Introduction
As an accessory phase, apatite (Ca5(F,Cl,OH)(PO4)3) is among the most important minerals controlling trace-element variation in igneous and metamorphic rocks (e.g. Watson and Harrison, Reference Watson and Harrison1984; Cherniak, Reference Cherniak2000; Spear and Pyle, Reference Spear and Pyle2002). Apatite has been defined as a supergroup of minerals that is divided into five groups on the basis of composition (Pasero, Reference Pasero, Kampf, Ferraris, Pekov, Rakovan and White2010). In this work we use the term apatite generally to refer to all Ca phosphates in the apatite group and for discussion of older papers. Because of its ability to incorporate and possibly concentrate a range of minor and trace elements, apatite is potentially a good indicator of P-T-X (pressure-temperature-composition) changes during magmatic and metamorphic processes (Korzhinsky, Reference Korzhinsky1981; Yardley, Reference Yardley1985; Gottesmann and Wirth, Reference Gottesmann and Wirth1997; Smith and Yardley, Reference Smith and Yardley1999; Sallet, Reference Sallet2000; Sun et al., Reference Sun, Tang, Sun, Xu, Zhai, Liang, Liang, Shen, Zhang, Zhou and Wang2007). In metamorphic rocks, apatite occurs over a wide P–T range from very low-grade conditions through amphibolite to high-temperature granulite facies, and even to ultrahigh-pressure metamorphic rocks (e.g. Liou et al., Reference Liou, Zhang, Ernst, Rumble, Maruyama and Hemley1998). By changing the temperature and/or pressure conditions, the crystal structure of the host mineral with ions of minor and trace elements becomes unstable and those elements incorporate into various phases created by exsolution (unmixing) (Yund and McCallister, Reference Yund and McCallister1970; Liou et al., Reference Liou, Zhang, Ernst, Rumble, Maruyama and Hemley1998). Exsolution lamellae in apatite have been reported from several high- to ultrahigh-grade rocks (Chen et al., Reference Chen, Zeng, Chen and Liang2006; Zeng et al., Reference Zeng, Chen, Liang and Xu2006; Sun et al., Reference Sun, Tang, Sun, Xu, Zhai, Liang, Liang, Shen, Zhang, Zhou and Wang2007; Liu et al., Reference Liu, Yang, Zhang, Chen, Wang and Yang2009; Broska et al., Reference Broska, Krogh Ravna, Vojtko, Janák, Konečný, Pentrák, Bačík, Luptáková and Kullerud2014), and are composed of crystallographically-orientated minerals such as: monazite; hematite; magnetite; baryte; pyrrhotite; anhydrite; and dolomite. In addition to their formation from the host apatite in a closed system (e.g. Sun et al., Reference Sun, Tang, Sun, Xu, Zhai, Liang, Liang, Shen, Zhang, Zhou and Wang2007), they are interpreted to be as a result of metasomatic reaction (Harlov et al., Reference Harlov, Förster and Nijland2002; Harlov, Reference Harlov2015; Broska et al., Reference Broska, Krogh Ravna, Vojtko, Janák, Konečný, Pentrák, Bačík, Luptáková and Kullerud2014).
In this paper, we present micro-textures of apatite with exsolution lamellae of various minerals in eclogite from the Moldanubian Zone in the Bohemian Massif. Using compositional maps of the host crystal and cathodoluminescence imaging, we document the relationship between the distribution of exsolution lamellae and compositional zoning of minor and trace elements. In addition to the question of a closed, or open system, the formation of apatite and exsolution within, we discuss the process of unmixing in relation to the P-T trajectory constrained for the host rocks (Faryad et al., Reference Faryad, Dolejš and Machek2009; Faryad et al., Reference Faryad, Jedlicka and Ettinger2013a).
Geological setting
The Bohemian Massif is located at the eastern end of the European Variscan orogenic belt. It is formed by two zones (the Moldanubian and Saxothuringian) with high- to ultrahigh-pressure (HP-UHP) metamorphic rocks and two blocks (the Teplá-Barrandian and Brunovistulian) that are free of HP-UHP rocks (Fig. 1a). The Moldanubian Zone consists mostly of amphibolite- and granulite-facies metamorphic rocks that contain bodies of garnet and/or spinel peridotite and eclogite (for more information see Medaris et al., Reference Medaris, Wang, Mísař and Jelínek1990; Schulmann et al., Reference Schulmann, Kröner, Hegner, Wendt, Konopásek, Lexa and Štípská2005; Faryad et al., Reference Faryad, Dolejš and Machek2009; Reference Faryad, Jedlicka and Collett2013b; Svojtka et al., Reference Svojtka, Ackerman, Medaris, Hegner, Valley, Hirajima, Jelinek and Hrstka2016). This zone is intruded by late Variscan granitoid plutons in its western and central parts. The garnet peridotite blocks reach up to 2 km x 4 km in size and occur sporadically within granulites and migmatitic gneisses (e.g. Medaris et al., Reference Medaris, Wang, Mísař and Jelínek1990). They may contain lenses and layers of eclogite and garnet pyroxenite. The garnet peridotite is lherzolite, harzburgite and rarely dunite in composition and has been extensively affected by serpentinisation.

Fig. 1. (a) Simplified geological map of the Bohemian Massif, modified after Cháb et al. (Reference Cháb, Stráník and Eliáš2007) and Faryad and Kachlík (Reference Faryad and Kachlík2013). CBPC = Central Bohemian Plutonic Complex, MPC = Moldanubian Plutonic Complex. (b) Detail of garnet peridotite body with eclogite in Nové Dvory. The open star indicates the location of the sample from the eclogite body with apatite.
Ultra-high pressure conditions for both garnet peridotites (5.5 GPa / 1100°C) and eclogites (5 GPa / 1000°C) were estimated by Medaris et al. (Reference Medaris, Wang, Mísař and Jelínek1990) and Nakamura et al. (Reference Nakamura, Svojtka and Naemura2004), respectively. Faryad et al. (Reference Faryad, Dolejš and Machek2009) studied exsolution lamellae of garnet in clinopyroxene from clinopyroxenite within garnet peridotite. On the basis of textural and compositional relations they concluded that fragments of original mantle wedge peridotite with lenses and layers of mafic rocks (Medaris et al., Reference Medaris, Wang, Mísař and Jelínek1990; Svojtka et al., Reference Svojtka, Ackerman, Medaris, Hegner, Valley, Hirajima, Jelinek and Hrstka2016) were incorporated in crustal rocks during subduction, shared the same P-T trajectory to peak pressure and were exhumed to the surface (Faryad et al., Reference Faryad, Jedlicka, Hauzenberger and Racek2018). Recent studies (Perraki and Faryad, Reference Perraki and Faryad2014; Jedlicka et al., Reference Jedlicka, Faryad and Hauzenberger2015) indicated that the peak pressures were reached by the rocks at a lower temperature (650–800°C) than that (1200–1300°C) estimated by Medaris et al. (Reference Medaris, Wang, Mísař and Jelínek1990) and Nakamura et al. (Reference Nakamura, Svojtka and Naemura2004) and the granulite-facies metamorphism (850–950°C / 1.0–1.6 GPa) occurred after partial exhumation of the UHP rocks into the lower and/or middle crustal levels. This high-temperature granulite-facies overprint resulted from mantle upwelling that occurred due to slab break-off during a collision orogeny (Faryad et al., Reference Faryad, Kachlík, Sláma and Hoinkes2015).
The garnet peridotite body at Nové Dvory is serpentinised garnet lherzolite (Medaris et al., Reference Medaris, Wang, Mísař and Jelínek1990) within migmatitic and granulite gneisses (Fig. 1b). It contains several large (up to 500 m long) lenses of eclogite (Fig. 1b) and thin layers of clinopyroxenite. On the basis of their geochemistry the lenses and layers of mafic rocks with peridotite formed by melt that migrated through the lithospheric mantle wedge above the subduction zone (Medaris et al., Reference Medaris, Beard and Jelínek2006; Ackerman et al., Reference Ackerman, Jelínek, Medaris, Ježek, Siebel and Strnad2009). Exsolution lamellae of garnet in clinopyroxene from clinopyroxenite layers were investigated by Faryad et al. (Reference Faryad, Dolejš and Machek2009) who concluded that formation of the lamellae by cooling was when the hot mantle rocks became involved with down-going crustal material during subduction. The eclogite investigated in this present work is exposed in the southern part of the garnet peridotite body as indicated in Fig. 1b.
Analytical methods
Compositional data and X-ray mapping were obtained using a JEOL JXA-8530F field-emission gun electron microprobe analyser (FEG-EMPA) equipped with wavelength- and energy-dispersive spectrometers (WDS and EDS) at the Institute of Petrology and Structural Geology, Charles University in Prague. The analyses were performed on polished thin-sections with normal operating conditions for spot and analyses with a defocused beam (5–10 μm wide) with 15 kV and 30 nA beam current and 30 s counting time on peak and total background. Typical detection limits for these analytical conditions were in the range of 50–150 ppm (3σ). The SPI #02753–AB 53 mineral standards were used for following elements: Si (Kα) – quartz; Al (Kα) – corundum; Mg (Kα) – periclase; Fe (Kα) – magnetite; Mn (Kα) – rhodonite; Ca (Kα) – calcite, diopside; Ti (Kα) – rutile; Cr (Kα) – chromium oxide; Na (Kα) – albite; P (Kα) – apatite; Ni (Kα) – nickel oxide; Y (Lα) – YAG. These elements were measured by the WDS crystals: TAP (Na, Mg, Al, Si, Y, P); PET (Ti, Cr, Ca, V); LIF (Mn, Fe, Ni). The ZAF algorithm was applied for correction of raw data. The same method was applied for detailed compositional profiles of major and trace elements. For high-resolution compositional mapping of major and trace elements, analytical conditions of 20 kV and 120 nA were used. The rare-earth elements (REE), As, Sr, F, Cl and S, detected in apatite and its exsolutions, were measured quantitatively only by WDS.
The Raman spectra of lamellae in apatites were obtained with a Renishaw Ramascope RM1000® Raman micro-spectrometer at the School of Mining and Metallurgical Engineering of the Technical University of Athens, Greece. Spectra were excited at room temperature with the 632.8 nm line of a red 19 mW He–Ne laser through an OLYMPUS® ×100 objective. The numerical aperture of the objective is 0.9. The laser spot on the surface has a diameter of ~1 μm and a power of 4 mW. The entrance slit into the spectrometer was set to 4 μm. Light was dispersed by a holographic grating with 1800 grooves/mm. Three accumulations of 20 s each were made to provide a good signal-to-noise ratio. The wavenumber calibration was checked regularly by measuring the position of the Rayleigh line and the LO phonon mode of a silicon single-crystal wafer.
Petrology
The eclogite with apatite is medium-to-coarse grained with garnet porphyroblasts between 1 and 3 mm in size and locally up to 8 mm. It is weakly foliated and in addition to garnet (up to 70 vol.% of the rock) contains omphacite and accessory amounts of amphibole, biotite, K-feldspar, ilmenite, rutile, apatite and spinel. Omphacite is relatively fresh and it is replaced partly by a very fine-grained symplectite of plagioclase, diopside and/or an amphibole. In contact with garnet, it is rimmed by green amphibole. Brown amphibole is present at the contact with ilmenite. The amphibole is partly replaced by biotite. Biotite with green spinel forms pseudomorphs after an unknown phase (probably phengite). In some cases, the boundaries between minerals are formed by thin coronae with symplectite of amphibole (?) and plagioclase and the fine grains in the symplectite have an orientation perpendicular to the interface of garnet and/or omphacite. Rutile is rarely present in garnet, but that occurring in the intergranular spaces between garnet and omphacite is rimmed or totally replaced by ilmenite.
Garnet from eclogite is zoned with high pyrope contents (ca. 36 mol.%) in the core which decrease towards the mantle before increasing at the outermost part of the rim (Fig. 2). Both almandine and grossular components show an increase towards the rims, but grossular additionally shows a decrease at the outermost part of the rim. Spessartine content is very low (~0.6 mol.%) and shows an almost flat compositional profile. The decrease in pyrope and increase in grossular content from core to the mantle part of garnet indicates a temperature decrease and pressure increase as result of the exchange reaction between garnet and clinopyroxene (Ellis and Green, Reference Ellis and Green1979), the process similar to that described for formation of the exsolution lamellae in clinopyroxene from clinopyroxenite (Faryad et al., Reference Faryad, Dolejš and Machek2009). This differs from subduction of cold crustal rocks heated by pressure increase during subduction and show increase of both Mg and Ca towards the rims (Jedlicka et al., Reference Jedlicka, Faryad and Hauzenberger2015). The increase in pyrope and decrease in grossular at the outermost rim part of garnet (Fig. 2) signify granulite-facies metamorphism occurring at lower pressure. The low grossular content is due to stabilisation of plagioclase in granulite-facies conditions.
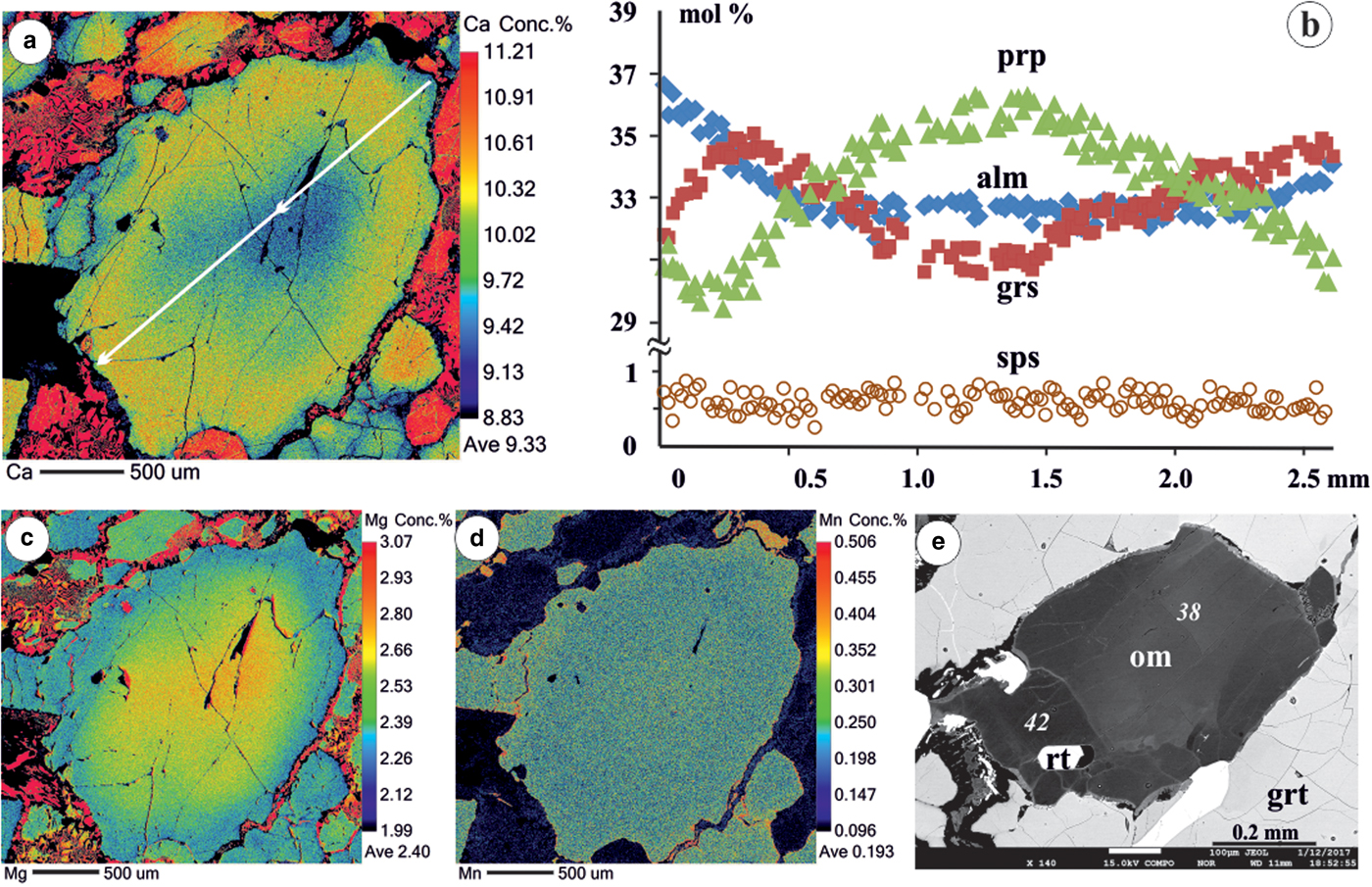
Fig. 2. False-colour compositional maps of garnet (a, c, d) with qualitative concentration of elements in wt.% and profile (b) which shows the zoning pattern (in mol.%) of pyrope (prp), almandine (alm), grossular (grs) and spessartine (sps). (e) BSE image of compositionally zoned omphacite with dark and light domains having different jadeite contents (in mol.%). Omphacite contains rutile (rt) inclusions and at the rim is replaced by a symplectite of plagioclase + amphibole + diopside).
Omphacite has a jadeite content up to 42 mol.%. The ferrous and ferric iron contents were calculated by stoichiometric and charge-balance criteria. Compositional maps of some omphacite grains show zoning with slightly jadeite-rich domains at the rims (Fig. 2e). Omphacite shows a thin diffusion rim with small amounts of jadeite. The amphibole is pargasite in composition and occurs in the intergranular spaces; some relic grains have rutile needles.
Apatite and exsolved minerals
Apatite is a common phase in the rock, occurring in the matrix and also as inclusions in garnet and omphacite. It forms clusters or separate grains with euhedral- to- subhedral habits and is up to 1 mm x 0.3 mm in size (Fig. 3). In polarised-light microscopy the grains appear to have domains, visible as fine dusty particles. The modal abundance of apatite in the rock is ~1 vol.%. The apatite contains F (2–3 wt.%) and has a maximum Cl content of 0.4 wt.% (Table 1).

Fig. 3. Plane polarised light images. Apatite (ap) forming clusters (a) or separate grains as inclusions (c–f) in garnet (grt). Note parallel inclusion patterns and variously shaded domains in a single grain of apatite. The white arrow in (a) indicates an exsolution-free track (probably healed fractures).
Table 1. Representative compositions from microprobe analyses of apatite from eclogite in the Nové Dvory.

bdl – below the detection limit.
Apatite contains very fine needle-like lamellae, and in rare cases, rods, that are oriented crystallographically parallel to the c axis (determined by polarised-light microscopy). Together with the fine particles, they give a dusty appearance to the apatite grains (Figs 3 and 4). The particles are very small and their nonlinear shapes on the surface could be due to the orientation of apatite grains (not parallel to the crystallographic planes). When comparing microphotographs and cathodoluminescence (CL) images, the dusty domains match a CL response with different intensity of luminescence (Fig. 5); the dusty domains have sharp boundaries with lower luminescence in the CL images. X-ray maps of apatite grains (Fig. 6) indicate that the dusty domains have a high concentration of Fe and S. No relation between F or Cl distribution and dusty domains in apatite (Fig. 6d, e) was observed. Plane-polarised-light microphotographs (Fig. 6a) and X-ray maps of S and Fe (Fig. 6b, c) indicate exsolution free tracks that probably represent former fractures in apatite crystals that were later healed. Some of these healed fractures (Fig. 5c) are also visible in CL images (Fig. 5f).

Fig. 4. BSE images of apatite inclusions in garnet. Note that the lamellae of different sizes in apatite are oriented parallel to the c axes of crystals. The black areas are holes created during sample preparation. (a) Scale bar = 100 μm; (b) scale bar = 10 μm.
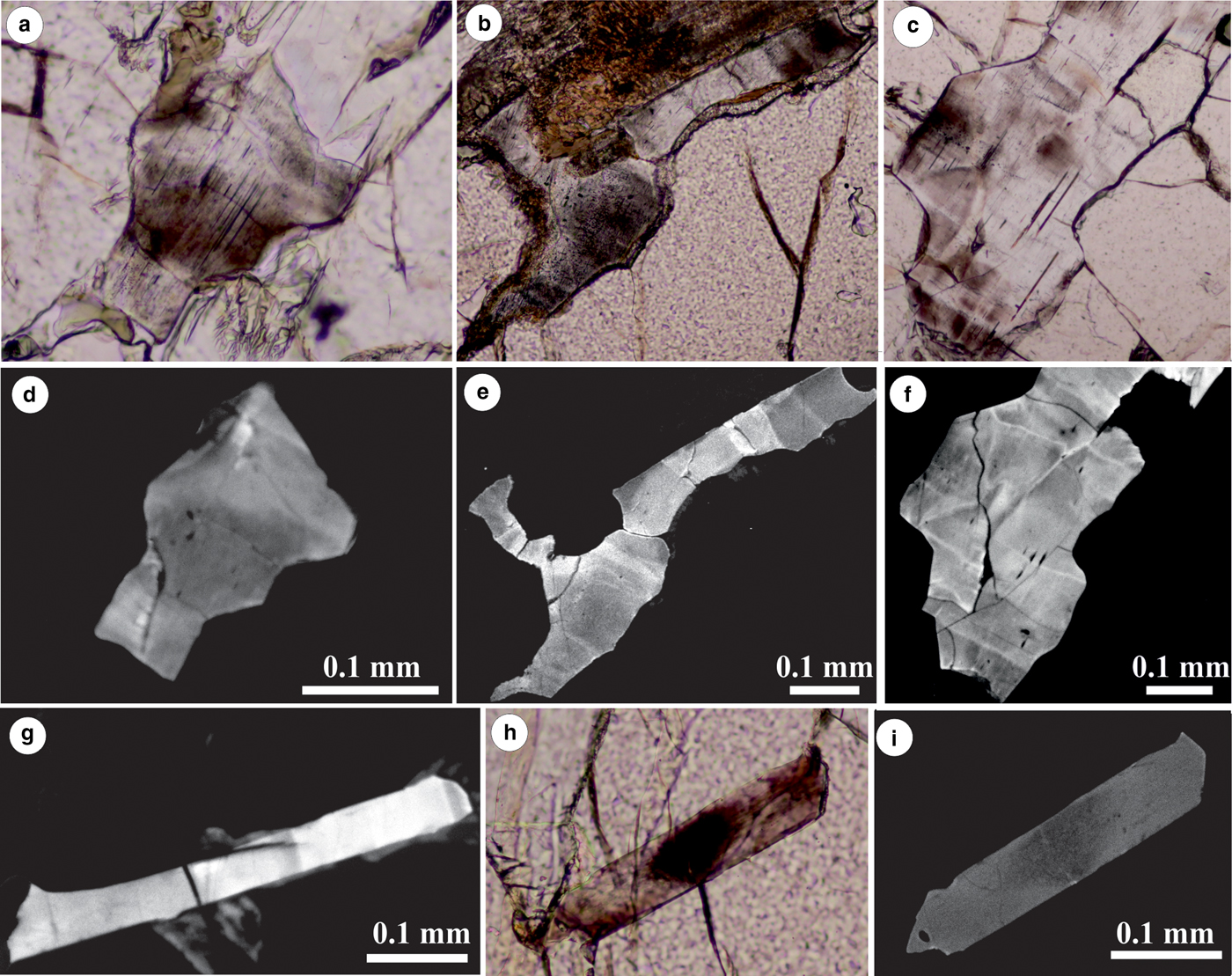
Fig. 5. Microphotographs (a, b, c, h) and respective cathodoluminescence (CL) images (d, e, f, g, i) of dusty domains in apatite. Note that the areas of high concentration of dusty domains show a lower luminescence intensity in CL images. Some domains have a sharp boundary and appear as sector zoning of the apatite crystals.

Fig. 6. Apatite with an inclusion of dusty domains (a) and corresponding false-colour compositional maps (qualitative concentration of elements in wt.%) of S (b), Fe (c), F (d) and Cl (e, inset). Note that domains with high concentration of inclusions have high Fe and S contents. The white arrows in (a), (b) and (c) indicate exsolution-free tracks.
Oriented lamellae and rods in apatite
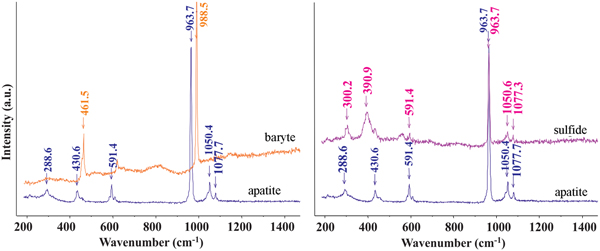
Microphotographs and back-scatter electron (BSE) images (Figs 3 and 4) indicate that, in addition to dusty particles, relatively large oriented rods and lamellae are present. Detailed Raman spectroscopy and microprobe analyses indicated that they represent various mineral phases. As the phases are very small, the true composition is difficult to determine, but by a combination of all these methods, five types of inclusions were observed. They are: (1) Fe sulfide; (2) Cu–Fe sulfide; (3) Ni–Co–Fe sulfide; (4) Fe oxide; and (5) baryte (Figs 7 and 8). Because of the small size of the lamellae, most analyses contain P and Ca from the host apatite. In such cases, P and Ca structurally bound to apatite were subtracted from the analyses to determine the composition of the lamellae. Most of the inclusions are rich in Fe. They generally form lamellae (Fig. 7b), but some polyhedral grains are also present (Fig. 7e). In addition to almost pure Fe oxide, Fe sulfide was also analysed. It has higher Fe content than common natural sulfide minerals (FeS, FeS2). On the basis of normalised Fe and S contents (Fe6.80S8.19), it is close to monoclinic pyrrhotite (Fe7S8). In addition to S, some lamellae contain Cu that range from 5.37 to 28.81 wt.% and minor Ni and Co. The high Cu content in the lamellae probably indicates the presence of other phases such as chalcopyrite, pentlandite or possible solid solutions between two phases. The sulfide and baryte lamellae were also identified from Raman spectra (Fig. 8). The best data (without excitation of the host apatite) were obtained for the large baryte lamellae. This has a small amount of Sr (Ba0.979Sr0.066S0.985O4) or Ca (Ba0.973Ca0.066S0.999O4) (Table 2). Both baryte and sulfide lamellae occur in a single apatite grain.

Fig. 7. BSE images of apatite crystal (a) with exsolution lamellae of baryte (b–d) and Fe, Cu sulfides (e and f). Note that some, relatively large inclusions (c and d) are multiphase. The BSE image in (a) is from that in Fig 3e. The crosses and squares are positions from where analyses and spectra were acquired.

Fig. 8. Raman spectra of baryte and sulfide lamellae in apatite. The sulfide lamella analysed is indicated in Fig. 7a (Cu, Fe, S).
Table 2. Representative compositions from microprobe analysis of lamellae in apatite.

b.d.l. = below detection limit.
Discussion
Relationship between density of exsolved phases and compositional zoning in apatite
Microphotographs indicate that the lamellae and submicroscopic (dusty) exsolutions are not distributed regularly throughout the apatite crystals and show no relation to the core or rim of grains. Similarly, no relationship was found in the density and distribution of the exsolved phases between the apatite grains forming inclusions in garnet or omphacite and those occurring in the matrix. However, a combination of optical microscopy with compositional mapping revealed that the dusty domains have high concentrations of Fe, S and other elements (Fig. 6a–c). Because of the very small sizes of the dusty particles, it was not possible to determine if the high concentrations of Fe and S observed in the maps are only from the fine particles or also from the host apatite. Detailed microprobe analyses of relatively large lamellae show a wide range of composition that includes variable amounts of Fe, S, Sr, Cu, Ni, Co, Al, Ba and Mg. The contents of P, Ca, F and Cl in the analysis of the small inclusions originate from the host apatite. Pan and Fleet (Reference Pan, Fleet, Kohn, Rakovan and Hughes2002) and Hughes and Rakovan (Reference Hughes and Rakovan2015) note that all these elements are common in apatite-supergroup minerals. According to the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification nomenclature (Pasero et al., Reference Pasero, Kampf, Ferraris, Pekov, Rakovan and White2010) the apatite supergroup contains five groups: apatite, belovite, britholite, ellestadite and hedyphanite. Most of the elements (Ca, Sr, Ba, Fe, Cu, Co, Ni and Mg) occupy the M1 and M2 sites of the apatite crystal structure, while P, S and Si are positioned in the tetrahedral (T) site. Fluorapatite, chlorapatite and hydroxylapatite (M1 = M2 = Ca, T = P) are most common in the apatite-group minerals and in addition to Ca and P, they may contain Ba, Pb, As, Mn, Sr and V. Other groups, e.g. the belovite and britholite groups may incorporate Ce, Sr, Nd, Y, Na, Ba or B and those of the ellestadite and hedyphanite groups can contain Si, S, Pb, Na, Bi, As and Ba.
Another observation from the apatite microtextures is the relationship between dusty domains with a high density of exsolutions and sector zoning. The dusty domains with high concentrations of Fe and S are only developed in some sectors. This is consistent with the observation of Rakovan (Reference Rakovan, Kohn, Rakovan and Hughes2002), suggesting that, in addition to a number of factors, the crystal growth of apatite is controlled by surface structural and chemical effects. A very close relationship was found between the concentration of REE, mainly La, Sm, Nd, etc. and sector zoning in apatite (Rakovan and Reeder, Reference Rakovan and Reeder1996). Therefore, the surface of an apatite crystal defines the interface with surroundings that may have very different characteristics at the contact with other phases. We assume that the sector zoning in apatite was developed, similar to that noted in other minerals (e.g. tourmaline, Van Hinsberg et al., Reference Van Hinsberg, Schumacher, Kearns, Mason and Franz2006), by preferential uptake of elements on the growth planes, resulting from a combined effect of differences in surface charge and morphology of these planes.
Origin and formation of the exsolution lamellae
According to Harlov et al. (Reference Harlov, Förster and Nijland2002) and Harlov (Reference Harlov2015), variation of trace-element concentrations in apatite could be mostly a result of metasomatic processes. They studied partially metasomatised chlorapatite from Ødegårdens Verk in Norway and experimentally investigated metasomatised apatite in an H2O- and F-rich system. On the basis of their BSE images, of natural apatite and experimental products (Harlov et al., Reference Harlov, Förster and Nijland2002), the apatite grains have clearly reacted along their rims or been pervasively penetrated by reaction fronts. The study found that the reacted areas with high F and OH contents were depleted in REE, which is explained to be a result of ‘chlorapatite’ replacement by OH- and fluorapatite. A metasomatic origin for the exsolution lamellae in apatite was also adopted by Broska et al. (Reference Broska, Krogh Ravna, Vojtko, Janák, Konečný, Pentrák, Bačík, Luptáková and Kullerud2014), who investigated pyrrhotite, anhydrite and dolomite oriented rods in apatite in silicate-bearing carbonate rocks associated with UHP eclogites in the Tromsø Nappe of the Scandinavian Caledonides in Norway. They considered the lamellae formed in a fluid-aided regime during amphibolite-facies metamorphism, subsequent to the UHP event.
In contrast to metasomatised apatite with replacement along the rims and penetrative zones by Harlov et al. (Reference Harlov, Förster and Nijland2002, Reference Harlov2015), we observed no relationship between the concentration of cations and fluorine or chlorine contents. In addition, concentration of the exsolution lamellae or dusty domains correlate well with the high trace-element concentration in the compositional maps (Fig. 6). We assume therefore that the elements present in the exsolution lamellae or identified in the compositional maps are the original impurities in the apatite crystal structure (Hughes and Rakovan, Reference Hughes and Rakovan2015). Both lamellae and fine particles were then formed by exsolution in a closed system and originated from the original impurities in the apatite crystal. As shown by Parsons and Brown (Reference Parsons, Brown and Ganguly1991), the process of unmixing of a single-phase solid solution into a two-phase assemblage is driven by free-energy minimisation and occurs by diffusion. Once two phases become established, the exsolution microtexture coarsens with time, also by diffusion. This seems to be the reason for the presence of fine particle exsolutions in the apatite crystals studied.
Formation of apatite and exsolutions in relation to the metamorphic P-T path of the host eclogite
Considering the eclogite and host garnet peridotite in Nové Dvory are fragments of subcontinental lithospheric mantle (Medaris et al., Reference Medaris, Beard and Jelínek2006; Ackerman et al., Reference Ackerman, Jelínek, Medaris, Ježek, Siebel and Strnad2009) and that they were recrystallized during subduction (Faryad et al., Reference Faryad, Dolejš and Machek2009; Reference Faryad, Jedlicka and Collett2013b), it is needed to clarify textural relations of apatite to other HP-UHP phases before the formation of apatite and exsolved inclusions is discussed. The occurrence of apatite within garnet and omphacite, even in central parts of the grains, indicates it is either a relic phase or crystallised during garnet and omphacite formation. In both cases, it could be formed by interaction of fluids generated in a subduction zone and infiltrated into the hot mantle wedge (Fig. 9). As shown by many experimental studies (Watson and Green, Reference Watson and Green1981; Fleet and Pan, Reference Fleet and Pan1997; Prowatke and Klemme, Reference Prowatke and Klemme2006), the incorporation of trace elements in apatite is, in addition to partition coefficients of the elements, controlled by their abundance in a melt or in fluid and by temperature changes. It is therefore probable that apatite, having such a high concentration of trace elements, formed at relatively high temperatures, when the mantle rocks were incorporated into subduction as it is assumed from the garnet zoning.
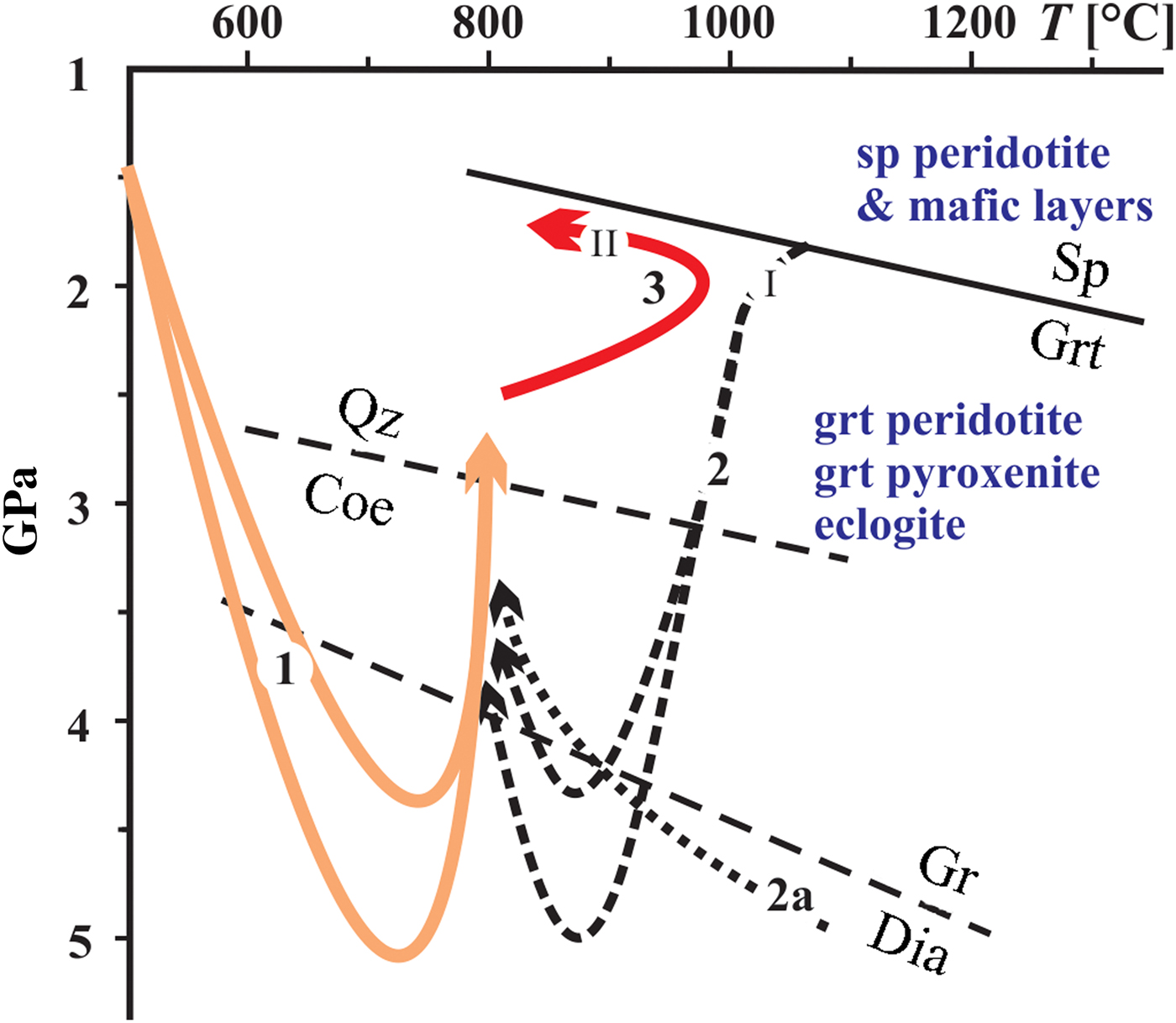
Fig. 9. Schematic diagram showing P-T paths of eclogite and host garnet peridotite and granulite (Faryad et al., Reference Faryad, Jedlicka, Hauzenberger and Racek2018). (1) Indicates subduction of felsic crustal rocks (now granulite) that took hot (or partially cooled fragments, due to the subduction geotherm) mantle peridotite with lenses and layers of mafic rocks which cooled down during pressure increase (2). Path (2a) shows involvement of mantle rock at maximum depths. After exhumation of crustal and mantle rocks to crustal levels, they underwent granulite-facies metamorphism (3) that was a result of slab break-off and mantle upwelling. (I) and (II) indicate possible crystallisation of apatite and formation of exsolution lamellae in apatite, respectively. Mineral abbreviations are: coe – coesite, dia – diamond, gr – graphite, grt – garnet, qz – quartz, sp – spinel.
The formation of exsolution lamellae in apatite could occur either during cooling or decompression of the host rocks. In most minerals, exsolution occur upon cooling below the temperature of mutual solubility or stability of the solution, but they could be also occur during decompression. The presence of pyrrhotite and other phases in apatite from many granitoids (e.g. Gottesmann and Wirth, Reference Gottesmann and Wirth1997; Krneta et al., Reference Krneta, Ciobanu, Cook, Ehrig and Kontonikas-Charos2016) is explained by cooling rather than by pressure decrease. The formation of sulfide exsolution lamellae in apatite from some UHP rocks are assumed to be a result of decompression (Chen et al., Reference Chen, Zeng, Chen and Liang2006; Zeng et al., Reference Zeng, Chen, Liang and Xu2006; Sun et al., Reference Sun, Tang, Sun, Xu, Zhai, Liang, Liang, Shen, Zhang, Zhou and Wang2007). The mechanism of exsolution is assumed to be similar to that in other minerals, e.g. quartz and K-feldspar lamellae in clinopyroxene or clinopyroxene exsolution in garnet, which are reported from UHP rocks (Zhang and Liou, Reference Zhang and Liou1999; Katayama et al., Reference Katayama, Patkinson, Okamoto, Nakajima and Maruyama2000; Dobrzhinetskaya et al., Reference Dobrzhinetskaya, Schweinehage, Massonne and Green2002; Zhang et al., Reference Zhang, Song, Liou, Ai and Li2005). Decompression-derived quartz or coesite lamellae are explained by the stabilisation of the Ca-Eskola component at high-pressure conditions (Gasparik, Reference Gasparik2003; Zhao et al., Reference Zhao, Nee, Green and Dobrzhinetskaya2011). However, the exsolution mechanism of sulfides and other phases in apatite during decompression has not yet been satisfactorily explained.
We do not exclude exsolution in apatite during decompression (exhumation), but for the case of their origin by cooling, two scenarios exist in regard to the above mentioned P–T path of the host rocks. The first is their formation in the subduction zone (I in Fig. 9), just after the apatite was formed by the interaction of subduction fluids with hot mantle. The second is that the exsolution occurred during rapid cooling from granulite-facies metamorphism (II in Fig. 9) in crustal levels. A short-lived granulite-facies metamorphism in the Moldanubian Zone was confirmed by the preservation of prograde zoning in felsic granulite (Jedlicka et al., Reference Jedlicka, Faryad and Hauzenberger2015) and by recrystallisation of retrograde phases formed in the cracks of eclogite-facies garnet into granulite-facies assemblages (Faryad and Fišera, Reference Faryad and Fišera2015). In the case of exsolution formation during cooling from the peak temperatures in granulite-facies conditions, the tracks after former cracks (Figs 3a, 5c,f and 6b,c) could also be healed during granulite-facies metamorphism.
Acknowledgements
This work was supported by the Czech Science Foundation (research project number 18-03160S), the Charles University (project GA UK no. 84217) and by institutional project Progres Q45. We thank H.-P Schertl and an anonymous reviewer for very helpful reviews. M. Arima is thanked for editorial handling.