Published online by Cambridge University Press: 22 February 2005
Parasitism often influences the phenotype of individuals. Many of the resulting changes are due to changes in resource allocation that come with infection. Here we examine the effect of a trematode parasite on the shape and defence morphology of a New Zealand freshwater snail, Potamopyrgus antipodarum. The trematode Microphallus sp. asexually produces hundreds of metacercarial cysts in the snail. The length, width and 2-dimensional area of each snail were measured. Snails were also assessed for their degree of spininess. Snails were dissected to determine gender, brooding condition and parasitism. Snails infected with Microphallus sp. were found to be significantly less spiny than uninfected snails. Microphallus-infected snails were also found to have a significantly greater width to length ratio at larger sizes than their uninfected counterparts. These trends could be explained in at least 3 ways. (1) Infection causes the snails to not produce spines and to become wider. (2) Spiny and narrower snails are more likely to die when they become infected. (3) Spiny and narrower snails are more resistant to infection. The changes in phenotype observed are unlikely to be adaptive for either the host or parasite and probably represent physiological by-products of the host-parasite relationship.
Infection by parasites has been shown to influence many aspects of host phenotype including physiology (Thompson, 1990), life-history (Minchella, 1985), behaviour (Moore, 2002), sexually selected traits (Zuk, 1992), and morphology (McCarthy, Fitzpatrick & Irwin, 2004). These effects can change the mean value of a trait, the variance, or both (Poulin & Thomas, 1999). Relatively little work has been done to examine the effect of parasitism on the morphology of molluscs (but see Krist, 1998; McCarthy et al. 2004). Changes in morphology can include changes in shell size, shell shape or shell ornamentation.
Most studies examining the effect of parasitism on shell morphology in molluscs have studied the growth rate. Outcomes vary from no effect of parasitism on growth rate (Fernandez & Esch, 1991), to decreased growth rates (Sousa, 1983; Crews & Yoshing, 1989; Krist & Lively, 1998) to increased growth rates (Rothschild & Rothschild, 1939; Mouritsen & Jensen, 1994; Ballabeni, 1995; Krist, 2000), which is often referred to as gigantism. Gigantism has been interpreted in several ways: (1) as a by-product of the parasitic castration where hosts that no longer invest energy in reproduction divert at least some of this energy into increasing the growth rate, (2) as an adaptation of the host to outlast the parasite (Minchella, 1985), and (3) as an adaptive manipulative effort of the parasite to increase space within the host for parasite growth and reproduction (McCarthy et al. 2004). It is also possible that parasitism may affect the shape by altering the rates of growth in one dimension but not another. A change in growth rate in length but not width, or vice versa, would change the shape and potentially the volume of the host individual. Such changes have been interpreted as adaptive when the volume of the host increased with parasitism and was related to an increase in parasite reproduction (McCarthy et al. 2004).
Parasites may also influence shell ornamentation. Many gastropods produce spiny extensions of the shell as defence or stabilizing structures (Vermeij, 1993). The construction of shell material in molluscs is energetically costly (Brusca & Brusca, 2003). The energetic drain caused by parasitism may leave little energy available for the production of spines.
Here we examine the effect of castrating trematode parasites on two aspects of the morphology of the New Zealand mud snail, Potamopyrgus antipodarum. Potamopyrgus antipodarum is a small Hydrobiid snail commonly found in New Zealand lakes and streams where it grazes on algae and detritus. It is an intermediate host to a suite of castrating trematode parasites. In Lake Alexandrina on the South Island, the most common parasite by far is Microphallus sp., which ranges in infection rate from about 3% to over 50% depending upon time and location within the lake (Jokela & Lively, 1995a; Levri unpublished data). Microphallus has a 2-host life-cycle. Adult worms reproduce sexually in the guts of waterfowl. Eggs are passed out with the faeces and consumed by Potamopyrgus. The eggs eventually produce hundreds of metacercarial cysts castrating the snail host. The life-cycle is completed when infected snails are eaten by waterfowl.
Microphallus has been demonstrated to influence the behaviour (Levri & Lively, 1996; Levri, 1998a,b; Levri, 1999; Levri & Fisher, 2000) and life-history (Lively, 1987; Jokela & Lively, 1995b; Krist & Lively, 1998) of this snail species. The snail varies in maximum length by geographical location (Winterbourn, 1970), and even by depth within a lake (Jokela & Lively, 1995a). Populations vary considerably in spine production, both in frequency of individuals with spines and the size of the spines (Winterbourn, 1970). Both environmental and genetic influences have been implicated in determining the degree of spininess in individuals (Winterbourn, 1970). In the shallow waters of Lake Alexandrina spine production is modest compared to other lakes, as the majority of snails do not produce spines or ridges.
We assessed the effect of Microphallus on the snail's shape (e.g. length to width ratio) and defence morphology (spine production). Potamopyrgus produces spines primarily composed of the periostracum layer of the shell, which is largely protein and thus would be energetically costly to produce (Winterbourn, 1970). The snail also shows substantial variation in shape between locations (Winterbourn, 1970). This variation in shape leads to differences in overall shell volume at a given length. Asexual reproduction by Microphallus within the snail results in hundreds of metacercarial cysts. Theoretically, this reproduction may be limited by shell volume, which has been found in other microphallid-infected gastropods (McCarthy et al. 2004).
Snails were collected from shallow-water habitats (<1 m) in Lake Alexandrina, South Island, New Zealand using dip nets in December of 2002. The snails were preserved in 70% ethanol and returned to the lab for measurement and dissection. Prior to dissection a digital photograph was taken of each snail using a SPOT Insight Digital camera. For the photograph, each snail was oriented in the same way. All were placed with the shell opening facing up. Each snail was measured using SPOT Insight software. Three measurements were taken for each snail (Fig. 1): length from one end to the other, the width of the most recently grown whorl (whorl 1), and the 2-dimensional area of the entire snail from the digital image.

Fig. 1. Drawing of a snail showing the various measurements taken to examine shape. The area of the snail was measured by tracing the outline of the entire 2-dimensional image.
Each snail was then assessed for the degree of spininess. We subjectively categorized each snail prior to dissection using a scale from 0 to 3 (Fig. 2). A score of 0 indicated no spines or ridges. A score of 1 was given to snails with a ridge, a 2 was given to snails with short spines, and a 3 was given to snails with long spines. The spininess score was given based on assessment of the most recently grown whorl of the snail (whorl 1 in Fig. 1). This was done because, in some snails, the spininess changed as the snail grew.

Fig. 2. Photographs of typical snails representing each of the four spininess categories.
Each snail was then dissected and gender, brooding condition, infection and type of parasite was noted for each snail. Parasites were identified in part using the descriptions provided by Winterbourn (1974). The snails were separated into 5 mutually exclusive classes including uninfected non-brooding females, uninfected brooding females, uninfected males, Microphallus-infected snails, and snails infected with parasites other than Microphallus. Snails infected with Microphallus and other parasites were rare and were eliminated from the study.
The effect of parasitism on shell shape was analysed using ANCOVA with the length of the snail used as a covariate, width of the first whorl and the square root of the area of the snail used as dependent variables, snail class as an independent variable, and a Type I sum of squares. The various classes of snails were compared in a pair-wise manner. A Levene's test for homogeneity of variance test was performed for each comparison to test for violations of this assumption of ANCOVA. Each pair of classes was first compared by testing for significant differences in the slopes of the regression lines produced by each class. This was done by examination of the interaction between snail class and length. A significantly different slope indicates a significant difference in shapes between the two classes. If there was no significant difference in slopes between the two classes (or the test yielded marginally significant results [0·01<P<0·10]), a second test was performed without using the snail class by length interaction term in the model. Here a significant effect of class would indicate a significant vertical shift in the regression lines between the two classes and would also indicate a significant difference in shape between the two groups.
The effect of parasitism on spininess was examined by using log-linear analyses comparing the proportion of spiny individuals in each snail class using pair-wise comparisons. In each class of snails, snails between 3 and 3·9 mm in length were compared to snails greater than 3·9 mm in length to determine if size and/or age influenced defence structures. Since this population of snails has relatively few snails with long spines (score of 3), snails with scores of 2 and 3 were grouped together.
A total of 375 snails were measured and dissected for this part of the study, of which 96 were infected by Microphallus, and 15 were infected by other castrating trematodes. These other infections included Telogaster opistorchis, Furcouscercaria, Gymnocephalous, and one or two undescribed species of monostomes (listed in Winterbourn, 1974). An ANCOVA with length as a covariate and the width of the first (most recently grown) whorl as an independent variable was used to compare various groups of snails. Since it is known that the probability of infection increases with age (length) (Jokela & Lively, 1995a), we were concerned that if the relationship between length and width (or square root of the area) was not precisely linear, then differences between groups may be an artifact of differences in length distribution. Thus, the statistical analyses were run under two different conditions. First, only snails greater than 3·0 mm for males and snails greater than 3·7 mm for females (females are on average larger than males) were used in this analysis. Then all snails of all lengths were used in the analysis. Since the results did not differ between the two analyses in every case but one, we present most of the data using all lengths of snails. The only exception is in comparing uninfected non-brooding female snails to uninfected brooding female snails. In this case, the analysis using snails of all sizes did not meet the assumption of equality of variances, but using sizes greater than 3·0 mm and less than 4·5 mm did meet the assumption. Detailed results from the statistical analysis of shape can be found in the Appendix.
Uninfected brooding females were found to be significantly longer than uninfected non-brooding females (P<0·001), uninfected females were found to be significantly longer than uninfected males (P<0·001), and infected snails were found to be significantly longer than uninfected snails (P<0·001). No difference in shape was found between brooding and non-brooding females (see Tables 1 and 2) thus brooding and non-brooding females were grouped together in subsequent analyses. Uninfected female snails were found to have a significantly greater width per unit length than uninfected males (Table 1). Because of this, infected and uninfected males and females were not grouped in subsequent analyses. Microphallus-infected female snails were found to have significantly different slopes from uninfected female snails (Tables 1 and 2). On average, infected snails had a greater width to length ratio as length increased (Fig. 3A). Microphallus-infected males were not significantly different from uninfected males (Tables 1 and 2). However, there was a very low sample size of infected males (7). Low sample sizes of snails infected by other castrating trematodes limited our ability to detect differences between them and other classes. However, the regression line of snails infected with other parasites was very similar to Microphallus-infected snails (Fig. 3B). An analysis with length as the covariate and the square-root of the 2-dimensional area yielded similar results to the analysis utilizing the width of the first whorl (Table 2).

Fig. 3. The relationship between length and width of whorl 1 in Microphallus-infected and uninfected snails (A) and Microphallus-infected snails and snails that are infected with parasites other than Microphallus (B). Microphallus-infected snails show a significantly different shape than uninfected snails. P-values on the graphs represent the tests performed to determine differences in slopes.

Table 2. Results of ANCOVA using length as a covariate and the square root of the 2-dimensional area as an independent variable (Vertical shift was not tested for uninfected females compared to Microphallus-infected snails because of the strongly significant differences in slopes.)

A total of 2574 snails were scored and dissected for this part of the study, of which 1129 were infected by Microphallus, and 154 were infected by other castrating trematodes.
No effect of length was found when comparing large to small individuals in any snail class (P>0·33 in all cases). Thus, large and small individuals were grouped together for subsequent analyses. The uninfected classes (non-brooding females, brooding females, and males) showed no differences in their proportion of spiny individuals (at least P>0·24 in all cases). Significant differences were found between all infected snails and all uninfected snails (Table 3; Fig. 4), as well as between Microphallus-infected snails and all uninfected snail classes (P<0·01 in all cases). In general, there were fewer spiny individuals found in the infected classes. No difference was found between Microphallus-infected snails and snails infected with other castrating trematodes, but no difference was also found between snails infected with trematodes other than Microphallus and uninfected snails. The sample size of snails infected with parasites other than Microphallus was limited here, however.

Fig. 4. The proportion of uninfected, Microphallus-infected, and snails infected with other parasites within each spininess class. Microphallus-infected snails are significantly less spiny than uninfected snails.

Snail classes could have different shapes in two possible ways. First, the slopes of the regression lines of the classes could be significantly different. This was the case for snails infected with Microphallus compared to uninfected females. Here the slope of the line for Microphallus-infected snails was significantly greater than the slope of uninfected females, indicating greater width to length and square root area to length ratios. This result is probably best explained by the fact that infected snails were once uninfected. The probability of infection increases with age and older (longer) snails are more likely to have been infected for a longer time than younger (shorter) snails. Thus the smaller infected snails used in the analysis were likely only recently infected. At small sizes there was little difference between infected and uninfected snails due to the lack of time for the parasite to have any pronounced effect on growth. However, for larger snails, a significant proportion of snails were infected for a longer period of time, and the difference between infected and uninfected snails is more pronounced. The regression lines of Microphallus-infected snails and uninfected females intersect at about 3·7 mm in length. Detectable Microphallus-infection does not usually become common in snails until a length of about 4·0 mm.
If the slopes of the regression lines were not significantly different, an ANCOVA was performed to test for a vertical shift in the regression lines. Such a shift would indicate a significantly greater width to length ratio (or square root area to length ratio) at all lengths. Such a difference was found between uninfected males and uninfected females. Since some of the differences in slopes were marginally significant, the ANCOVA was performed as a precaution in case the slope differences were simply due to chance.
Here we show that Potamopyrgus antipodarum infected with Microphallus are shaped differently from and are less likely to produce spines than uninfected snails. These results could be explained in at least three ways. (1) The infection influences the growth pattern and spine production in the snails. (2) Wider and smoother snails are more likely to become infected. The probability of this is reasonable in this system because the snails in Lake Alexandrina (as well as other lakes) live in a mixed population of sexual and asexual individuals, and the frequency of clones varies over time. Some clones tend to be spinier than others, and some clones have shown greater abilities than others to resist infection (Lively & Dybdahl, 2000; Lively, personal communication). (3) Parasitism results in differential mortality due to higher parasite-induced death rates in narrower and spinier snails. Spiny snails are allocating more energy to spine production than smooth snails. When infected, the parasite usurps a certain amount of resources from the snail, and if the snails make spines as well, there may not be enough resources remaining to sustain the snail, thus increasing mortality. With regard to shape, if a certain number of metacercariae are always produced, that number in a wider snail may be able to be sustained, while in a narrow snail that number of metacercariae may stress the snail to a greater degree, increasing mortality. This seems unlikely from an evolutionary standpoint however. It would make more sense for the parasite to adjust the number of metacercariae based on the size of the snail. To produce too many may result in the death of the parasite as well. Although, if a certain number of metacercariae are required to reach a critical threshold population size in the waterfowl gut, selection may act to maintain a high number of metacercariae despite the mortality cost to the snail and parasite.
Since this study was performed on snails captured and preserved in the field, we cannot differentiate between these alternatives here. Anecdotally, however, with regard to spine production, we noticed that infected snails often had a smooth first (most recently produced) whorl, but would have spiny second or third whorls. This suggests that spininess changed since the time of infection in these individuals. Unfortunately, this was not quantified during this study. We plan future studies to quantify the change in spininess within snails as they age and future experiments utilizing experimental infections to distinguish among the above three hypotheses.
If infection does cause changes in shape and defence morphology in individuals, then these changes could result in reduced fitness consequences for the snails. Spines in this system are hypothesized to reduce the probability of being eaten by predatory fish. The most common fish in Lake Alexandrina, the common bully (Gobiomorphus cotidianus), has been shown to eat Potamopyrgus in relatively large numbers (Levri, 1998a). Gobiomorphus is gape limited and has been shown to consume only snails that are as long or smaller than the width of its mouth (Levri, 1998a). Thus an increase in width of the snail caused by spines or ridges may decrease the probability of predation by this fish. Infection resulting in reduced spininess may increase the mortality rate of the snails due to predation and also increase the mortality rate of the parasite in the process. Changes in shell shape can influence fitness by changing the ability of the shell to withstand the force of crushing predators and thus increase the likelihood of surviving an attack (Appleton & Palmer, 1988; Krist, 1998, 2002).
In this study, uninfected females were found to be significantly longer than uninfected males, uninfected brooding females were found to be significantly longer than uninfected non-brooding females, and infected snails were found to be significantly longer than uninfected snails. All of these results are consistent with the results of previous studies utilizing this system (Jokela & Lively, 1995b; Levri & Lively, 1996). Since females brood their offspring in a brood chamber, there is likely to be a reproductive advantage for larger females. Larger females have on average larger broods (Levri, unpublished data). As females get larger, a greater proportion of them can be found brooding, thus increasing the mean length of brooding females compared to non-brooding females (Levri, unpublished data). The reason for a greater length of infected snails is less straightforward. Infected snails grow at a slower rate than uninfected snails, at least when infected as juveniles (Krist & Lively, 1998). The most likely explanation is simply that the probability of having encountered a parasite increases with age. Thus older (longer) snails have a greater probability of being infected (Jokela & Lively, 1995a).
It cannot be determined for either shape or spininess whether the effects are Microphallus-specific or not. In both cases, snails infected with parasites other than Microphallus were not significantly different from Microphallus-infected snails and uninfected snails. The lack of differences here are likely due to the limited sample sizes of snails infected with other parasites. However, visually, snails infected with other parasites appear more similar to Microphallus-infected snails than to uninfected snails. This suggests, inconclusively, that parasitism, in general, results in changes in shape and defence morphology. If true, this would make explanations for the changes due to adaptations on the part of Microphallus less likely.
McCarthy et al. (2004) found that Littorina saxatilis infected by Microphallus piriformes were shaped differently than uninfected snails. They concluded that the effect of the parasite was adaptive in that it increased the volume of infected snails resulting in increased space for the asexual production of metacercariae. Results here are a bit different. McCarthy et al. (2004) found that parasitism resulted in snails with a greater length to width ratio, while here we found that infected snails have a lower length to width ratio. Also, Krist & Lively (1998) found that juvenile Potamopyrgus antipodarum infected with Microphallus grew at a significantly slower rate than uninfected snails. Coupled with these results, it appears that the parasite may decrease the rate of growth in length to a greater degree than it influences the growth in width. This appears to make the adaptive manipulation of shape by the parasite in this system less likely. However, it is possible that given reduced growth rate caused by parasitism, the parasite may be making the best of a bad situation by increasing the growth in width and thus volume.
In conclusion, parasitism in Potamopyrgus is related to shell shape and defence morphology. Infected snails show a lower probability of being spiny and tend to be wider for a given length as length increases. The mechanism that results in this relationship cannot be determined from this study.
We would like to thank Carolyn Itle for help with snail collection in the field, Becky Carson, Tessa Strittmatter, Shane Lunnen, Erica Meyers, Leocadia Mosquea, Brian Kinkade, Robert Platt, and Justin Winters for assistance in the lab, and Jukka Jokela and Jeffrey Plochocki for statistical advice. We are indebted to Jan MacKenzie and the Edward Percival Field Station and Jack van Berkel for logistical support in New Zealand. The manuscript was improved by comments from Maureen Levri. This project was supported by grants from Penn State – Altoona and the Darbaker Research Grant from the Pennsylvania Academy of Science.
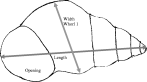
Fig. 1. Drawing of a snail showing the various measurements taken to examine shape. The area of the snail was measured by tracing the outline of the entire 2-dimensional image.

Fig. 2. Photographs of typical snails representing each of the four spininess categories.
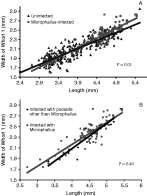
Fig. 3. The relationship between length and width of whorl 1 in Microphallus-infected and uninfected snails (A) and Microphallus-infected snails and snails that are infected with parasites other than Microphallus (B). Microphallus-infected snails show a significantly different shape than uninfected snails. P-values on the graphs represent the tests performed to determine differences in slopes.

Table 1. Results of ANCOVA using length as a covariate and width of whorl 1 as an independent variable

Table 2. Results of ANCOVA using length as a covariate and the square root of the 2-dimensional area as an independent variable

Fig. 4. The proportion of uninfected, Microphallus-infected, and snails infected with other parasites within each spininess class. Microphallus-infected snails are significantly less spiny than uninfected snails.

Table 3. Results of pair-wise log-linear analyses comparing proportion of spiny individuals between snail classes