Introduction
The semiarid interior of north-eastern Brazil represents the largest and most biologically diverse block of seasonally dry tropical forest in the Neotropics (Pennington et al. Reference Pennington, Lavin and Oliveira-Filho2009) and is a known area of avian endemism (Cracraft Reference Cracraft1985, Haffer Reference Haffer1985). Part of this diversity is likely due to the caatinga’s vegetation heterogeneity, which represents a mosaic of different habitats, associated with varying degrees of topographic and edaphoclimatic conditions (Vasconcelos et al. Reference Vasconcelos, de Araújo and Lopes2010, Queiroz et al. Reference Queiroz, Cardoso, Fernandes and Moro2017). Within the caatinga, there are several different phytophysiognomies, ranging from open and shrubby woodlands to ecologically more complex arboreal dry forests, and enclaves of wet forests (Giulietti et al. Reference Giulietti, Bocage Neta, Castro, Gamarra-Rojas, Sampaio, Virgínio, Queiroz, Figueiredo, Rodal, Barbosa. and Harley2004, Queiroz et al. Reference Queiroz, Cardoso, Fernandes and Moro2017).
While many of the caatinga avian endemics are restricted to the typical open shrubby woodlands found on the lowlands (<500 m above sea level), other endemics and dry forest specialists seem to favour denser forests, often present in higher plateaus (>600 m asl) and table-top mountains (known in Brazil as chapadas). On top of some of these plateaus, vegetation differs from that of the surrounding lowlands, including rocky outcrops (campos rupestres) and carrasco vegetation (Araújo et al. Reference Araújo, Sampaio, Figueiredo, Rodal and Fernandes1998, Alves and Kolbek Reference Alves and Kolbek2010).
The carrasco, also known as sedimentary forest, is a dense shrubby vegetation type, with a xerophilous flora that grows on top of quartz sandy soils, generally between 700 and 900 m above sea level (Araújo et al. Reference Araújo, Martins and Shepherd1999). Carrascos are mostly found on the Ibiapaba and Araripe plateaus in the Brazilian semiarid region, although isolated patches are also present as far south as northern Minas Gerais (Araújo et al. Reference Araújo, Martins and Shepherd1999, Vasconcelos et al. Reference Vasconcelos, de Araújo and Lopes2010) (Figure 1). Despite being in the heart of the caatinga realm, this vegetation type has biogeographical and ecological affinities with both the cerrado of central Brazil and the caatinga dry forests (Figueiredo Reference Figueiredo1986). These biogeographic affinities, however, remain poorly understood, particularly for animals (Araújo et al. Reference Araújo, Martins and Shepherd1999, Vasconcelos et al. Reference Vasconcelos, de Araújo and Lopes2010).

Figure 1. Map of South America, highlighting the caatinga Dry Forest (light green), the cerrado (dark green), and the distribution of the carrasco vegetation (in yellow); adapted from Araújo (Reference Araújo, Sampaio, Figueiredo, Rodal and Fernandes1998). In detail, the Fazenda Pau D’Arco, with the 162 sampling points where we conducted point counts (all dots) and the 115 sampling points where we conducted mobbing experiments (black dots). The white line represents the main dirt road that gives access to the entire area, numbered lines (1 to 12) represent secondary roads that give access to the management plots. Red plots represent areas that had already been managed by the time of the beginning of our study (2014), whereas green plots represent unmanaged areas. RL1 and RL2 represent unmanaged control areas (Reserve Legal).
Worryingly, these thick patches of forest are under threat; anthropogenic disturbance includes extraction of wood for fuel, habitat conversion for crops, and grazing by goats (Chaves and Barros Reference Chaves and Barros2008). Some carrascos, such as those located on top of the Araripe Plateau are deforested due to local economic activities, such as gypsum mining and firewood gathering to feed industrial ovens. These kinds of anthropogenic disturbances are common in tropical dry forests and often lead to severe soil impoverishment and degradation (Shahabuddin and Kumar Reference Shahabuddin and Kumar2006, Venter et al. Reference Venter, Sanderson, Magrach, Allan, Beher, Jones, Possingham, Laurance, Wood, Fekete, Levy and Watson2016). Such disturbances can cause local extinctions, changes in species composition, and alterations in important ecological processes and ecosystem services, such as pollination and seed dispersion, which in turn, may compromise the sustainability of native forests (Wiens Reference Wiens1994, Castelletti et al. Reference Castelletti, Silva, Tabarelli and Santos2003, Sekercioglu Reference Sekercioglu2006).
The dense woodlands of the carrascos are also feeding the wood market as part of legal management logging programmes, allowed under Brazilian law. One such area is located at the Fazenda Pau D´Arco, a privately owned 2,125 ha property located on top of the Araripe Plateau, in the Brazilian state of Ceará. This area has been managed since 2001, with nearly 5% of the property logged every year and left to regrow for a new cutting cycle of 25 years. In 2013, we initiated avian surveys to evaluate the effects of this legal forest management programme on the avifauna. According to the management programme provided by the owners, by the end of our fieldwork, a total of 1,150 ha had already been logged, and another 520 ha are planned to be logged by 2026, when a new cutting cycle will be initiated. Due to the management programme, a network of dirt roads has been created in the area, resulting in ~40 km of roads, which offer access to an otherwise almost impenetrably dense thorny forest. From the original forest cover, nearly half of the area remains in a natural condition, offering a unique opportunity to sample its avifauna. Despite the longstanding interest in the effects of deforestation and forest fragmentation on tropical rainforest avian communities (Stouffer Reference Stouffer2020), little attention has been given to evaluate the effects of anthropogenic changes in tropical dry forests (Shahabuddin and Kumar Reference Shahabuddin and Kumar2006).
In this article, our main goals are two-fold. First, we aim to describe in detail the avifauna of a well-preserved patch of carrasco dry forest, allowing us to better understand the ecological and biogeographical affinities of its avifauna. Second, we provide avian baseline data that will be useful to understand the effects of management on a forest that is currently being managed and will be almost completely converted into a secondary forest by 2026. To assess the biogeographical affinities of its avifauna, we provide a regional comparison of the carrasco avifauna, in relation to several areas of caatinga and cerrado along the South American Dry Diagonal. The avian baseline data provided is the result of a variety of sampling methods, resulting in what we believe is a fairly complete and well-documented inventory of its avifauna. We present abundance estimates based on quantitative surveys and use an indicator species analysis to evaluate associations between species and forest management. Finally, we provide details on the presence of 16 bird species that are either dry forest specialists, caatinga endemics, and/or threatened taxa. For these species, we include detailed maps of their presence in the study area. This represents the first avian inventory of an area exclusively dominated by carrasco vegetation that is also subject to a long-term forest management program and aims to provide the necessary grounds to establish a long-term species monitoring programme.
Methods
Study area
This study was conducted at the Fazenda Pau D´Arco (7°18’S, 39°33’W), located on top of the Araripe Plateau, in the municipality of Crato, in the Brazilian state of Ceará (Figure 1). The entire area of the Fazenda is covered by carrasco vegetation. Its north-eastern boundary is the Araripe-Apodi National Forest (FLONA Araripe-Apodi), which has a taller forest with a more open understorey locally known as cerradão and harbours a quite different set of bird species. The Fazenda Pau D´Arco covers 2,125 ha, 1,670 of which are part of a management programme aiming to provide charcoal and feed local industrial ovens. According to the management programme provided by the owners, nearly 425 ha were set aside as protected areas (Reserva Legal), which should go untouched under the management scheme proposed. Until 2017, when we conducted our last fieldwork in the area, a total of 1,240 ha had already been managed, and 90% of the area (excluding the Reserva Legal) will be logged by 2026. The region has a semiarid and hot tropical climate (mean annual temperature between 24 and 26ᵒ C), with a mean annual rainfall of ~1,090 mm, concentrated between January and May, with a peak in March (IPECE 2012).
Fieldwork and sampling of the avifauna
We conducted avian surveys at the Fazenda Pau D´Arco between 2013 and 2017 using multiple complementary methods, including: i) systematic surveys (point counts); ii) mobbing experiments; iii) mist-netting, and iv) opportunistic observations (Appendix 1). While opportunistic observations were conducted mostly along the main road and surroundings, point counts and mobbing experiments were conducted along secondary roads, covering the entire area (Figure 1).
We first visited the area in August 2013, when we made opportunistic observations and deployed mist-nets during field courses of the Federal University of Pernambuco (UFPE). In 2014, we initiated quantitative surveys, when we conducted a total of 295 5-min point counts at 162 points, systematically distributed every 250 m along the 12 parallel roads that cross the entire extension of the study area (black and white dots in Figure 1). Point counts started before sunrise (~05h00) and lasted till mid-morning (~ 09h00). A subset of these sampling sites (115 white dots in Figure 1) was surveyed through mobbing experiments. For the mobbing experiments, we excluded sites at the edges of each transect to avoid confounding variables, such as proximity to the main road. Mobbing experiments consisted of broadcasting the call of the Ferruginous Pygmy-Owl Glaucidium brasilianum for 5 mins to disclose the presence of birds in the thick vegetation (see Lima et al. Reference Lima, Las-Casas, Ribeiro, Gonçalves-Souza and Naka2018 for further details on this methodology). Each point was subject to these experiments twice (early morning and late afternoon) on different days.
Whenever possible, we documented our observations with photographs and audio recordings, and also collected a limited number of bird specimens to provide a reference collection of the study area. All specimens, collected under SISBIO license no. 36496, were captured using mist-nets, and are currently held at the UFPE Bird Collection (Appendix S1 in the online supplementary material). Voucher samples are now part of the permanent collection that aims to characterize and preserve morphological and genetic diversity in the Brazilian north-east, following the general guidelines extensively discussed by Collar (Reference Collar2000). Given that this area is often visited by birdwatchers, we also included documented material obtained by other people and available in public databases, such as Wikiaves (www.wikiaves.com.br) and eBird (https://ebird.org/), for audio recordings and photographs. Taxonomy and nomenclature follow the Brazilian Committee of Ornithological Records (Piacentini et al. Reference Piacentini, Aleixo, Agne, Maurício, Pacheco, Bravo, Brito, Naka, Olmos, Posso and Silveira2015), and its forthcoming update (CBRO in prep). Endemism was classified based on Pacheco (Reference Pacheco, Silva, Tabarelli, Fonseca and Lins2004) and Billerman et al. (Reference Billerman, Keeney, Rodewald and Schulenberg2020), and threatened status according to Brazilian (MMA 2018, Machado et al. Reference Machado, de Araújo, Alves and Mendes2018) and global (IUCN 2020) Red Lists. The specific effects of forest management on species density and abundance and on avian species composition are being presented elsewhere (Ribeiro et al. Reference Ribeiro, Las-Casas, Silva and Nakain press a and Reference Ribeiro, Las-Casas, Lima, Silva and Nakab).
Carrasco avifauna affinities
In order to evaluate the biogeographical affinities of the avifauna of the Fazenda Pau D’Arco, in comparison with other dry forests in the Brazilian northeast and cerrado sites from central Brazil, we compiled bird species lists from 18 protected areas along the South American Dry Diagonal, including 11 caatinga and seven cerrado sites (Figure 2). We searched for published articles and complemented these from bird lists available in online databases, such as Wikiaves (www.wikiaves.com.br) and Ebird (ebird.org) (Table S1). For lists published in online databases, we double-checked the consistency of each record, ensuring its plausibility and preferably choosing lists that accurately discriminated its exact location. With this information, we generated a presence/absence matrix of species occurrence, which included a total of 677 bird species present in the carrasco, caatinga, and cerrado locations (Figure 2, Table S1). We then compared the bird species recorded at the Fazenda Pau D’Arco to evaluate biogeographical avian affinities. We used Jaccard’s index (Sneath Reference Sneath1957) to create a similarity matrix to compare sites. This index ranges from 0 to 1; values close to 0 indicate high composition similarity and values close to 1 indicate different communities. We then performed a hierarchical cluster analysis and a non-metric multidimensional scaling (NMDS) (Gower Reference Gower1966) to visualize similarities. We also applied a permutational analysis of variance (PERMANOVA) (Anderson Reference Anderson2001) to verify the robustness of the groups formed. Although there are obvious sampling differences among sites, we believe that if biogeographical affinities are strong enough, they should be less influenced by sampling methods.
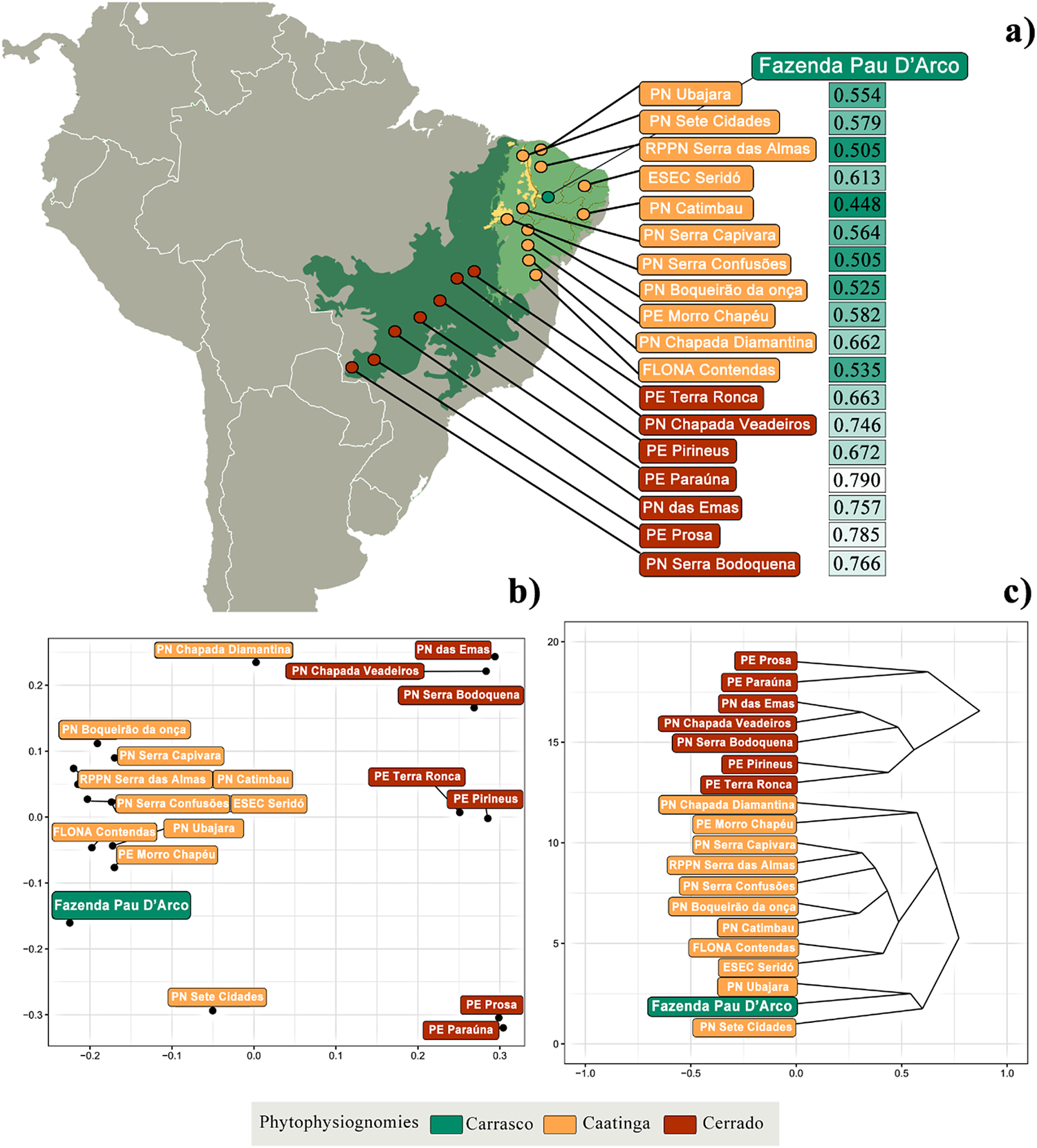
Figure 2. (a) Map of geographic localities selected to compare species similarity between and within biomes, (b) Multidimensional scaling, and (c) Cluster analysis showing the avian similarity between the Fazenda Pau D’Arco and 11 caatinga and seven cerrado localities. We indicate Jaccard’s index values next to the localities’ names (a). Dark green values represent more similar and light green values more dissimilar areas, in relation to carrasco avifauna.
To detect a potential effect of isolation by distance, we calculated the correlations between geographic distance and bird species dissimilarity using Mantel tests (Smouse et al. Reference Smouse, Long and Sokal1986). For this, we used the geographic coordinates of each location and Jaccard’s index as a response variable. We compared the effect of the geographic distance in the similarity of species between all locations and between carrasco and caatinga localities. All analyses were performed in the software R 4.0.1 (R Core Team 2020).
Management and bird species associations
We used all our standardized data (point counts and mobbing experiments) in both managed and unmanaged areas, to perform an Indicator Species Analysis (ISA) (Dufrêne and Legendre Reference Dufrêne and Legendre1997) to identify those species that appear to have a tight relationship to habitat condition (managed or unmanaged). ISA can be used to objectively identify those species that present higher fidelity to one kind of habitat over another. This analysis generates values between 0 and 1, where 1 indicates complete fidelity to one of the groups and presence in every site in that given group. Statistical significance was obtained by conducting 9999 permutations. ISA was conducted using the multipart function of the indicspecies package in R (Cáceres and Legendre Reference Cáceres and Legendre2009). For this specific analysis, we used only 141 sampling points, 84 of them in unmanaged areas, and 57 in managed areas, excluding surveys conducted along trail 7 and half of trail 6, which had managed areas on one side and unmanaged areas on the other side of the trail.
Species accounts
Intending to provide baseline data for future studies, we also include a species accounts section, where we mapped results from point counts and mobbing experiments, for species that fulfill one of the requirements of being: i) globally or nationally threatened; ii) representative of caatinga or dry forest specialists (Stotz et al. Reference Stotz, Fitzpatrick, Parker and and Moskovits1996, Araújo and Silva Reference Araújo and da Silva2017); or, iii) showed significant association with unmanaged areas in the ISA analysis. When appropriate, we included current threatened status at the Brazilian (MMA 2018) and global levels (IUCN 2020). Taxonomy and nomenclature follow the Brazilian Committee of Ornithological Records (Piacentini et al. Reference Piacentini, Aleixo, Agne, Maurício, Pacheco, Bravo, Brito, Naka, Olmos, Posso and Silveira2015), and its forthcoming update (CBRO in prep).
Results
Avian surveys
A total of 148 species of birds have been recorded at the Fazenda Pau D´Arco, including 144 species recorded by ourselves and four species by other researchers/observers. Our most efficient surveying method was the opportunistic observations, which resulted in the detection of 129 species, including 36 species not detected by any other quantitative or standardized method (Appendix S1). Point counts were also quite effective, resulting in the detection of 108 species, and provided quantitative georeferenced data on individual species. Mobbing experiments also provided georeferenced data of 72 species (Appendix S1). With 44 species captured, mist-nets were an essential method for collecting specimens but did not contribute species solely recorded by this survey method (Appendix S1). We present documented evidence for more than half (95 spp or 64%) of the species recorded, including 44 species with collected specimens, 59 with audio recordings, and 57 with photographs (Appendix 1). Unfortunately, we lack documented evidence for the Fazenda Pau D’Arco for a relatively large number of species (53), although most of these represent non-controversial records, and most of them are already known from and have been documented at the Araripe Plateau.
Species abundance and seasonality
Our quantitative surveys allowed us to offer a rough characterization of avian abundance at the Fazenda Pau D’Arco, which will be particularly important for future comparisons following the completion of the first management cycle. Only 18 species (12.1%) were fairly abundant, being recorded in more than 20% of our surveys (Appendix S1). On the other hand, 71 species (47.9%) were considered rare and were recorded in less than 1% of our systematic surveys or only recorded through opportunistic observations. Four species (2.7%) were detected and documented by other observers but not by any of our survey methods and were considered occasional (Appendix S1). We acknowledge that these categories are rather subjective, and present continuous abundance values in Appendix S1.
Most species (129 species or 87%) recorded at our study site are likely residents (Appendix S1). However, we recorded at least 19 species that are known to engage in some sort of seasonal movements in the Neotropics, including 18 austral migrants and one longitudinal migrant (Ash-throated Casiornis Casiornis fuscus). We recorded 23 Brazilian endemic species at our study site, including eight caatinga endemics and seven north-eastern Brazilian endemics (Appendix S1).
Dry forest avian affinities
The avifauna of the Fazenda Pau D´Arco represents a subsample of the caatinga avifauna, which is quite clear from our ordination and clustering analyses (Figures 2b and 2c). The avian composition of this carrasco site was significantly grouped with other Caatinga localities, rather than with cerrado localities (PERMANOVA, R²=0.26, P <0.01) (Figure 2b). The avifauna at the Fazenda Pau D’Arco is more similar (measured by Jaccard’s Index) to the avifauna recorded in areas such as, the Catimbau National Park (0.448), Serra das Confusões National Park (0.505), and Serra das Almas Private Reserve (0.505), all of which harbour a mix of shrubby and arboreal caatinga, including carrasco vegetation (Figure 2a). On the other hand, the avifauna of the Fazenda Pau D’Arco is more different from Serra da Bodoquena National Park (0.766), Prosa State Park (0.785), and Paraúna State Park (0.790), areas where cerrado is predominant (Figure 2a, Table S1). Avian similarity, however, has a significant spatial autocorrelation (species dissimilarity and geographic distance), when both major biomes (caatinga and cerrado) are included (Mantel statistic, r = 0.615, P <0.001) (Figure S1). When only the caatinga localities are included, this spatial correlation is no longer significant (Mantel statistic, r = 0.174, P = 0.120) (Figure S1), suggesting that other environmental or ecological variables may be important to define species composition.
Forest management and bird species associations
We found that 24 species of birds recorded during our standardized surveys (point counts and mobbing experiments) had significant associations with either unmanaged or managed areas (Table 1). Fourteen species showed significant associations with unmanaged areas, including six that were not recorded in managed areas at all. These numbers suggest that unless these species are able to colonize old-growth forests, they may go locally extinct when the management cycle is over (Table 1). On the other hand, 10 species showed significant associations with managed areas (Table 1), indicating that some of them may actually benefit from forest management.
Table 1. Indicator Species Analysis (ISA) showing species with significant associations with unmanaged and managed areas, including individual records and p values. The total number of areas in our surveys included 84 unmanaged and 57 managed points. IndVal refers to the Indicator Value index measuring the association between a species and a site group.

Species accounts
We include species accounts for 16 avian taxa (species or subspecies), which include species that are most likely to be affected by forest management in the study area and which can be used as proxies to understand the influence of forest management on the avian community. We present species maps for 14 of those species, based on our quantitative censuses and mobbing experiments in the study area (Figure 3). We do not present occurrence maps for the Yellow-legged Tinamou Crypturellus zabele and Greater Wagtail-tyrant Stigmatura budytoides since neither of them was recorded during our standardized surveys.

Figure 3. Local distribution maps of selected species at the Fazenda Pau D’Arco. Each point represents a sampling point surveyed by quantitative censuses (point counts) and mobbing experiments. Light green points with a white border represent presence points, whereas dark green points depict the absence of records.
Yellow-legged Tinamou Crypturellus zabele (NT/VU)
This taxon, recently elevated to full species (C. zabele) (Tomotani and Silveira Reference Tomotani and Silveira2016,CBRO, in prep.), is endemic to NE Brazil, and a rare inhabitant of caatinga and Atlantic Forests. Although we consider this species as being occasional at the Fazenda Pau D’Arco (Appendix S1), there are several records at this site (W. Girão, pers. obs.). The main threats to this taxon are hunting, trapping, and habitat loss, with population trends decreasing (IUCN 2020).
Rusty-margined Guan Penelope superciliaris
Although this species is widespread in the Neotropics, several taxa have been described for eastern Brazil and the caatinga (del Hoyo and Kirwan Reference del Hoyo, Kirwan, Hoyo, Elliott, Sargatal, Christie and Juana2020), where it is considered the main source of bushmeat for local human populations. Within the caatinga, the Rusty-margined Guan inhabits different types of phytophysiognomies, such as dry and gallery forests. This species was uncommon at the Fazenda Pau D’Arco, where we detected its presence in only a few of our surveys (5/162 points) (Figure 3a), mostly in unmanaged areas. One of our records, however, was obtained in an area that had been managed 12 years before our surveys, suggesting that this species may use second-growth forests. Although this species is not currently considered threatened, population trends appear to be negative (IUCN 2020). Being one of the few species at the site that is currently hunted for meat, we believe that the evidence of hunting found at our study site was likely targeting this species.
Spotted Piculet Picumnus pygmaeus
This caatinga endemic occurs in dry open and dense woodlands (Winkler et al. Reference Winkler, Christie, Bonan, Hoyo, Elliott, Sargatal, Christie and Juana2020a). The Spotted Piculet was uncommon at the Fazenda Pau D’Arco, where we detected its presence in only a few of our surveys (9/162 points) (Figure 3b), including unmanaged and relatively old (>10 yr) second-growth managed areas. It seems to avoid recently logged areas, where we failed to record this species.
Ochraceous Piculet Picumnus limae
This north-east Brazilian endemic inhabits deciduous, semi-deciduous, and secondary stands in the Atlantic Forest. Within the caatinga, it inhabits forested and shrubby dry forest vegetation up to ~1,000 m (Winkler et al. Reference Winkler, Christie, de Juana, Hoyo, Elliott, Sargatal, Christie and Juana2020b). This species was uncommon at the Fazenda Pau D’Arco, where we recorded it in only a few of our surveys (3/162 points) (Figure 3c), in both managed and unmanaged areas at low densities. Recent taxonomic studies have suggested that P. limae is conspecific with P. fulvescens and comprises a single species with a high degree of colour variation and a clinal distribution (Lima et al. Reference Lima, Tomotani and Silveira2020), a treatment adopted by the South American Classification Committee (SACC; https://www.museum.lsu.edu/~Remsen/SACCBaseline05.htm) and by the upcoming checklist of the Brazilian Committee of Ornithological Records (CBRO in prep).
Stripe-backed Antbird Myrmorchilus strigilatus
This dry forest specialist has two allopatric populations in the caatinga and the chaco. The nominate form, endemic to the caatinga, favours dry woodlands up to 1,200 m (Zimmer et al. Reference Zimmer, Isler, Kirwan, Hoyo, Elliott, Sargatal, Christie and Juana2020). With its constant vocalization, the Stripe-backed Antbird had the highest relative abundance at the Fazenda Pau D’Arco, where we detected its presence in most of our surveys (154/162 points) (Appendix S1, Figure 3d), both in managed and unmanaged areas.
Caatinga Antwren Herpsilochmus sellowi
Besides a few restricted Amazonian populations, this species favours the middle and upper strata of Caatinga scrub and deciduous woodlands up to 1,100 m (Zimmer and Isler Reference Zimmer, Isler, Hoyo, Elliott, Sargatal, Christie and Juana2020a). The Caatinga Antwren was abundant (among the 10 most frequent species) at the Fazenda Pau D’Arco, where we detected its presence in most of our surveys (139/162 points) (Figure 3e). Although it does occur in managed areas, our data suggest that this species is more abundant and presents higher individual densities in unmanaged forests (Ribeiro et al. Reference Ribeiro, Las-Casas, Silva and Nakain press a).
Silvery-cheeked Antshrike Sakesphorus cristatus
This caatinga endemic inhabits the understory and mid-storey of arid lowland caatinga, up to 1,100 m (Zimmer and Isler Reference Zimmer, Isler, Hoyo, Elliott, Sargatal, Christie and Juana2020b). The Silvery-cheeked Antshrike was abundant (being among the 10 most frequent species) at the Fazenda Pau D’Arco, where we detected its presence in most of our surveys (151/162 points) (Figure 3f), both in managed and unmanaged areas.
Caatinga Antshrike Thamnophilus capistratus
This caatinga endemic, sometimes considered conspecific with the Barred Antshrike T. doliatus, inhabits dense undergrowth and the mid-storey of deciduous woodland, second-growth forests, and dry scrub (Koloff and Mennill Reference Koloff, Mennill and Schulenberg2020). This species was among the 20 most abundant species at our study site, where we detected its presence at 50/162 points (Figure 3g). Although we detected this species throughout the study area, our data suggest that it is significantly associated with managed areas (Table 1), possibly benefiting from management conditions.
White-browed Antpitta Hylopezus ochroleucus (NT/-)
This caatinga endemic inhabits deciduous and semideciduous dry forests at ~400-1,000 m (Greeney Reference Greeney and Schulenberg2020). We considered this species abundant at the Fazenda Pau D’Arco, where we detected its vocalizations in most of our surveys (144/162 points) (Figure 3h). Although it does occur in both managed and unmanaged areas, this species is more abundant and occurs in higher densities in unmanaged areas (Ribeiro et al. Reference Ribeiro, Las-Casas, Silva and Nakain press a). This species is not currently considered threatened by Brazilian authorities (MMA 2018), but the fragmentation of its populations and the transformation of natural habitats have rendered it a ‘Near Threatened’ global status (IUCN 2020).
Ceara Leaftosser Sclerurus cearensis (VU/VU)
This species, recently split from Sclerurus scansor based on biogeography, genetics, morphology, coloration, and vocalizations (d’Horta et al. Reference d’Horta, Cuervo, Ribas, Brumfield and Miyaki2013), is a NE Brazilian endemic that inhabits both humid and dry forests on a few 1,000-m high plateaus surrounded by caatinga scrub vegetation (del Hoyo et al. Reference del Hoyo, Remsen, Kirwan, Collar, Billerman, Keeney, Rodewald and Schulenberg2020). Its current ‘Vulnerable’ status is mainly due to having a highly fragmented population coupled with habitat loss through logging and wood harvesting. The Ceará Leaftosser was common at the Fazenda Pau D’Arco, where we detected its presence in about a fifth of our surveys (33/162 points) (Figure 3i), but only in areas that have not undergone any forest management (Appendix S1, Table 1). The local distribution at the study site suggests that this species has disappeared from managed areas, demonstrating the importance of forested vegetation patches and conserved areas to the species’ occurrence and persistence.
Great Xenops Megaxenops parnaguae
This caatinga endemic occurs in the interior of north-eastern Brazil, where it favours humid and dense dry woodlands, between 200 and 1,100 m (Remsen Reference Remsen, Hoyo, Elliott, Sargatal, Christie and Juana2020a). Great Xenops was abundant at the Fazenda Pau D’Arco, where we detected its presence in about a third of our surveys (55/162 points) (Figure 3j). Although it does occur in both managed and unmanaged areas, our data suggest that this species is more abundant in unmanaged taller forests (Ribeiro et al. Reference Ribeiro, Las-Casas, Silva and Nakain press a).
Red-shouldered Spinetail Synallaxis hellmayri
This caatinga endemic inhabits several types of dry forest throughout the Brazilian north-east (Remsen Reference Remsen, Hoyo, Elliott, Sargatal, Christie and Juana2020b). The Red-shouldered Spinetail was abundant (among the 10 most frequent species) at the Fazenda Pau D’Arco, where we detected its presence in about a third of our surveys (56/162 points), in both managed and unmanaged areas (Figure 3k).
Ochre-cheeked Spinetail Synallaxis scutata
This species occurs in low dense forests in central and north-eastern Brazil up to 1,700 m (Remsen Reference Remsen, Hoyo, Elliott, Sargatal, Christie and Juana2020c). The Ochre-cheeked Spinetail was uncommon at the Fazenda Pau D’Arco, where we detected its presence in about a fifth of our surveys (31/162 points) (Figure 3l), occurring mostly in unmanaged areas. Although it does occur in some previously logged areas, our data suggest that this species is significantly associated with unmanaged forests (Table 1), possibly avoiding management conditions.
Lesser Wagtail-Tyrant Stigmatura napensis
The subspecies bahiae, sometimes considered a full species, represents a Brazilian and caatinga endemic taxon that inhabits dense, brushy, low vegetation (Fitzpatrick et al. Reference Fitzpatrick, del Hoyo, Kirwan, Collar, Billerman, Keeney, Rodewald and Schulenberg2020). The Lesser Wagtail-Tyrant was uncommon at the Fazenda Pau D’Arco, where we detected its presence in about a tenth of our surveys (15/162 points), mostly in managed areas of at least five years old. We found that the distribution of this species at our study site was significantly associated with managed areas (Table 1), suggesting that it may benefit from forest management (Figure 3m).
Greater Wagtail-Tyrant Stigmatura budytoides
The subspecies gracilis represents a Brazilian and caatinga endemic taxon that inhabits arid scrub, deciduous woodlands, from sea-level to ~1,000 m (Fitzpatrick Reference Fitzpatrick, Hoyo, Elliott, Sargatal, Christie and Juana2020). The Greater Wagtail-Tyrant was rare at the Fazenda Pau D’Arco, where we detected its presence at a single managed site.
Green-winged Saltator Saltator similis
This species is widely distributed in South America occurring in Brazil, Bolivia, Paraguay, Argentina, and Uruguay (Brewer Reference Brewer, Hoyo, Elliott, Sargatal, Christie and Juana2020). It is also widely distributed in Brazil, especially in the central-southern portion of the country, where it inhabits woodlands, gallery forests, forest edges, and clearings, up to 1,200 m. This species was first found and documented by FMGLC at the Fazenda Pau D’Arco in December 2014 (audio recording WA1550839), representing the first record for the state of Ceará. The Green-winged Saltator was uncommon at the Fazenda Pau D’Arco, where we detected its presence at only a few of our surveys (11/162 points) (Figure 3n), mostly in managed areas.
Discussion
This study represents the first attempt to offer quantitative data to characterize the avifauna of the carrasco, an often-neglected vegetation type within the caatinga dry forests. As far as we are aware, all previous ornithological studies conducted in the semiarid interior of north-eastern Brazil that included this vegetation type, also included other phytophysiognomies, preventing thorough analyses on this specific vegetation type (Nascimento et al Reference Nascimento, Nascimento and de Azevedo Júnior2000, Farias et al. Reference Farias, Girão e Silva and Albano2006, Santos et al. Reference Santos, Santana, Soares and Sousa2012, Schunck et al. Reference Schunck, Piacentini, de Souza, de Sousa, Rego, Albano, Nunes, de Lima Favaro, Neto, Mariano and Lima2012, Sousa et al. Reference Sousa, Lima and Lyra-Neves2012, Vasconcelos et al. Reference Vasconcelos, Souza, Duca, Pacheco, Parrini, Serpa, Albano, Abreu, dos Santos and Neto2012). Therefore, by sampling an area exclusively dominated by carrasco vegetation, with relatively low habitat heterogeneity, we can offer a clear description of what we believe is a typical carrasco avifauna. There are three key topics that are worth highlighting. First, despite the suggestion that the carrasco may be biogeographically related to the cerrado of central Brazil, we found that the carrasco avifauna actually represents a subsample of the caatinga one. Second, we obtained quantitative data suggesting that the kind of forest management being conducted at the Fazenda Pau D’Arco does affect its avifauna in significant ways. Third, while some non-forest bird species may benefit from forest management, we found that a larger number of bird species avoid managed areas, including species of conservation concern. Therefore, the results of our surveys are important for the understanding of the current and future effects of forest management, providing baseline data on an avian community that will have ~ 90% of its entire vegetation removed in the next decade.
Biogeographical affinities of the carrasco avifauna
The dense arboreal carrasco vegetation on top of the Araripe Plateau presents a relatively high species diversity for a 2,000-ha dry forest, composed of a typical arboreal caatinga avifauna. Based on our results, it is clear that the avifauna of the Fazenda Pau D’Arco shows biogeographic affinities with other caatinga sites, rather than to any cerrado locality (Figure 2). Although we found a significant spatial correlation (we cannot rule out the effect of distance on species composition; Figure S1), we recorded 19 avian taxa (including species and subspecies) endemic to the caatinga at the Fazenda Pau D’Arco (Appendix S1), and none that is generally considered a cerrado specialist. Interestingly, when considering only caatinga localities, we found no significant effect of distance on avian composition similarities, suggesting that unaccounted ecological factors, such as type of forest cover, altitude, or rainfall, may be involved in defining avian composition patterns. However, our results show the importance of the carrasco vegetation for the overall maintenance of avian caatinga diversity.
The presence of some species in our study area is as interesting as the absence of several others. Many caatinga endemics, which often dominate most of the lowland crystalline basin, were either completely absent (e.g. Caatinga Cacholote Pseudoseisura cristata) or rare (e.g. Red-cowled Cardinal Paroaria dominicana, Campo Troupial Icterus jamacaii, Pale Baywing Agelaioides fringillarius) on the top of the plateau dominated by denser forests. Similarly, dry forest specialists such as White-browed Antpitta Hylopezus ochroleucus or Great Xenops Megaxenops parnaguae (Stotz et al. Reference Stotz, Fitzpatrick, Parker and and Moskovits1996), which are often absent or hard to find in areas of open shrubby caatinga (Mazar Barnett et al. Reference Mazar Barnett, Ingels, Ros and Naka2014), thrive at the Fazenda Pau D’Arco and even represent some of the most abundant species in our quantitative surveys.
Effects of forest management on the avifauna
The Fazenda Pau D’Arco is currently under a forest management program, under which its native vegetation cover will be completely removed from most of its area (except for ~425 ha of protected “legal reserve”). This means that by 2026, 75% of the original forest cover will be replaced by different levels of second-growth forest. While this represents a conservation issue, it also offers a unique opportunity to reach a better understanding of the effect of forest management on dry forest biodiversity.
Our results have shown that several species, such as the Ochre-cheeked Spinetail and the Great Xenops, are already suffering from this management, showing lower densities in managed areas or even being completely absent from them, as was the case of the Ceara Leaftosser (a globally ‘Vulnerable’ species) that was only recorded in areas that were not previously logged. We consider these results problematic, suggesting that the current management scheme may be further threatening degradation-intolerant species that are already threatened by forest loss (del Hoyo et al. Reference del Hoyo, Remsen, Kirwan, Collar, Billerman, Keeney, Rodewald and Schulenberg2020). Furthermore, our results suggest that even after a decade of forest recovery, the Ceara Leaftosser has not recolonized these areas. Although, we cannot rule out that this species may be able to recolonize forest patches after a 25-year recovery cycle, as planned by the management programme, there may not be many individuals left in the area to lead this hypothetical recovery. We believe that is more likely that this species will eventually be extirpated from the entire area unless more areas are left unmanaged at this specific site. The high level of habitat specificity showed by many bird species from other arid environments (Pavey and Nano Reference Pavey and Nano2009) could lead to further losses, including Blue-crowned Trogon Trogon curucui, Flavescent Warbler Myiothlypis flaveola, Red-billed Scythebill Campylorhamphus trochilirostris, or Olivaceous Woodcreeper Sittasomus griseicapillus, all of which are associated with arboreal environments and seem to be affected by the management programme and therefore may be extirpated from the area in the next few years.
On the other hand, we found that some species, such as the Caatinga Antshrike Thamnophilus capistratus and Lesser Wagtail-Tyrant Stigmatura napensis, apparently benefit from forest management. It is known that management frequently causes a reduction of habitat for arboreal species and also a reduction of the soil seed bank, which can directly affect species that are dependent on a dense environment, as well as granivorous species (Zwarts et al. Reference Zwarts, Bijlsma and van der Kamp2018). Meanwhile, it may favour species from other guilds, as appears to be the case at the Fazenda Pau D’Arco.
By providing baseline data and documenting the avifauna before the complete exclusion of the original vegetation, we are providing the means for a full evaluation of the sustainability of this kind of forest management. Our preliminary results indicate that some bird species of conservation concern are significantly affected by forest management and point out the necessity of keeping unmanaged forest patches to avoid local extinctions. Therefore, our work represents an initial first step towards reaching a better understanding of the carrasco avifauna and evaluating the effect of forest management on its biodiversity.
Supplementary Material
To view supplementary material for this article, please visit http://doi.org/10.1017/S0959270921000101.
Acknowledgements
We are extremely grateful to the owners and employees of the Fazenda Pau D’Arco for allowing our access to the area and their sincere interest in the sustainability of their activities. Paulo Maier, former head of the Chapada do Araripe APA and Flávia Domingos, were vital to the success of our activities, offering transport and logistical support during fieldwork. Willian Brito, former head of the Araripe National Forest, allowed us to use their accommodations. We thank the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBIO) and also Aquasis/Araripe Manakin Project for financial and logistical support. We also thank the Brazilian birdwatching community for making their records readily available through online platforms, such as Wikiaves, Xeno-canto, and ebird, which allowed us to obtain data for this work. Finally, we would like to thank the many professionals and students that shared part of the fieldwork with us, including Jefferson Luiz Gonçalves de Lima, Andrei Arrais, Bruna Costa, Lays Viturino de Freitas and Yuri Raia. Stephannie Targino generously helped us gather locality data for our biogeographical comparisons. American Bird Conservancy funded a substantial part of this research. We are grateful to Dr. Pedro Develey of the Society for the Conservation of Birds in Brazil (SAVE Brazil) and Bennett Hennessey, Coordinator of the Brazilian Conservation Program of the American Bird Conservancy for support in this project. Additional funding for this research was granted to LNN by the Fundação de Amparo a Ciência e Tecnologia de Pernambuco FACEPE (APQ- 0337-2.04/15) and the Brazilian Council for Research (CNPq, 432630/2016-3). FMGLC was funded by a CNPq/FACEPE post-doctoral grant (DCR-0018-2 05/15). HL was funded by a CNPQ technical support grant (Apoio técnico à Pesquisa, proc. nº 372272/2018-5 and is currently being funded by a CAPES/PROEX doctoral grant (PROEX Regulation - Public HEIs 88887.373219/2019-00). JRR was funded by a CAPES/CNPQ MSc. grant (130684/2016-3) and by grants provided by CNPq of technical support (372602/2019-3).






