Introduction
Babies who are thin or light at birth are much more likely to develop cardiovascular disease, hypertension, insulin resistance and type 2 diabetes as adults.Reference Barker, Osmond, Golding, Kuh and Wadsworth 1 – Reference Storgaard, Poulsen, Ling, Groop and Vaag 3 The early postnatal environment also independently influences the risk of adult diseases, with lower infant weight gain in the first year of life increasing the risk of adult cardiovascular diseaseReference Eriksson, Forsen, Tuomilehto, Osmond and Barker 4 , Reference Forsen, Eriksson, Osmond and Barker 5 and accelerated weight gain after the first year of life further increasing the risk of adult cardiovascular disease in those of low birth weight.Reference Eriksson, Forsen, Tuomilehto, Osmond and Barker 4 In humans, uteroplacental insufficiency is the major cause of foetal growth restriction in the western world.Reference Barker, Osmond, Golding, Kuh and Wadsworth 1 – Reference Storgaard, Poulsen, Ling, Groop and Vaag 3 Our group, and others, have found in adult rats that restriction of growth in prenatal life induced by uteroplacental insufficiency adversely impacts on later cardiovascular and metabolic health including increased rates of hypertension,Reference Wlodek, Mibus and Tan 6 – Reference Wlodek, Westcott, Siebel, Owens and Moritz 8 cardiac hypertrophy,Reference Wlodek, Westcott, Siebel, Owens and Moritz 8 myocardial insulin resistanceReference Tsirka, Gruetzmacher and Kelley 9 and glucose intoleranceReference Siebel, Mibus and De Blasio 10 as well as reducing skeletal muscle mitochondrial biogenesis.Reference Wadley, Siebel and Cooney 11
In the heart, the functions of the signaling kinases AMP-activated protein kinase (AMPK), the serine/threonine kinase Akt (otherwise known as protein kinase B), p38 mitogen activated protein kinase (p38 MAPK), extracellular signal regulated kinases (ERK1 and 2) and c-Jun N-terminal kinases (JNK1, 2 and 3) are yet to be fully defined, but include the regulation of cardiac hypertrophy, apoptosis, remodeling, gene transcription, protein synthesis and glucose uptake.Reference Dyck and Lopaschuk 12 – Reference Young, Li, Baron and Russell 20 Given the well-described effects of prenatal and postnatal growth restriction on cardiovascular health,Reference Wlodek, Mibus and Tan 6 – Reference Tsirka, Gruetzmacher and Kelley 9 many of the signaling kinases involved in the regulation of these processes are likely to be altered in the adult heart following growth restriction. Indeed, phosphorylation of Akt is upregulated in the adult rat heart in models of growth restriction that involve maternal and postnatal undernutritionReference Gavete, Agote, Martin, Alvarez and Escriva 21 and maternal dexamethasone exposure.Reference Langdown, Holness and Sugden 22 However, the impact of growth restriction on the phosphorylation of Akt in the adult rat heart using a model of uteroplacental insufficiency, which is more relevant to the western world, is not known. Surprisingly, no study of growth restriction has investigated the impact of growth restriction on the phosphorylation of AMPK, p38 MAPK and JNK in the adult heart.
Oxidative stress has been implicated in the pathogenesis of left ventricular hypertrophyReference Takimoto and Kass 23 , Reference Seddon, Looi and Shah 24 and is implicated in the cardiac remodeling of adult female rats exposed to the low maternal sodium exposure growth restriction model.Reference Battista, Calvo and Chorvatova 25 Furthermore, reactive oxygen species activate AMPK, p38 MAPK and Akt in the heartReference Horie, Ono and Nagao 26 and these kinases are implicated in the development of cardiac hypertrophy.Reference Young, Li, Baron and Russell 20 , Reference Takimoto and Kass 23 , Reference Dolinsky and Dyck 27 Therefore, we propose that the elevated oxidative stress and subsequent phosphorylation of AMPK, p38 MAPK and Akt may be a molecular mechanism to explain our previous findings of prenatal and postnatal growth restriction programming left ventricular hypertrophy in adult male rats.Reference Wlodek, Westcott, Siebel, Owens and Moritz 8 However, the level of oxidative stress in the hearts of adult rats that were exposed to growth restriction by uteroplacental insufficiency has not previously been reported. Thus, this study investigated the effect of uteroplacental insufficiency on the signaling pathways (phosphorylated AMPK, p38 MAPK, ERK, JNK and Akt) that are responsive to oxidative stress and are implicated in the development of cardiac hypertrophy in the left ventricle (LV) of adult offspring.
Since, we have previously shown that reductions in litter size independently programs postnatal growth restriction and hypertension in males,Reference Wlodek, Westcott, Siebel, Owens and Moritz 8 , Reference O’Dowd, Kent, Moseley and Wlodek 28 all comparisons were made between offspring of sham operated controls of normal litter size (Controls) and a separate group of postnatal growth restriction induced by reduced litter size, which underwent sham surgery (termed Reduced Litter), to litters exposed to uteroplacental insufficiency (termed Restricted), who have prenatal and postnatal growth restriction. Importantly, we also studied male and female offspring separately due to the marked sex differences in a range of metabolic and cardiovascular outcomes following uteroplacental insufficiency.Reference Wlodek, Mibus and Tan 6 , Reference Siebel, Mibus and De Blasio 10 , Reference Wadley, Siebel and Cooney 11
We have previously shown that improving postnatal nutrition and growth by cross-fostering growth-restricted pups onto a different sham operated mother with normal lactation prevents or ameliorates a number of adverse outcomes including the adult onset of hypertension, nephron deficitReference Wlodek, Mibus and Tan 6 and glucose intolerance.Reference Siebel, Mibus and De Blasio 10 , Reference Siebel, Gallo, Guan, Owens and Wlodek 29 Thus, it is possible that cross-fostering may also ameliorate any dysregulated kinase signaling in the adult rat heart following growth restriction. Therefore, another aim of this study was to examine, if any dysregulated kinase signaling in the LV of adult rats following prenatal or postnatal growth restriction could be ameliorated by cross-fostering.
We hypothesized, based on elevated Akt signaling in other models of growth restrictionReference Gavete, Agote, Martin, Alvarez and Escriva 21 , Reference Langdown, Holness and Sugden 22 and the aforementioned relationships between oxidative stress, signaling kinases and cardiac hypertrophy,Reference Young, Li, Baron and Russell 20 , Reference Takimoto and Kass 23 , Reference Seddon, Looi and Shah 24 , Reference Horie, Ono and Nagao 26 , Reference Dolinsky and Dyck 27 that prenatal and postnatal growth restriction would increase phosphorylation of AMPK, p38 MAPK, ERK, JNK and Akt and this would be associated with elevated levels of oxidative stress. Furthermore, based on our previous findings that cross-fostering can ameliorate adverse outcomes such as hypertension, nephron deficit and glucose intolerance,Reference Wlodek, Mibus and Tan 6 , Reference Siebel, Mibus and De Blasio 10 , Reference Siebel, Gallo, Guan, Owens and Wlodek 29 we hypothesized that cross-fostering would ameliorate any dysregulated kinase signaling in the LV of prenatal or postnatal growth-restricted rats.
Methods
Animals
All experiments were approved by The University of Melbourne Animal Experimentation Sub-Committee. Wistar Kyoto rats (9–13 weeks of age) were obtained from the Australian Resource Centre (Murdoch, Western Australia) prior to mating. On day 18 of gestation, pregnant rats underwent bilateral uterine vessel (artery and vein) ligation to induce growth restriction, as previously describedReference Wlodek, Mibus and Tan 6 , Reference O’Dowd, Kent, Moseley and Wlodek 28 , Reference Wlodek, Westcott and O’Dowd 30 or sham surgery, which was identical except the uterine vessels were not ligated.
Experiment 1: uteroplacental insufficiency and reduced litter size
The male rats in this experiment were the same animals that were used in our previous study describing the impact of growth restriction on hypertension, left ventricular hypertrophy and nephron development.Reference Wlodek, Westcott, Siebel, Owens and Moritz 8 Importantly, the female rats in this experiment were subject to the same conditions at the same time as the male rats. Refer to Figure 1 for a schematic representation of the experimental model. Briefly, at birth, half of the litters from the sham surgery group (litter size 10–14 pups) had their litter size randomly reduced to five pups (Reduced Litter) to match the litter size of approximately five pups born to uteroplacentally restricted mothers (Restricted).Reference Wlodek, Mibus and Tan 6 , Reference O’Dowd, Kent, Moseley and Wlodek 28 The remaining half of the litters from the sham surgery were left with a normal litter size of 10–14 pups (Control). All pups were handled at similar times and in the same manner and remained with their own mothers until weaning at 5 weeks of age. For all groups, 7–8 offspring of each sex were studied, each arising from different mothers. At 6 months of age, male and female rats were killed with an intraperitoneal injection of xylazine (30 mg/kg) and ketamine (225 mg/kg). The heart was quickly dissected and the weights of the whole heart and LV were recorded, frozen in liquid N2 and stored at −80°C.

Fig. 1 Schematic representation of the experimental models. On day 18 of gestation, pregnant rats underwent bilateral uterine vessel ligation (BUVL) to induce growth restriction or sham surgery (Sham). In experiment 1, at birth, half of the litters from the Sham group (10–14 pups/l) had their litter size randomly reduced to five pups (Reduced Litter) to match the litter size of approximately five pups born to uteroplacentally restricted mothers (Restricted). The remaining half of the litters from the Sham were left with a normal litter size of 10–14 pups (Control). In experiment 2, pups from each of the three groups described above (Control (Con), Reduced Litter (Red) and Restricted (Res)) were cross-fostered at day 1 onto a different control or restricted mother. This procedure produced six groups (pup-on-mother). For both experiments, the rats were killed at the age of 6 months.
Experiment 2: cross-fostering
The rats in this cross-fostering experiment were the same animals that were used in our previous studies describing the impact of growth restriction on hypertension and nephron development in adulthood.Reference Wlodek, Mibus and Tan 6 , Reference Siebel, Gallo, Guan, Owens and Wlodek 29 , Reference Moritz, Mazzuca and Siebel 31 Refer to Figure 1 for a schematic representation of this experimental model. Briefly, male and female pups from each of the three groups described above (Control, Reduced Litter and Restricted) were cross-fostered at day 1 onto a different control or restricted mother as previously described.Reference Wlodek, Mibus and Tan 6 , Reference Siebel, Mibus and De Blasio 10 This procedure produced six groups (pup-on-mother): Control-on-Control, Control-on-Restricted, Reduced-on-Restricted, Reduced-on-Control, Restricted-on-Control, Restricted-on-Restricted with a similar number of male and female pups in each litter. At 6 months of age, rats were killed and the LV was excised, weighed, frozen in liquid N2 and stored at −80°C.
Immunoblotting
For immunoblotting, frozen LV was homogenized and analyzed as previously described.Reference Wadley, Siebel and Cooney 11 Antibodies for phospho-p38 Thr180Tyr182 MAPK (pThr180Tyr182 p38 MAPK), AMPKα PAN (α1 and α2 subunits), phospho-Akt Ser473 (pSer473 Akt), phospho-Thr202Tyr204 ERK1/2 (pThr202Tyr204 ERK1/2) and phospho Thr183Tyr185 JNK (pThr183Tyr185 JNK) were from Cell Signaling Technology (Hartsfordshire, England). Binding was detected with IRDye™ 800-conjugated anti-rabbit IgG (Rockland, Gilbertsville, PA, USA) or IRDye™ 680-conjugated anti-mouse IgG (Molecular Probes, Eugene, OR) fluorescent antibodies via infrared detection (Odyssey Imaging system, LI-COR Biosciences, Lincoln, NE). Membranes were then stripped (2% SDS (w/v) in 25 mM glycine, pH 2.0) and re-probed with antibodies for phospho-AMPKα Thr172 (pThr172 AMPKα from Upstate Biotechnology (NY, USA) and Akt, p38 MAPK, ERK1/2 and JNK from cell signaling. For this study, all phosphorylation is expressed relative to protein abundance.
Validation of tissue harvesting for measurement of kinase phosphorylation
Since the LVs in our study were harvested for weighing prior to being frozen, it is possible that the time taken (∼2 min) to freeze the cardiac tissue may have resulted in a hypoxic/ischemic stress. Indeed, myocardial AMPK is activated by ischemia and anoxia.Reference Beauloye, Marsin and Bertrand 32 , Reference Altarejos, Taniguchi, Clanachan and Lopaschuk 33 Therefore, as an important control, we subsequently performed a validation experiment to examine the phosphorylation of these kinases and the metabolite levels at a range of time points following dissection. Male rats from sham surgery groups at 5 weeks of age were anaesthetized with an intraperitoneal injection of xylazine (30 mg/kg) and ketamine (225 mg/kg). Once pedal reflexes were lost, the chest cavity was quickly opened and while the heart was still beating, it was freeze-clamped while still in the chest or dissected, blotted free of blood and left at room temperature before being freeze-clamped immediately (i.e. 6 ± 1 s of dissection) and 60, 180 and 300 s following dissection (n = 6 rats per time point). Hearts were crushed into a powder under liquid N2 and stored at −80°C for later measurement of metabolites and kinase phosphorylation as previously described.Reference Wadley, Lee-Young and Canny 34 We examined whole-hearts (rather than LV), since this was the quickest method to extract and freeze cardiac muscle.
Five minutes delay in freezing did not significantly increase cardiac pThr172 AMPKα, pSer473 Akt, pThr183Tyr185 JNK, the ratio of free AMP/ATP or levels of creatine, nor did it significantly decrease cardiac levels of ATP, creatine phosphate or glycogen (data not shown). The cardiac ATP levels and ratio of free AMP/ATP for all timepoints were (mean ± s.e.m.) 13.2 ± 0.6 mmol/kg dry weight and 0.67 ± 0.12, respectively, and are consistent with normal non-ischemic myocardial levels that have been previously reported.Reference Altarejos, Taniguchi, Clanachan and Lopaschuk 33 , Reference Tian, Musi, D’Agostino, Hirshman and Goodyear 35 , Reference Xing, Musi and Fujii 36 Thus, the ∼2 min of delayed freezing in this study does not induce a significant ischemic or hypoxic stress on cardiac tissue. Similar findings of no change in high-energy phosphates in pig cardiac muscle with delays in freezing for up to 5 min have also been reported.Reference Botker, Helligso, Kimose, Thomassen and Nielsen 37 However, in this study, a delay in freezing time of 60 to 300 s significantly increased cardiac pThr180Tyr182 p38 MAPK, pThr202Tyr204 ERK1/2 and lactate levels (P < 0.05, data not shown).
The relatively stable levels of high-energy phosphates and the lack of increase in pThr172 AMPKα during 5 min of delayed freezing is likely due to the dramatically reduced energy demand of the myocardium following dissection in our validation study when compared to the energy demands of isolated perfused and contracting hearts subjected to ischemia and anoxia.Reference Beauloye, Marsin and Bertrand 32 , Reference Altarejos, Taniguchi, Clanachan and Lopaschuk 33 , Reference Xing, Musi and Fujii 36 Therefore, any differences in pThr172 AMPKα, pSer473 Akt and pThr183Tyr185 JNK that we observe between growth-restricted groups cannot, therefore, be an artifact of harvesting methodology.
Oxidative stress
The ratio of oxidized to total glutathione (GSSG/TGSH) is a commonly used marker of intracellular oxidative stressReference Sandstrom, Zhang and Bruton 38 , Reference Sandstrom, Zhang, Westerblad and Katz 39 and was measured using a commercially available kit (Biooxytech GSH/GSSG-412, Oxis Health Products, Portland, USA) as described by Sandstrom et al.Reference Sandstrom, Zhang and Bruton 38
Statistical analyses
Results were analyzed using one-way ANOVA, with Newman–Keuls post hoc analysis, where appropriate. All data are presented as mean ± s.e.m. The level of significance was set at P < 0.05.
Results
Experiment 1: effect of uteroplacental insufficiency and postnatal growth restriction (Reduced Litter) on heart mass and LV kinase phosphorylation and oxidative stress
Briefly, the adult female Restricted and Reduced Litter and adult male Restricted litter offspring had reduced body weight compared with Controls (Table 1) as previously described.Reference Wadley, Siebel and Cooney 11 Furthermore, we have previously shown that these male Restricted and Reduced Litter offspring have left ventricular hypertrophy and hypertension,Reference Wlodek, Westcott, Siebel, Owens and Moritz 8 while for adult females blood pressure is not altered.Reference Wlodek, Westcott, Siebel, Owens and Moritz 8
Table 1 Experiment 1: the effect of uteroplacental insufficiency (Restricted) and reducing litter size (Reduced Litter) on body and heart weight in adult offspring

LV = left ventricle.
Values are mean ± s.e.m.; n = 10 per group for Control and Reduced Litter and n = 8 for Restricted.
aBody weights of both sexes have been previously reported.Reference Wadley, Siebel and Cooney 11
bHeart weights of the adult male offspring have been previously reported.Reference Wlodek, Westcott, Siebel, Owens and Moritz 8
*P < 0.05 v. Control; **P < 0.05 v. Reduced Litter.
For adult female rats, this study found LV hypertrophy was not evident, since there was no significant difference in heart weight or LV weight between Control, Reduced Litter or Restricted rats, respectively (Table 1).
In females
Restricted adult offspring had increased pThr172 AMPKα (P < 0.05; Fig. 2) compared to Controls. In addition, AMPKα protein abundance was significantly lower in the Restricted and Reduced Litter offspring compared to Controls (P < 0.05, 0.99 ± 0.08 and 0.94 ± 0.06 compared to 1.17 ± 0.05 integrated intensity, respectively). However, the altered AMPKα protein abundance had no influence on the higher pThr172 AMPKα in Restricted adult offspring, as the effect was observed with or without normalization to AMPKα protein (data not shown). Both Restricted and Reduced Litter adult offspring had increased pThr180Tyr182 p38 MAPK and pSer473 Akt (P < 0.05; Fig. 2). Protein abundance of p38 MAPK and Akt was not altered between groups in the LV. There was no significant difference between the female offspring in LV pThr202Tyr204 ERK1/2 and pThr183Tyr185 JNK (data not shown). However, ERK 1/2 protein abundance was significantly lower in the Reduced Litter compared to Controls and Restricted offspring (P < 0.05, 23.2 ± 0.6 compared to 28.4 ± 0.7 and 25.5 ± 0.9 integrated intensity, respectively). Protein abundance of JNK was not altered between groups in the LV (data not shown). LV levels of oxidative stress were unaltered (Table 2).

Fig. 2 Experiment 1: The effect of uteroplacental insufficiency (Restricted) and reducing litter size (Reduced Litter) on LV phosphorylation of AMPKα Thr172 (pThr172 AMPKα), p38 MAPK Thr180Tyr182 (pThr180Tyr182 p38 MAPK) and Akt Ser473 (pSer473 Akt) in adult female (left) and male (right) offspring. Western blots are representative from one rat from each treatment group. Values are mean ± s.e.m.; n = 8 for all groups, except n = 7 for male and female Restricted groups. *P < 0.05 v. Control; LV = left ventricle.
Table 2 Experiment 1: the effect of uteroplacental insufficiency (Restricted) and reducing litter size (Reduced Litter) on left ventricle oxidative stress in adult female and male offspring
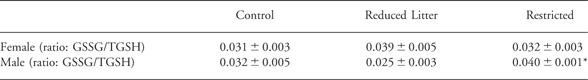
Values are mean ± s.e.m.; n = 5 per group for females and n = 7 per group for males.
*P < 0.05 v. Reduced Litter (one-way ANOVA).
In males
Reduced Litter offspring had increased pThr172 AMPKα, pThr180Tyr182 p38 MAPK and pSer473 Akt compared to Control and Restricted (P < 0.05; Fig. 2). Protein abundance of AMPKα, p38 MAPK and Akt were not altered between groups. There was no significant difference between the male offspring in pThr202Tyr204 ERK1/2 and pThr183Tyr185 JNK or ERK 1/2 and JNK protein abundance (data not shown). In addition, Restricted offspring had significantly increased oxidative stress in the LV compared to Reduced Litter offspring (P < 0.05; Table 2).
Experiment 2: the effect of cross-fostering and prenatal and postnatal growth restriction on LV kinase phosphorylation and oxidative stress
In females
All cross-fostered groups had significantly increased (approximately twofold) pThr172 AMPKα and pSer473 Akt compared to Control (non-cross-fostered) offspring (P < 0.05, Fig. 3). In addition, the pThr180Tyr182 p38 MAPK was significantly higher in the Con-on-Res and Res-on-Con offspring compared to the Controls (P < 0.05; Fig. 3). The pThr172 AMPKα, pThr180Tyr182 p38 MAPK and pSer473 Akt were not significantly different across these six cross-fostered groups even when a separate ANOVA was performed on these groups. In addition, protein abundance of AMPKα, p38 MAPK and Akt were not significantly different between any of the female groups (data not shown).

Fig. 3 Experiment 2: The effect of uteroplacental insufficiency (Restricted: Res) and reducing litter size (Reduced Litter: Red) and cross-fostering on LV phosphorylation of AMPKα Thr172 (pThr172 AMPKα), p38 MAPK Thr180Tyr182 (pThr180Tyr182 p38 MAPK) and Akt Ser473 (pSer473 Akt) in adult female (left) and male (right) offspring. Western blots are representative from one rat from each group. Values are mean ± s.e.m.; n = 10 for all groups except n = 8 for Control group (CON). *P < 0.05 v. CON, +P < 0.05 v. Red-on-Res. For pThr172 AMPKα in males, P = 0.12 (one-way ANOVA); LV = left ventricle.
In males
All cross-fostered groups had significantly increased pSer473 Akt compared to Control offspring (P < 0.05; Fig. 3). Red-on-Con offspring had significantly higher pSer473 Akt compared to Red-on-Res (P < 0.05; Fig. 3). In addition, the pThr180Tyr182 p38 MAPK was significantly higher in the Con-on-Con and Con-on-Res offspring compared to the Controls (P < 0.05; Fig. 3). Furthermore, p38 MAPK and Akt protein abundance was significantly lower in the Control offspring compared to all six cross-fostered groups (P < 0.05, data not shown). Protein abundance of AMPKα was not significantly different between any of the male groups (data not shown).
Discussion
A major finding of this study was that early life perturbations of both uteroplacental insufficiency and restriction of postnatal growth by reductions in litter size, increased kinase signaling in the LV of adult female rats, while only reducing litter size and therefore postnatal growth restriction induced similar increases in male offspring. Another major finding was that compared to non-cross-fostered animals, the cross-fostering intervention exerts its own effects on the programming of kinase phosphorylation levels in the adult rat heart.
Oxidative stress is known to be an activator of AMPK, p38 MAPK and Akt in the heartReference Horie, Ono and Nagao 26 and is implicated in the pathogenesis of cardiovascular hypertrophy and remodeling.Reference Takimoto and Kass 23 , Reference Seddon, Looi and Shah 24 However, it is unlikely oxidative stress is responsible for the programming of LV hypertrophy we have previously observed in these male Restricted and Reduced Litter adult offspring,Reference Wlodek, Westcott, Siebel, Owens and Moritz 8 since only the Restricted offspring displayed increased oxidative stress (Table 2). Furthermore, although this study found uteroplacental insufficiency programs oxidative stress in the LV of Restricted male offspring, this does not account for the altered kinase signaling we observed in the males, since the increased pThr172 AMPKα, pThr180Tyr182 p38 MAPK and pSer473 Akt were only observed in the Reduced Litter and not the Restricted males. Since oxidative stress increases with ageing in the heart,Reference Alvarez, Caldiz and Fantinelli 40 it is possible that the oxidative stress observed in the male Restricted offspring at 6 months of age could have a much greater impact on cardiovascular health in much older rodents and further studies are required to examine this. Nevertheless, it would appear that the mechanism for the LV hypertrophy we have previously observed in these male Restricted and Reduced Litter ratsReference Wlodek, Westcott, Siebel, Owens and Moritz 8 is probably occurring independently of oxidative stress.
The functions of AMPK, p38 MAPK and Akt signaling in the heart are yet to be fully defined, but include the regulation of gene transcription, protein synthesis, apoptosis, cardiomyocyte hypertrophy and glucose uptake.Reference Dyck and Lopaschuk 12 – Reference Baines and Molkentin 17 However, the collective pattern of activation/phosphorylation of AMPK, p38 MAPK and Akt in the heart often differs depending on which of these pathways one is considering. For example, elevated cardiomyocyte hypertrophy in the adult heart is largely consistent with increased phosphorylation of Akt,Reference Clerk and Sugden 15 but reduced phosphorylation of AMPKReference Dyck and Lopaschuk 12 and p38 MAPK.Reference Liang and Molkentin 16 Anti-apoptotic signaling in the cardiomyocyte is also largely consistent with increased phosphorylation of AMPKαReference Shiojima and Walsh 14 and Akt,Reference Shiojima and Walsh 14 but decreased p38 MAPK phosphorylation.Reference Baines and Molkentin 17 However, the consequences of increased phosphorylation of AMPK, p38 MAPK and Akt in the heart all include increased glucose uptake.Reference Dyck and Lopaschuk 12 – Reference Shiojima and Walsh 14 Therefore, the largely consistent pattern of increased pThr172 AMPKα, pThr180Tyr182 p38 MAPK and pSer473 Akt in the LV of male and female Reduced Litter and female Restricted rats in this study tends to suggest a role for increased basal (non-insulin-stimulated) glucose uptake in the myocardium of these rats. Further work is now required to establish whether the elevated myocardial kinase signaling following prenatal and postnatal growth restriction in this study is also consistent with elevated basal heart glucose uptake.
AMPK has been implicated in altering substrate utilization during pressure overload hypertrophy.Reference Tian, Musi, D’Agostino, Hirshman and Goodyear 35 However, increased pThr172 AMPKα (Fig. 2) was found in the adult male offspring of Reduced Litters only, whereas we have previously reported that both these adult male Reduced Litter and Restricted offspring have hypertension and left ventricular hypertrophy.Reference Wlodek, Westcott, Siebel, Owens and Moritz 8 We also found increased pThr172 AMPKα (Fig. 2) in the Restricted adult female offspring despite unaltered left ventricular mass and blood pressure.Reference Wlodek, Westcott, Siebel, Owens and Moritz 8
This would suggest that the altered LV AMPK signaling we observed in Reduced Litter males and Restricted females is occurring independently from the hypertension and left ventricular hypertrophy previously observed in both these male growth-restricted groups.Reference Wlodek, Westcott, Siebel, Owens and Moritz 8
An important finding of this study was that reduced postnatal growth as a consequence of reduced litter size increases LV kinase signaling in adult female offspring independently of uteroplacental insufficiency. Similarly, we have previously shown reduced postnatal growth via reductions in litter size exerts its own influence on the metabolic characteristics of adult skeletal muscle.Reference Wadley, Siebel and Cooney 11 The mechanism(s) whereby an adverse postnatal environment can program later kinase signaling in heart and metabolic function in skeletal muscle are unclear, but are likely to include altered postnatal nutrition, due to the reduced quantity and altered composition of maternal milk.Reference O’Dowd, Kent, Moseley and Wlodek 28 Moderate reductions of litter size is also commonly used in many studies to control for the reduced number of pups following intrauterine growth restriction.Reference Vuguin, Raab, Liu, Barzilai and Simmons 41 – Reference Thamotharan, Shin and Suddirikku 44 It is important to note that if we had used the Reduced Litter group as the only control in this study, we would have reported impaired pThr172 AMPKα, pThr180Tyr182 p38 MAPK and pSer473 Akt in the male Restricted group (v. Reduced Litter; Fig. 2), rather than normal phosphorylation of these variables. Importantly, pups from Control, Restricted and Reduced Litter groups were all handled identically after birth, so our findings cannot be due to the stress on the mothers or pups caused by differences in handling. Thus, our present finding in the LV and previous findings in skeletal muscleReference Wadley, Siebel and Cooney 11 highlight the importance of including additional controls with unaltered litter size when investigating the impact of growth restriction in cardiac and skeletal muscle.
A surprising finding was that prenatal and postnatal growth restriction impacted the LV kinase signaling of adult female rats more than male rats, as evidenced by elevated pSer473 Akt and pThr180Tyr182 p38 MAPK in both Restricted and Reduced litter groups while males only displayed these elevated levels in the Reduced Litter group. Consistent with these findings, estrogen is known to increase pSer473 Akt in the heart.Reference Camper-Kirby, Welch and Walker 45 Furthermore, estrogen protects against the development of adult hypertension in the female growth-restricted rat.Reference Ojeda, Grigore, Robertson and Alexander 46 Thus, it is possible that these protective effects of estrogen in the female growth-restricted heart may have secondary consequences, such as the altered kinase signaling we have observed in the LV. Further experiments are now required to examine the possible role of estrogen on our gender-specific findings.
Cross-fostering is commonly used in growth restriction studies to control or manipulate postnatal nutrition.Reference Wlodek, Mibus and Tan 6 , Reference Siebel, Mibus and De Blasio 10 , Reference Simmons, Suponitsky-Kroyter and Selak 42 , Reference Thamotharan, Shin and Suddirikku 44 , Reference Simmons, Templeton and Gertz 47 It was concerning, therefore, that we found cross-fostering alone exerts effects on the programming of the rat heart, with pSer473 Akt, pThr172 AMPKα and pThr180Tyr182 p38 MAPK either significantly higher or tending to be higher in the LV of all cross-fostered groups of both male and female rats compared to Control non-cross-fostered animals (Fig. 3). Importantly, the effects of cross-fostering on LV kinase phosphorylation were minimal within the cross-fostered groups when compared to the Control (non-cross-fostered) offspring. These findings highlight the importance of adequate control groups for the interpretation of results and demonstrate the vulnerability of the early postnatal heart to programming from stresses related to cross-fostering alone (i.e. a control pup cross-fostered onto a different control mother). Indeed, maternal care such as the levels of licking and grooming provided by the mother is known to program altered gene expression in the brain of adult rat offspring,Reference Liu, Diorio and Tannenbaum 48 including genes in the MAPK pathway,Reference Diorio and Meaney 49 although its effects on the heart remain to be elucidated.
In conclusion, increased pThr172 AMPKα, pThr180Tyr182 p38 MAPK and pSer473 Akt can be programmed by uteroplacental insufficiency in a gender-specific manner in the adult rat LV. Surprisingly, the LV of adult rats is sensitive to postnatal growth restriction following reductions in litter size and also the postnatal intervention of cross-fostering alone. However, these results together with our previous studies suggest that the activation of these kinase signaling pathways by growth restriction occurs independently of high blood pressure,Reference Wlodek, Westcott, Siebel, Owens and Moritz 8 ventricular hypertrophyReference Wlodek, Westcott, Siebel, Owens and Moritz 8 or oxidative stress.
Acknowledgments
The authors would like to thank Kerryn Westcott and Dr Andrew Siebel for their technical assistance. All authors have contributed substantially to the scientific process leading up to the writing of the paper. GW, MW and GM contributed to the conception and design of the project. All authors contributed to the performance of experiments or the analysis and interpretation of data. All authors made a contribution to drafting of the manuscript. We the authors declare that we are entirely responsible for the scientific content of the paper. This work was supported by a grant (no. G06M 2597) from the Heart Foundation of Australia.
Statement of Interest
None.







