic observations of thallus discs from the incubator experiment, there were no significant differences in diatom coverage between gastropod or control treatments on either the tops or bottoms of the thallus discs held with E. calignosa (top: t5 = −0.210, P = 0.842; bottom: t5 = 0.841, P = 0.439; Fig. 2). Significant differences between treatment were apparent for diatom coverage on both the top and bottom of thallus discs held with E. kerguelenensis regularis (top: t5 = 2.587, P = 0.049; bottom: t5 = 3.371, P = 0.020; Fig. 2). For thallus discs held with M. antarctica, there was a significant difference on the tops (t4 = 5.942, P = 0.004) but not bottoms (t4 = −0.302, P = 0.778) of the discs (Fig. 2). Judged subjectively, the thickness of the diatom coverage was not as deep as that observed in the mesocosm experiments. Filament-like diatom chains as well as filamentous green algae (either Urospora penicilliformis (Roth) Areschoug or Ulothrix sp.) were occasionally observed on the tops or bottoms of thallus discs from all three sets of jars but never very many and with no apparent difference between gastropod or control treatments.
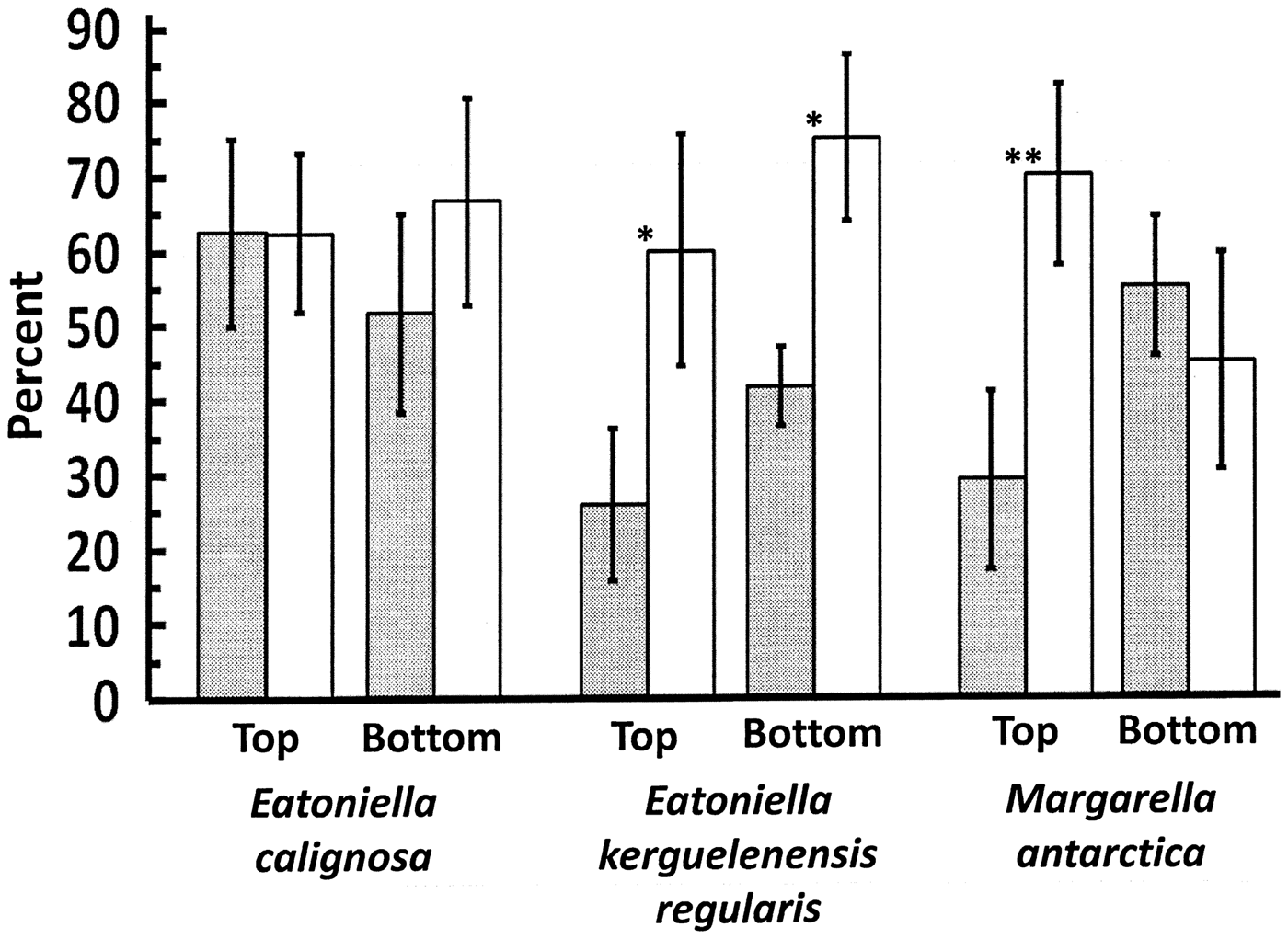
Fig. 2. Percentage cover of diatoms on the tops and bottoms of H. grandifolius thallus discs in the incubator experiment in the presence of the three species of gastropods used. Shaded bars indicate experimental jars with gastropods, open bars indicate gastropod-free control jars. Means ± 1 s.e. * indicates P < 0.05. ** indicates P < 0.01.
In no instance in either the mesocosm or incubator experiments were any wounds observed on H. grandifolius that would be consistent with direct grazing on the macroalga by the gastropods.
There was no significant difference in the residence times of sea-stars on the intact (18.2 ± 4.7 min, mean ± s.e.) vs extracted (16.4 ± 4.7 min) thallus discs (t11 = 0.275, P = 0.789). In the paired discs, the sea-stars left the intact disc first seven times and the extracted disc first five times (χ2 = 0.08, P = 0.7773).
Discussion
Our results support the hypothesis that gastropods can benefit the large brown alga H. grandifolius by reducing densities of biofouling diatoms. However, while significant, the reduction in diatom coverage observed in the mesocosm experiment discussed here was considerably less than observed for H grandifolius by Aumack et al. (Reference Aumack, Amsler, McClintock and Baker2011a) in a mesocosm experiment using natural densities of amphipods. Aumack et al. (Reference Aumack, Amsler, McClintock and Baker2011a) utilized the same mesocosm tanks positioned in the same place on the Palmer Station aquarium deck for seven weeks at the same general time of year. The primary difference was that the shade cloth used in the earlier experiment reduced irradiance to about 10% of the surface level compared to 25% in the experiment discussed in this paper. H. grandifolius in mesocosms with amphipods had a mean epiphyte cover of 8.1% compared to a mean of 53% in amphipod-free controls (Aumack et al. Reference Aumack, Amsler, McClintock and Baker2011a). The higher diatom coverage in both experimental and control treatments in the present experiment is probably, at least in part, because of the greater amount of light in the tanks, but the magnitude of difference between treatments with and without mesograzers suggests that amphipods probably had a larger impact on diatom coverage than did gastropods. In nature, of course, both amphipods and gastropods are present on H. grandifolius and so both presumably contribute to a reduction in diatom biofouling. Although, as noted previously, not nearly as many amphipods are observed on H. grandifolius as on branched macroalgae in this nearshore community, all observations of H. grandifolius in this study were made during daylight hours. It is known that amphipods move off branched macroalgae at night, presumably to forage elsewhere when the visually oriented predatory fish, which are their main predators, are not active (Aumack et al. Reference Aumack, Amsler, McClintock and Baker2011b). Accordingly, amphipods could have an even greater role in reducing fouling on H. grandifolius during the night than the daytime observations suggest.
The weight gains in H. grandifolius in both experimental and control mesocosms were almost certainly because of the heavy growths of diatoms on the thallus strips. The significantly greater weight gain in the control mesocosms was probably a result of the greater cover of both microscopic and filament-like diatoms, along with the apparently thicker layer of epiphytic diatoms on the tops of the strips. The thallus strips were cut from above the meristems, so while existing cells could potentially have been elongating (cf. Drew & Hastings Reference Drew and Hastings1992), no new cell growth should have been occurring. Aumack et al. (Reference Aumack, Amsler, McClintock and Baker2011a) also observed significantly greater weight gain in macroalgae in control mesocosms compared to mesocosms with amphipods in three of four macroalgal species examined, including H. grandifolius, which was attributed to diatom fouling on the algae in the amphipod-free controls.
A limitation of the mesocosm experimental design under discussion here was a relatively low rate of water flow in the tanks. In nature, H. grandifolius thalli are moved around by currents and surge, rubbing against other macroalgae and the bottom. The single-cell diatom coverage in the control mesocosms was so dense, and the filament-like diatoms covering the tops of the thallus strips so weakly attached, that at least some portion of the single-celled and most of the filament-like diatoms would probably have been abraded off in a more natural setting. Such a heavy diatom coverage in nature might also attract grazers from the adjacent benthos resulting in a higher number of grazers than the levels used to stock the experimental mesocosms. After processing the thallus strips as the experiment was being taken down, some were held overnight in a shallow aquarium that had numerous larger limpets (N. concinna), a variety of smaller gastropods, and a sea urchin (Sterechinus neumayeri Meissner 1900). The following morning, the thallus strips were covered by these grazers that had greatly reduced the diatom coverage.
The incubator experiment with individual species confirmed that different gastropod species appear to be differentially effective at reducing fouling diatoms on H. grandifolius. Although E. calignosa caused no significant reduction in diatom coverage, faecal pellets from the snails were apparent in most of the culture jars so clearly they were actively feeding. It is possible that these small snails (Fig. S3) were thinning but not completely removing the layer of fouling diatoms. The largest of the three gastropod species (Fig. S3) used in the experiment, M. antarctica, only significantly reduced diatom cover on the tops of the thallus disks. It is possible that the snails may have had a hard time getting under the H. grandifolius discs, which were only slightly smaller in diameter than the bottoms of the culture jars.
As mentioned, no evidence was noted of any grazing scars in H. grandifolius tissues in either the mesocosm or the incubator experiments. In feeding bioassays with fresh thallus material, H. grandifolius is unpalatable to sea-stars, amphipods, and fish (Amsler et al. Reference Amsler, Iken, McClintock, Amsler, Peters, Hubbard, Furrow and Baker2005, Reference Amsler, Amsler, McClintock and Baker2009). This unpalatability to all three consumers has a chemical basis as demonstrated by significant feeding deterrence in both hydrophobic and particularly hydrophilic crude extracts (Amsler et al. Reference Amsler, Iken, McClintock, Amsler, Peters, Hubbard, Furrow and Baker2005). Most of the gastropod species found by Amsler et al. (Reference Amsler, Huang, Engl, McClintock and Amsler2015) and in the 2017 collections that served as the basis for this experiment are relatively small and have taenioglossan radulae which are best suited for scraping diatoms and filamentous algae (based either on what is known for the species or inferred from the genus; Steneck & Watling Reference Steneck and Watling1982, M. Amsler unpublished). Of the larger and more conspicuous gastropods, M. antarctica has a rhipidoglossan radula which acts more like a broom, brushing thallus surfaces rather than cutting, but the largest and most obvious gastropod in the community, the limpet N. concinna, presumably (like other limpets; Steneck & Watling Reference Steneck and Watling1982) has a minerally hardened docoglassan radula that is well suited for digging into tough macroalgal thalli, but may not be as well suited for eating filamentous or other epiphytic algae. Nevertheless, N. concinna is an important consumer of benthic diatoms in the intertidal zone (Daglio et al. Reference Daglio, Sacristán, Ansaldo and Rodríguez2018, Valdivia et al. Reference Valdivia, Pardo, Macaya, Huovinen and Gómezin press). Grazing marks were occasionally observed from N. concinna on intertidal granite bedrock, suggesting that their radula are indeed tough but these marks are almost never seen in H. grandifolius that have even large N. concinna on them in nature or in laboratory aquaria. The instances where these grazing marks have been observed on H. grandifolius in nature were in areas of the thallus that appear otherwise damaged or unhealthy and so may not have been producing chemical defences at normal levels. Consequently, it is likely that many of the macroalgal-associated gastropod species are biophysically restricted to grazing epiphytes and, therefore, benefiting their macroalgal hosts regardless of host chemical defences. However, N. concinna probably could consume H. grandifolius if it was not chemically resistant to grazing.
The sea-star O. validus is a very common in shallow waters surrounding Antarctica and includes gastropods in its diet (McClintock Reference McClintock1994). The O. validus feeding bioassays previously conducted by the present researchers with fresh thallus and crude extracts of H. grandifolius (Amsler et al. Reference Amsler, Iken, McClintock, Amsler, Peters, Hubbard, Furrow and Baker2005) were the motivation for the simple behavioural experiment performed here. When held in aquaria, many O. validus individuals will climb the sides and extend one or more arms along the air–water interface with their chemosensory tube feet extended. In the bioassays, potential food items or artificial foods containing extracts are placed on the ambulacral groove of such arms, midway between the mouth and arm tip. The sea-stars then either move the item to their mouths to consume it or move it so that it drops off the side or end of the arm. Both fresh H. grandifolius thallus and otherwise palatable artificial foods with crude extracts of H. grandifolius were rejected in these assays (Amsler et al. Reference Amsler, Iken, McClintock, Amsler, Peters, Hubbard, Furrow and Baker2005). Although O. validus are occasionally seen on H. grandifolius blades in nature, it is hypothesized that since the defensive compounds in the thallus were being sensed as unpleasant by the sea-stars' tube feet, the sea-stars might be less likely to crawl onto the macroalgae than onto adjacent substrates. Hence, a gastropod on an alga would have a lower chance of being encountered by a sea-star and, therefore, be gaining somewhat of an associational refuge from sea-star predation. This would be analogous to the associational refuge that WAP amphipods (and by extension, presumably gastropods as well) gain from fish predation by living on branched, chemically defended macroalgae (Zamzow et al. Reference Zamzow, Amsler, McClintock and Baker2010, Amsler et al. Reference Amsler, McClintock and Baker2014). The behavioural experiment in the present work, however, did not support this hypothesis as there was no significant difference either between sea-star residence times on extracted vs intact H. grandifolius thallus or between which of the paired discs the sea-stars moved off first.
In summary, there has previously been strong evidence that amphipod mesograzers and branched macroalgae along the WAP, including the branched Desmarestia spp. that dominate in shallower depths, form a mutualistic relationship (Amsler et al. Reference Amsler, McClintock and Baker2014). The chemically defended macroalgae are not consumed by most amphipod species but benefit from the association because amphipods consume smaller algal epiphytes. The amphipods in turn benefit by receiving an associational refuge from predatory fish. A previous mesocosm experiment (Aumack et al. Reference Aumack, Amsler, McClintock and Baker2011a) demonstrated that amphipods can also reduce diatom fouling on strips of the huge, blade-forming macroalga H. grandifolius that dominates on hard substrates beneath the Desmarestia spp. zone. While it has not been practical to sample these huge macroalgae in a manner that would produce quantitative data on amphipod densities on the algae, the qualitative observations made in the present study during hundreds of hours of diving observations indicated that amphipods are relatively less abundant and gastropods relatively more abundant on H. grandifolius compared to the large, branched brown macroalgae. This raised the question of whether the macroalgal–gastropod interaction on larger, bladed macroalgae such as H. grandifolius might be an analogous, mutualistic relationship. The observations made by the current authors indicate that there are some similarities but also some differences between the amphipod and gastropod interactions with macroalgae. They are similar in that the gastropods can significantly reduce biofouling by smaller algae and also that the gastropods rarely if ever consume their macroalgal hosts. They differ in that while the one, simple behavioural experiment carried out in the present study by itself does not rule out a reciprocal benefit to the gastropods, neither it nor any other observation suggests that there is a benefit. The gastropods are using epiphytic microalgae as a food source, but these also occur on rocks and other benthic materials. In addition to sea-stars, the dominant fish species in the community, Notothenia coriiceps Richardson 1844, is also a major consumer of gastropods (Zamzow et al. Reference Zamzow, Aumack, Amsler, McClintock, Amsler and Baker2011). While gastropods on branched, chemically defended macroalgae presumably gain a refuge from fish (as do the amphipods), it seems - subjectively at least - that a fish could pick a gastropod off a H. grandifolius blade as easily as from a rock, and without also ingesting part of the chemically defended macroalga, as would be the case for a gastropod on a finely-branched macroalga.
Acknowledgements
The authors are grateful to S. Heiser, A. Shilling, C. Brothers, and K. Smith for assistance in the field as well as to the Antarctic Support Contract staff of Palmer Station for outstanding logistical support of this research. Thanks are also due to two anonymous reviewers for constructive comments that improved the final version of the manuscript. L. Miller performed preliminary experiments which informed the design of the behavioural experiment and collected gastropod samples in 2017 that were used for determination of gastropod densities in the mesocosm experiment. L. Miller and R. Edwards contributed to the enumeration of the 2017 gastropod samples. This research was funded by National Science Foundation awards PLR-1341333 (CDA, JBM) and PLR-1341339 (BJB) from the Antarctic Organisms and Ecosystems Program. JBM acknowledges the support of an Endowed University Professorship in Polar and Marine Biology provided by the University of Alabama at Birmingham.
Author contribution
CDA, MOA, JBM, and BJB designed the experiments. CDA, MOA, and MDC performed the experiments. CDA analysed the data and wrote the first draft of the manuscript. All authors edited the final version of the manuscript.
Supplemental material
Supplemental figures S1–S4 will be found at https://dx.doi.org/10.1017/S0954102019000014.