Introduction
Radiation therapy remains a vital modality in the management of head and neck cancer patients. However, there are numerous factors that can affect the accuracy of radiotherapy treatment delivery. Several studies have reported random or interfraction setup errors in head and neck cancer treatment and some have analysed the effects various setup margins used during planning.Reference Feng, Wang and Bai1–Reference Zeidan, Langen and Meeks4 Therefore, setting of appropriate margins is a crucial step in radiotherapy planning. Whereas smaller margins can affect target coverage which is related to treatment outcome, wider margins could result is in increased dose to the organs at risk (OAR).
Owing to the usually high doses of radiotherapy delivered in curative head and neck treatments, the need for accuracy is imperative. Nowadays, various treatment techniques can be used to reduce toxicity to the OAR while maximising the dose to the target volumes. For instance, intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy can be used to escalate doses to head and neck cancer while doses to critical organs can be maintained at acceptable levels.Reference Leclerc, Maingon and Hamoir5
Additional efforts to improve accuracy have been seen through image-guided radiotherapy (IGRT) techniques such as cone beam computed tomography (CBCT), which has seen better accuracy and precision in treatment delivery for head and neck cancers.Reference Zeidan, Langen and Meeks4 It offers increased geometric accuracy and precision with excellent bone and good soft tissue matching and fast online registration using low imaging doses.Reference Jaffray, Kupelian and Djemil6–Reference Walter, Boda-Heggeman and Wertz7 The excellent contrast resolution has enabled better visualisation of patient anatomy, which is necessary to produce accurate setups through bone and soft tissue registrations.Reference Zhang, Garden and Lo8–Reference Bel, Keus and Virjlbrief9
Despite the improved technology, availability of expertise and high workloads has resulted in different CBCT protocols being implemented in different radiotherapy departments. For instance, it is presently not clear if a daily cone beam imaging protocol results in clinically significant improvements in patient positioning in head and neck cancers compared to weekly imaging protocols.Reference Zeidan, Langen and Meeks4 Though several studies have been conducted on the impact of imaging frequency in head and neck patients,Reference Hong, Wofgang and Richard Chapelle3, Reference Walter, Boda-Heggeman and Wertz7 there is need for similar studies to be conducted using CBCT as an imaging modality in head and neck radiotherapy. Several reports have indicated that daily CBCT imaging can be associated with high costs in terms of machine time and increased dose to the patient. Therefore, if a daily protocol is to be used, there is need for evidence on clinical benefit.Reference Bel, Keus and Virjlbrief9 This can be in terms of the impact on the clinical target volume-planning target volume (CTV–PTV) margins and setup error reduction.
The aim of this study was to evaluate the impact of a daily and weekly guided radiotherapy (IGRT) protocol in reducing setup errors and setting of appropriate margins in head and neck cancer patients.
Materials and Methods
Patient selection and computed tomography (CT) simulation
A total of 31 head and neck cancer patients treated with external radiotherapy irrespective of the primary tumour location were selected. The average age was 58 years (range 25–80 years). A Varian Clinac IX OBI (v1·4) (Varian Medical Systems, Inc.) was used for treatment. All patients were simulated on a GE Lightspeed RT16 (GE Medical Systems) CT scanner and were immobilised with thermoplastic masks (WFR Aquaplastic mask, PA, USA), which cover the patient’s face, shoulders and customised head supports. A superior straightening mark was placed on the mask at the level of the supra-sternal notch and inferior straightening tattoo was placed over the xiphoid process in addition to lateral levelling tattoos to improve setup reproducibility in a supine position.
Target and OAR definition
The radiation oncologist outlined the gross target volumes (GTV1) that encompassed the primary tumour and involved lymph nodes. A 5 mm margin was used for expansion to the CTV1. A second CTV2 included all electively treated lymph nodes. The CTVs were expanded in all directions by a margin of 0·5 cm to form the PTV1 and PTV2. The OARs delineated include bilateral lens, spinal cord (SC), brainstem, optic nerves(ON), optic chiasm (OC), bilateral parotid glands and the oral cavity as per departmental protocol. A planning organ at risk volume was contoured for the SC by adding a 0·5 cm margin and 0·3 cm for the OC and the ON. The prescription dose was 70 Gy to PTV1, whereas the PTV2 was treated to 56–63 Gy.
Patient verification procedure
The CBCT images were registered to planning CT images and the setup errors were analysed based on the departmental protocol. The pre-treatment imaging protocol involved CBCT day 1–3 plus weekly till end of treatment. Daily imaging was done only to a small number of patients who had a high dose prescribed to tumours close to critical organs. In this study only the data from the daily imaged patients were used. All images were automatic vertebrae matched to eliminate inter-user variability. The second cervical vertebra (C2) was the main part of the neck that was to match head and neck images based on Zhang et al.’s study.Reference Zhang, Garden and Lo8 At the author’s institution the isocenters for the head and neck patients were often close to C1–C4. Therefore, the C2 vertebral body was considered the landmark of choice for all patients except for those whose target was distant from C2. A tolerance of 3 mm was used and translational couch shifts were applied if they were >3 mm as per departmental protocol. Approval by radiation oncologists was required for first day images only and the rest of the images were assessed by two competent radiation therapists.
Based on the author’s institutional protocol, mean couch shifts for the first three CBCTs’ was used to estimate and correct for the presumed systematic setup errors. If the average is <3 mm in S-I, A-P and L-R direction, no repeat imaging was done and this was followed by weekly imaging. If the average was >3 mm, images would be repeated for 3 more days and an average would be taken and used to correct for presumed systematic error. In the current study we investigated the daily imaging protocol and the day 1–3 plus weekly imaging protocol. In addition, we calculated and analysed residual errors for day 1–3 plus weekly imaging data by finding the average shifts of the first three imaged fields.
Determination of residual errors
We calculated and analysed residual errors for day 1–3 imaging data by finding the average shifts of the first three imaged days in the S-I, L-R, and A-P direction. Residual mean of random errors gave an estimate of systematic error. This average was then subtracted from the shift correction obtained using daily CBCT for each subsequent fraction to obtain the residual error. The residual error showed the magnitude of difference between shifting based on daily CBCT and the shift that would have been made if the patient was moved a fixed amount based on the average couch shifts calculated from the first three imaged fields.Reference Hong, Wofgang and Richard Chapelle3 The equation below was used to calculate population mean setup errors (M) and population systematic error (Σ) and population random error (σ) for residual error protocol.
As per Zeidan et al.,Reference Zeidan, Langen and Meeks4 if for one patient the k th number of treatment is delivered with dk (where dk is the shift in cm) setup error, then the individual mean set-up error (m) of n (total number of fractions) fractions is given by:
For the analysed group of p (total number of patients), the overall population mean setup error M pop is:
The systematic error for the population (Σpop) is defined as the standard deviation (SD) of the individual mean setup errors about the overall population means M pop is:
 $${\sum}\limits_{{{\rm pop}}} = \sqrt {{\matrix{ (m_{1} {\minus}M_{{\rm pop}} )^{2} {\plus}(m_{2} {\minus}M_{{\rm pop}} )^{2} {\plus}(m_{3} {\minus}M_{{\rm pop}} )^{2} \hfill \cr {\plus}...{\plus}(m_{p} {\minus}M_{{\rm spop}} )^{2} \hfill \cr} \over {p{\minus}1}}} $$
$${\sum}\limits_{{{\rm pop}}} = \sqrt {{\matrix{ (m_{1} {\minus}M_{{\rm pop}} )^{2} {\plus}(m_{2} {\minus}M_{{\rm pop}} )^{2} {\plus}(m_{3} {\minus}M_{{\rm pop}} )^{2} \hfill \cr {\plus}...{\plus}(m_{p} {\minus}M_{{\rm spop}} )^{2} \hfill \cr} \over {p{\minus}1}}} $$
The individual random error (σ 1) is the SD of individual setup error:
 $$\sigma _{i} =\sqrt {{{(d_{1} {\minus}m)^{2} {\plus}(d_{2} {\minus}m)^{2} {\plus}(d_{3} {\minus}m)^{2} {\plus}...{\plus}(d_{n} {\minus}m)^{2} } \over {n{\minus}1}}} $$
$$\sigma _{i} =\sqrt {{{(d_{1} {\minus}m)^{2} {\plus}(d_{2} {\minus}m)^{2} {\plus}(d_{3} {\minus}m)^{2} {\plus}...{\plus}(d_{n} {\minus}m)^{2} } \over {n{\minus}1}}} $$
The population random error (σ pop) is the mean of all the individual random errors:
Determination of PTV margins
Applying suitable PTV margin facilitates coverage of geometric errors during treatment delivery while reducing radiation doses to surrounding areas. After calculating the interfraction and systematic errors, the PTV margins were calculated from the setup data. This estimate was based on the need to cover the empirical 3D margins from the CTV in 90% of the patients with the 95% isodose. We used van Herk’s formulaReference van Herk and Remeijer10 as follows:
Where m is the mean systematic error, Σ is the systematic setup error and σ is random setup error, both given as 1 SD. We calculated Σ as the population SD from individual patients weighted by the number of acquired images. Whereas, σ was calculated as root mean square value over all displacements around the systematic setup errors. The CTV to PTV margins were calculated from the daily, day1–3 plus weekly and from day 1–3 residual imaging data.
Statistical analysis
The mean differences in setup errors between interfractional daily imaging and hypothetical day 1–3 and weekly imaging were calculated using a paired t-test from Microsoft excel version 2010 to determine whether the differences between the daily imaging and day 1–3 plus weekly imaging protocols were statistically significant.
A p-value of ≤0·05 was considered as statistically significant.
Results
Comparison of shifts from different protocols
A total of 964 individual CBCT scans were analysed. The pre-treatment daily CBCT scans were analysed stratified into ≥3 and ≥5 mm shifts. The mean pre-treatment daily with shifts ≥3 mm was in 16·6, 16 and 3% of setups in the S-I, L-R and A-P directions, respectively. Shifts ≥5 mm were 5, 25 and 0·8% in the S-I, L-R and A-P directions, respectively. In comparison, the hypothetical ‘day 1–3 plus weekly’ interfraction shifts ≥3 mm occurred in 16, 18 and 33% of the S-I, L-R and A-P direction. Residual error shifts ≥3 mm occurred in 25, 27 and 24% of the S-I, L-R and A-P directions, respectively. The calculated mean residual errors ≥5 mm occurred in 7, 10 and 4% in the S-I, L-R and A-P directions, respectively. The p-values of daily versus day 1–3 plus weekly imaging are shown in Table 1. The differences for daily versus day 1–3 residual errors are shown in Table 2.
Table 1 Comparison of daily versus day 1–3 plus weekly imaging

Abbreviations: S-I, superior-inferior; L-R, left-right; A-P, anterior-posterior.
Table 2 Comparison of daily versus day 1–3 residual imaging

Abbreviations: S-I, superior-inferior; L-R, left-right; A-P, anterior-posterior.
Mean shifts for ‘daily’ and hypothetical ‘day 1–3 residual’ imaging data
Figures 1a–1c show the mean shifts for daily versus hypothetical day 1–3 residual CBCT imaging protocols. All fractions per patient were included in this analysis. Results are for S-I, L-R and A-P directions. Results show that daily imaging has fewer systematic errors ≥3 and ≥5 mm in all three dimensions than day1–3 plus weekly protocol and day 1–3 residual protocol. Figures 2a–2c show the mean random error shifts for daily versus day 1–3 plus weekly imaging CBCT protocols of 31 patients.

Figure 1 (a) Daily versus day 1–3 residual shifts in the S-I direction (n=31), (b) Daily versus day 1–3 residual shifts in the L-R direction (n=31) and (c) Daily versus day 1–3 residual shifts in the A-P direction (n=31). Abbreviations: S-I, superior-inferior; L-R, left-right; A-P, anterior-posterior.

Figure 2 (a) Mean shifts for daily versus day 1–3 plus weekly S-I direction (n=31), (b) Mean shifts for daily versus day 1–3 plus weekly L-R direction (n=31) and (c) Mean shifts for daily versus day 1–3 plus weekly A-P direction (n=31). Abbreviations: S-I, superior-inferior; L-R, left-right; A-P, anterior-posterior.
CTV to PTV margin expansion
Table 3 shows the calculated CTV–PTV margins based on the data from the interfraction and systematic errors in the daily imaging protocol. Tables 4 and 5 shows similar calculations based on the day 1–3 plus weekly and the day 1–3 residual protocols.
Table 3 CTV–PTV expansions: daily imaging protocol

Abbreviations: CTV, clinical target volume; PTV, planning target volume.
Table 4 CTV–PTV expansions: day 1–3 plus weekly protocol

Abbreviations: CTV, clinical target volume; PTV, planning target volume.
Table 5 CTV–PTV expansions: day 1–3 residual protocol
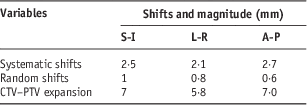
Abbreviations: CTV, clinical target volume; PTV, planning target volume.
Discussion
The treatment of head and neck tumours requires greater accuracy due to the numerous OAR, which are often in close proximity to the tumour. The results in this study show that less imaging resulted in large systematic errors and no difference in random errors. A large proportion of errors could be picked if daily imaging is used. This is consistent with findings of other researchers.Reference Qi, Wu and Newman11–Reference Oita, Ohmori and Obinata13 No significant difference was found in the mean setup error for the daily and hypothetical day 1–3 plus weekly protocols. This is in agreement with Laurence et al.Reference Laurence, Court and Luciant14 who showed that imaging protocols less than daily do not eliminate random error but can effectively reduce systematic errors.
de Boer et al.’s studyReference de Boer and Heijmen15–Reference de Boer and Heijman17 showed that day 1–3 plus weekly are sufficient for head and neck cancers using site-specific tolerances determined locally and in the range of 2–3 mm tolerance. de Boer used no-action-level (NAL) protocol that calculates the mean setup error over a fixed number of fractions and corrects it to reduce systematic patient setup errors with minimal portal imaging workload. He evaluated the protocol by applying it to a database of measured setup errors from 600 patients (with on average ten imaged fractions). Results showed the NAL protocol was efficient in decreasing required portal images.Reference de Boer and Heijman17 The Royal College of Radiologists, Institute of Physics and Engineering in Medicine Society and College of Radiographers; 200818 recommended imaging for head and neck cancers to be days 1–3 and weekly using site-specific tolerances.
The calculated residual errors for the daily imaging protocols correlates to the studies conducted by Zeidan et al.Reference Zeidan, Langen and Meeks4 and Den et al.Reference Den, Demer and Kubicek19 Zeidan et al.Reference Zeidan, Langen and Meeks4 assessed the residual setup error in different image–guidance protocols in the alignment of patients with head and neck cancer patients. Residual errors for different protocols were retrospectively calculated using data from patients who were treated with daily imaging. According to Zeidan et al.’sReference Zeidan, Langen and Meeks4 imaging on every other treatment day resulted in 11% of all treatments subject to setup errors of ≥5 mm in dimensions in head and neck patients. His results showed reduction in residual setup errors with increased frequency of imaging for head and neck cancers. Results seem to prove that the author’s head and neck setups are more rigid and reproducible than Zeidan et al.’s.Reference Zeidan, Langen and Meeks4 Both studies proved that residual setup errors reduce with increasing frequency of imaging for head and neck cancers.
Den et al.Reference Den, Demer and Kubicek19 studied 28 head and neck patients who were daily imaged with CBCT. The average interfraction shifts in his study were in the 1·4±1·4, 1, 7±1·9 and 1 ·8±2·1 mm in the L-R, S-I and A-P direction. His shifts were similar to those obtained in this study. There was difference in residual data since he measured residual errors from CBCT after treatment. His residual results were more accurate. In this study, we did not include data of pre- and post-CBCT because it was not available; this limited the accuracy in the calculation residual errors. An estimate formula for residual errors similar to the one used by Zumsteg et al.Reference Zeidan, Langen and Meeks4 was used. The major limitation to his formula is that it assumes that residual errors remain constant though in actual fact they can vary each day according to the patient’s motion during treatment.
Challenges in the treatment of head and neck are organ and tumour motion induced by deglutition support the need for daily. As reported by Bradley et al.Reference Bradley, Paulson and Ahunbay20 and Stenson et al.Reference Stenson, McCracken and List21deglutition-induced tumour displacements are larger and significant compared with resting motion. In addition, rotational errors also play an important role in accurate delivery of treatment to patients. As reported by Guckenberger et al.Reference Guckenburger, Meyer and Vordermark22, a large head and neck target volume of 10-cm long with a rotation of 5% can result in a 4·4 mm displacement at the target end and this may lead to under dosage of the target or increased doses to adjacent OAR when using IMRT with steep dose gradients.
Kim et al.Reference Kim, Pawlicki and Le23 showed that unadjusted rotational displacements caused an increase in the dose to the SC dose of 6·4% and contra-lateral parotid of 2·7%. On the other hand Li et al.’sReference Li, Qi and Pitterle24 study proved that although rotational correction may slightly offset the deviations in translational shifts, there was negligible impact on the accurate setup. Though the results show that it is better to image daily to reduce treatment setup errors and setup margins. Clinicians should be aware of the increased dose to the patient and it is more time consuming compared with the day 1–3 plus weekly protocols. Therefore, radiotherapy departments may choose to incorporate the daily imaging to the dose delivered to the patient.
As shown in Tables 3–5 interfraction protocols, the use of daily CBCT resulted in reduction of PTV margins compared with day 1–3 plus weekly interfraction and day 1–3 residual protocols. The largest margins were in the L-R direction. For this direction only, daily imaging would require 3·8 mm margins whereas day 1–3 plus weekly interfraction and day 1–3 residual protocols needed a 5 mm PTV margins. These results are similar to Qi et al.’sReference Qi, Wu and Newman11 study that proved that frequent imaging reduces systematic error and PTV margins. Based on the results it appears beneficial to reduce PTV margins on treatment planning when daily imaging is used. However, use of a stable setup positions and immobilisation devises is recommended.
Limitations in the study
In this study, rotational errors were not considered, only translational shifts were recorded due to the lack of a hexagon couch. In addition, the accuracy in calculating residual errors could have been improved by imaging before and after each treatment. This was not possible because only pre-treatment imaging data were available.
Conclusion
Finding the most suitable imaging protocol for head and neck cancers is still a controversial issue. Some authors have reported results that support the use of daily imagingReference Zeidan, Langen and Meeks4, Reference Zhang, Garden and Lo8, Reference Bel, Keus and Virjlbrief9, Reference Qi, Wu and Newman11, Reference Bradley, Paulson and Ahunbay20 whereas other studies were not in favour of daily imaging.Reference de Boer and Heijmen15–18 Some researchers reported average doses per scan for head and neck imaging of 0·07 and 0·03 cGy, therefore reduction in imaging frequency would be preferable.Reference Li, Qi and Pitterle24, Reference Daly, Siewerdsen and Morsely25 However, the results of this study show that a daily CBCT protocol reduces setup errors and allows setup margin reduction in head and neck radiotherapy compared to a weekly imaging protocol. In addition to the increased dose to the patient, the impact of daily imaging on workflow and availability of resources could be factors in considering whether a daily imaging protocols could be implemented.
Acknowledgements
The authors would like to thank Dr Andrew Pearce, Gemma Burke, Dr Shuying Wan, Dr Mike Oliver and William Tran and colleagues at the Sudbury Regional Hospital, Canada for their support during this project.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.